Rich or poor: the impact of electron donation and withdrawal on the photophysical and photocatalytic properties of copper(i) complexes†
Abstract
Motivated by the need to design efficient photocatalysts based on earth-abundant metals and to elucidate essential structure–property relationships, this study deals with heteroleptic copper(I) complexes. A systematic series of four novel diimine–diphosphine copper(I) complexes of the type [Cu(N^N)(P^P)]+ with different electron donating and withdrawing substituents in the 4 and 7-positions of the phenanthroline moiety has been prepared and compared to those with substituents in the 5 and 6-positions. In addition, two crystal structures were obtained, including that of the important synthesis intermediate [Cu(MeCN)2(P^P)]+. Accompanying DFT calculations revealed the orbital energies and HOMO–LUMO gaps, which are strongly dependent on the substituents. Most strikingly, substitution at the 4 and 7-positions leads to better absorption properties of the resulting complexes than that at the 5 and 6-positions. Within the series, the bistrifluoromethyl groups (CF3)2 play a special role, e.g. the Cu(CF3)2 complex is the only one that can be reduced twice. Moreover, Cu(CF3)2 represents the strongest excited state oxidant, whose potential is shifted by 90 mV compared to the reference complex CuH lacking substituents. The production of reactive singlet oxygen (1O2), including the catalytic oxygenation of 2,5-diphenylfuran (DPF), and the photocatalytic reductive dehalogenation of aryl halides were then chosen to evaluate the activity of these complexes. In fact, all complexes are good sensitizers generating 1O2 with singlet oxygen quantum yields 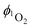 of up to 54% and a DPF oxygenation efficiency that depends on the electron donation ability of the respective substituents. A screening of different substrates, including the challenging 2-chloropyridine, and catalysis parameters demonstrated that these copper(I) complexes are very potent photocatalysts for reductive dehalogenations. They enable high yields >90% and fast reactions with maximum turnover frequencies of 1180 h−1 (Ar–Br) and 2939 h−1 (Ar–I), exceeding previous benchmark complexes.
of up to 54% and a DPF oxygenation efficiency that depends on the electron donation ability of the respective substituents. A screening of different substrates, including the challenging 2-chloropyridine, and catalysis parameters demonstrated that these copper(I) complexes are very potent photocatalysts for reductive dehalogenations. They enable high yields >90% and fast reactions with maximum turnover frequencies of 1180 h−1 (Ar–Br) and 2939 h−1 (Ar–I), exceeding previous benchmark complexes.



 Please wait while we load your content...
Please wait while we load your content...