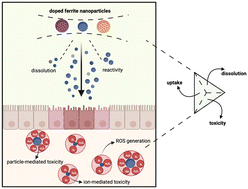
Owing to their remarkable properties in terms of electrical resistivity, chemical stability, and saturation magnetisation, ferrite nanoparticles are being increasingly used for a wide range of applications. This study looks to investigate as to whether ferrite nanoparticles can be safely and viably doped with transition metal elements without adversely affecting the stability and toxicity of the nanoparticles. Monodispersed and phase pure variants of ferrites (MxFe3−xO4 where M = Co, Cu, Zn, Mn) were synthesised with a size range of 9–11 nm using a wet chemistry route. The doping % within the ferrites was within the range of 15–18% for all the dopants. Compared to ferrite nanoparticles, Co and Mn doping significantly enhanced the dissolution, whereas doping with Cu and Zn had an opposite effect to dissolution. DFT calculations performed on the ferrites to calculate the vacancy formation energy of Fe and dopant atoms substantiated the experimental dissolution data. A549 cells showed a dose dependent response (10–200 μg mL−1) and the reduction in cell viability followed the trend of MnxFe3−xO4 > CoxFe3−xO4 > ZnxFe3−xO4 > CuxFe3−xO4 > Fe3O4. A correlation study between dissolution, cell viability and uptake indicated cell viability and dissolution had a strong negative correlation for Fe3O4, and CoxFe3−xO4 whereas for CuxFe3−xO4 this correlation was very weak. We conclude by providing an overview of the impact of doping on the safety of other metal-oxide nanoparticles (CuO, ZnO, TiO2 and CeO2) in comparison to ferrite nanoparticles.