The charge effects on the hydrogen evolution reaction activity of the defected monolayer MoS2†
Abstract
Doping engineering has proven to be an effective way to tune the hydrogen evolution reaction (HER) activity of MoS2. Introducing these defects could cause the overall charge imbalance of MoS2, which makes MoS2 charged. In order to understand the effect of charge on the HER activity of the defected MoS2, we systematically investigate the formation energies, hydrogen adsorption Gibbs free energy (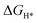 ), and electronic structures of 3d, 4d, and 5d transition metal (TM) doped monolayer MoS2 with S vacancies (Svac) based on the density functional theory (DFT) calculations. According to the formation energy calculation, Svac in the 0 and −1 charge states (S0vac and Svac1−) is found to be stable.
), and electronic structures of 3d, 4d, and 5d transition metal (TM) doped monolayer MoS2 with S vacancies (Svac) based on the density functional theory (DFT) calculations. According to the formation energy calculation, Svac in the 0 and −1 charge states (S0vac and Svac1−) is found to be stable. 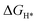 of Svac1− is −0.16 eV, suggesting its HER catalytic activity is lower than that of Pt (
of Svac1− is −0.16 eV, suggesting its HER catalytic activity is lower than that of Pt (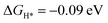 ), which is consistent with the experimental results. By substituting the Mo atom with TM atoms, we found that the TM atoms in groups VB-VIIB can promote the generation of Svac, forming defect complexes (TMMoSvac).
), which is consistent with the experimental results. By substituting the Mo atom with TM atoms, we found that the TM atoms in groups VB-VIIB can promote the generation of Svac, forming defect complexes (TMMoSvac). 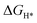 is greatly affected by the charge state of defects; TMMoSvac defects (TM = V, Nb, Ta, Cr, W, Mn, and Re) in −1 charge states exhibited excellent HER activity (
is greatly affected by the charge state of defects; TMMoSvac defects (TM = V, Nb, Ta, Cr, W, Mn, and Re) in −1 charge states exhibited excellent HER activity (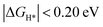 ). Significantly, W and Re doping can promote the HER activity of MoS2 independent of the charge state and the Fermi level, which suggests that W and Re doping are most beneficial to improve the HER activity of MoS2. Therefore, the HER activity of defected MoS2 is not only influenced by
). Significantly, W and Re doping can promote the HER activity of MoS2 independent of the charge state and the Fermi level, which suggests that W and Re doping are most beneficial to improve the HER activity of MoS2. Therefore, the HER activity of defected MoS2 is not only influenced by 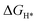 as previously thought, but also by formation energies, charge state and Fermi level position of defects. The underlying physics could be deduced from the charge-induced changes in electronic structures. Our work highlights the defect charge effects on the electrochemical reactions and offers plausible mechanisms of defect charge effects.
as previously thought, but also by formation energies, charge state and Fermi level position of defects. The underlying physics could be deduced from the charge-induced changes in electronic structures. Our work highlights the defect charge effects on the electrochemical reactions and offers plausible mechanisms of defect charge effects.



 Please wait while we load your content...
Please wait while we load your content...