A first-principles alternative to empirical solvent parameters†
Abstract
The use of solvents is ubiquitous in chemistry. Empirical parameters, such as the Kamlet–Taft parameters and Gutmann donor/acceptor numbers, have long been used to predict and quantify the effects solvents have on chemical phenomena. Collectively however, such parameters are unsatisfactory, since each describes ultimately the same non-covalent solute–solvent and solute–solute interactions in completely disparate ways. Here we hypothesise that empirical solvent parameters are essentially proxy measures of the electrostatic terms that dominate solvent–solute interactions. On the basis of this hypothesis, we develop a new fundamental descriptor of these interactions, 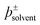 , and show that it is a self-consistent, probe-free, first principles alternative to established empirical solvent parameters.
, and show that it is a self-consistent, probe-free, first principles alternative to established empirical solvent parameters.

- This article is part of the themed collection: Showcasing Physical Chemistry research in Australia and New Zealand


 Please wait while we load your content...
Please wait while we load your content...