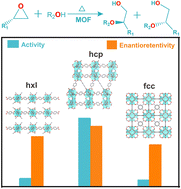
Highly enantioretentive alcoholysis of epoxides is an important way to synthesize enantiopure β-alkoxy alcohols, which are irreplaceable intermediates demanded by biomedicines, fine chemicals and other industries. In this report, we exploit a series of Zr-based metal–organic frameworks (Zr-MOFs) as the catalysts to achieve high activity and enantioretentivity in the alcoholysis of styrene oxide via modulating their assembly fashions. It is explored that hcp-UiO-66 not only exhibits a ∼10 fold improved catalytic activity than both hxl-CAU-26 and fcc-UiO-66 of varied assemblies but also maintains superior product enantioretentivity. Theoretic calculations together with experimental proof discloses the origin of distinct catalytic activity caused by different assembly fashions. This assembly modulation strategy offers a potential protocol for seeking high-performance catalysts among MOFs by virtue of their rich polymorphisms.