Prediction of second-order rate constants of the sulfate radical anion with aromatic contaminants using the Monte Carlo technique†
Abstract
It has been proved that the removal of aromatic contaminants from the environment with sulfate radical anion (SO4˙−)-based advanced oxidation technology is an effective method. Thus, knowing the second-order rate constants between aromatic contaminants and a sulfate radical 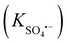 is important for evaluating the advanced oxidation processes. In the present manuscript, the quantitative structure–property relationship (QSPR) technique using the inbuilt Monte Carlo algorithm of CORAL software is employed to model the second-order rate constants of the sulfate radical for a dataset of 88 aromatic contaminants. The dataset is used to construct ten random splits and each of them is divided into four sets (i.e. active training, passive training, calibration, and validation). To construct QSPR models, a hybrid optimal descriptor is applied using a combination of SMILES and HFG (hydrogen-filled graph). Four target functions i.e. TF0 (WIIC = WCII = 0), TF1 (WIIC = 0.5 and WCII = 0), TF2 (WIIC = 0 and WCII = 0.3) and TF3 (WIIC = 0.5 and WCII = 0.3) are employed to build 40 QSRR models. The statistical outcomes of each target function are compared with each other. The better predictive potential is obtained for the models generated by the combination of the index of ideality of correlation (IIC) and correlation intensity index (CII). The statistical quality of the established QSPR model of split 5 calculated by TF3 is better than that of the other models, so it is considered to be the best model (Rvalidation2 = 0.8162, IIC = 0.6554, CII = 0.8787, CCC = 0.8734, Q2 = 0.7778 and RMSE = 0.2213). The promoters of increase and decrease of rate constants are also extracted from three splits 4, 5 and 8.
is important for evaluating the advanced oxidation processes. In the present manuscript, the quantitative structure–property relationship (QSPR) technique using the inbuilt Monte Carlo algorithm of CORAL software is employed to model the second-order rate constants of the sulfate radical for a dataset of 88 aromatic contaminants. The dataset is used to construct ten random splits and each of them is divided into four sets (i.e. active training, passive training, calibration, and validation). To construct QSPR models, a hybrid optimal descriptor is applied using a combination of SMILES and HFG (hydrogen-filled graph). Four target functions i.e. TF0 (WIIC = WCII = 0), TF1 (WIIC = 0.5 and WCII = 0), TF2 (WIIC = 0 and WCII = 0.3) and TF3 (WIIC = 0.5 and WCII = 0.3) are employed to build 40 QSRR models. The statistical outcomes of each target function are compared with each other. The better predictive potential is obtained for the models generated by the combination of the index of ideality of correlation (IIC) and correlation intensity index (CII). The statistical quality of the established QSPR model of split 5 calculated by TF3 is better than that of the other models, so it is considered to be the best model (Rvalidation2 = 0.8162, IIC = 0.6554, CII = 0.8787, CCC = 0.8734, Q2 = 0.7778 and RMSE = 0.2213). The promoters of increase and decrease of rate constants are also extracted from three splits 4, 5 and 8.



 Please wait while we load your content...
Please wait while we load your content...