The formation and stability of fluoxetine HCl cocrystals investigated by multicomponent milling†
Abstract
Competitive milling (CM) and stability milling (SM) mechanochemical reactions are used to comprehensively assess the relative thermodynamic stabilities and cocrystallization affinities of three pharmaceutical cocrystals (PCCs) of fluoxetine HCl (X) with three different pharmaceutically acceptable coformers (PACs, i.e., benzoic acid (B), fumaric acid (F), and succinic acid (S)). CM reactions, which involve milling X in the presence of two or more different PACs, were used to determine cocrystallization affinities, whereas SM reactions, which involve milling a PCC of X with a different coformer, were used to determine relative thermodynamic stabilities. In certain cases, SM reactions exhibited a remarkable solid-state exchange of coformers, yielding new cocrystalline forms. 35Cl (spin I = 3/2) SSNMR is used as the primary probe of the products of CM and SM reactions, providing a reliable means of identifying and quantifying chloride ions in unique hydrogen bonding environments in each reaction mixture (13C SSNMR spectra and pXRD patterns are used in support of these data). On the basis of these reactions and data, the PAC cocrystallization affinities with X are B > F ≈ S (most to least preferred), and the PCC stabilities are XB > X2F ≈ X2S (most to least preferred), corresponding to enthalpies of cocrystallization ranked as ΔHCCXB < 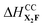 ≈
≈ 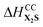 . PAC affinities and PCC stabilities were found to be the same for products of analogous slow evaporation experiments and mechanochemical reactions with extended milling times (i.e., 90 minutes). Preliminary plane-wave DFT-D2* calculations are supportive of cocrystal formation; however, challenges remain for the quantification of relative enthalpies of cocrystallization. This work demonstrates the great potential of CM and SM reactions for providing pathways to the rational design, discovery, and manufacture of new cocrystalline forms of APIs.
. PAC affinities and PCC stabilities were found to be the same for products of analogous slow evaporation experiments and mechanochemical reactions with extended milling times (i.e., 90 minutes). Preliminary plane-wave DFT-D2* calculations are supportive of cocrystal formation; however, challenges remain for the quantification of relative enthalpies of cocrystallization. This work demonstrates the great potential of CM and SM reactions for providing pathways to the rational design, discovery, and manufacture of new cocrystalline forms of APIs.



 Please wait while we load your content...
Please wait while we load your content...
