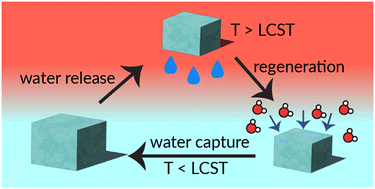
Water-harvesting polymer materials have the potential to create new sources of potable water. However, a holistic understanding of the relationship between polymer structure and water-harvesting properties is lacking compared to studies on specific materials. In this work, we synthesised a library of methacrylic acid-co-poly(ethylene glycol) methyl ether methacrylate)-based hydrogels (poly(MAA-co-PEGMA)) with directed modifications, including composition, crosslinker lengths, crosslinking density and preparation of the hydrogels. MAA serves as a hygroscopic monomer while PEGMA provides hydrophilicity and thermoresponsive properties. The water uptake and release capabilities of all materials was also assessed. The optimised composition of the copolymer (75 : 5 : 20 MAA : EGDMA : PEGMA, mole%) has a water uptake of 98 mg g−1 polymer at 60% RH after 24 hours. The poly(MAA-co-PEGMA) materials also show a capability for water release, showing no significant decrease in water uptake capacity after repeated uptake-release cycles. Minimum temperatures for water release could easily be adjusted with polymer composition, ranging from 50–70 °C. The data presented in this body of work serves as a foundation for future efforts in creating thermoresponsive, water-harvesting polymers with real-world applications.