Copper-doped LaCoO3 for direct propylene epoxidation: a DFT study†
Abstract
There are few reports on the direct epoxidation of propylene catalyzed by LaCoO3 perovskite to form propylene oxide (PO) (both experimental and theoretical studies), especially the promoting effect of Cu doping. Herein, we report a comprehensive mechanistic study using both DFT calculations and microkinetic simulations for undoped and Cu-doped LaCoO3(110)–Cl to explore the effects of Cu doping in LaCoO3 perovskite towards PO selectivity. The propylene oxidation process consists of two parallel pathways, i.e., allylic hydrogen stripping and propylene oxametalcycle (OOMMP) intermediate mechanisms. Our results indicated that doping Cu has little effect on the selectivity for PO on LaCoO3 without Cl due to its very low reactivity. Alternatively, in the presence of Cl, copper doping not only lowers the strength of the Brønsted base of molecular 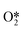 , and thus disfavors the propylene α-H striping process, leading to higher OOMMP intermediate formation selectivity, but also enhances the secondary chemistry, improving both the selectivity and activity for PO formation. Moreover, the microkinetic modelling results showed that the Cu-doped LaO-terminated LaCoO3(110)–Cl surface has higher selectivity for PO than that of the Cu-doped CoO-terminated LaCoO3(110)–Cl surface. It is hoped that the present work will help researchers better understand the mechanism of Cu doping in LaCoO3-like perovskite catalysts for PO formation reactions.
, and thus disfavors the propylene α-H striping process, leading to higher OOMMP intermediate formation selectivity, but also enhances the secondary chemistry, improving both the selectivity and activity for PO formation. Moreover, the microkinetic modelling results showed that the Cu-doped LaO-terminated LaCoO3(110)–Cl surface has higher selectivity for PO than that of the Cu-doped CoO-terminated LaCoO3(110)–Cl surface. It is hoped that the present work will help researchers better understand the mechanism of Cu doping in LaCoO3-like perovskite catalysts for PO formation reactions.



 Please wait while we load your content...
Please wait while we load your content...