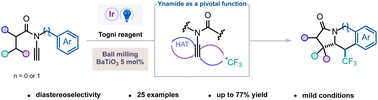
Radical cyclization cascades triggered by a trifluoromethyl radical represent a valuable tool for accessing highly complex and biologically relevant scaffolds. Herein, we introduce a strategy employing ynamides as pivotal functions for the diastereoselective synthesis of γ-lactam polycyclic compounds incorporating a trifluoromethyl group. Using photoredox conditions, this cascade featuring notably a 1,5 hydrogen atom transfer proceeds with high efficiency and diastereoselectivity. Additionally, a mechanoredox approach utilizing piezocatalysis proved to be competent in providing the same highly functionalized polycyclic products.