Solvent-polarity reconfigurable fluorescent 4-piperazino-N-aryl-1,8-naphthalimide crown ether logic gates†
Abstract
Four compounds 1–4 were designed and synthesised, comprising a 4-amino-N-aryl-1,8-naphthalimide fluorophore, a piperazine receptor, and an aryl group, as fluorescent logic gates. At the imide position, the substituent is phenyl (1), 1,2-dimethoxyphenyl (2), benzo-15-crown-5 (3), or benzo-18-crown-6 (4). Molecules 1 and 2 are constructed according to a fluorophore–spacer–receptor format, while 3 and 4 are engineered according to a receptor1–spacer1–fluorophore–spacer2–receptor2 format based on photoinduced electron transfer and internal charge transfer mechanisms. The compounds were studied in water, water/methanol mixtures of different ratios, and methanol by UV-visible absorption and steady-state fluorescence spectroscopy, as a function of pH, metal ions and solvent polarity. The excited state 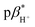 of 1–4 is 8.4 ± 0.2 in water, 7.6 ± 0.1 in 1 : 1 (v/v) water/methanol, and 7.1 ± 0.3 in methanol. The
of 1–4 is 8.4 ± 0.2 in water, 7.6 ± 0.1 in 1 : 1 (v/v) water/methanol, and 7.1 ± 0.3 in methanol. The 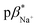 of 3 in water is 0.92 and the
of 3 in water is 0.92 and the 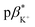 and
and 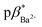 of 4 in water are 2.3 and 2.9. 1H NMR data in D2O and CD3OD confirm H+ interaction at the piperazine moiety, and Na+ and Ba2+ binding at the benzo-15-crown-5 and benzo-18-crown-6 moieties of 3 and 4. By altering the solvent polarity, the fluorescent logic gates can be reconfigured between TRANSFER logic and AND logic. Molecules with polarity reconfigurable logic could be useful tools for probing the microenvironment of cellular membranes and protein interfaces.
of 4 in water are 2.3 and 2.9. 1H NMR data in D2O and CD3OD confirm H+ interaction at the piperazine moiety, and Na+ and Ba2+ binding at the benzo-15-crown-5 and benzo-18-crown-6 moieties of 3 and 4. By altering the solvent polarity, the fluorescent logic gates can be reconfigured between TRANSFER logic and AND logic. Molecules with polarity reconfigurable logic could be useful tools for probing the microenvironment of cellular membranes and protein interfaces.



 Please wait while we load your content...
Please wait while we load your content...