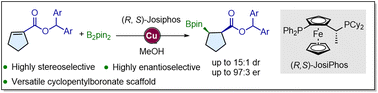
The optically-enriched cyclopentylborane derivatives containing consecutive stereogenic centers are of great use for the construction of chiral cyclopentanes. The preceding examples are limited to specialized substrates and suffer from a lack of generality, and the access to consecutive stereogenic centers remains elusive. Here we disclose a copper catalyzed conjugative borylation of cyclopentenylcarboxyesters, for accessing cyclopentyl boronates bearing a consecutive stereogenic center in a diastereoselective and enantioselective fashion. This method could be scaled up and the C–B bond in products could be easily transformed in late-stage functionalization. Furthermore, modeling for rationalization of the enantioselectivity and diastereoselectivity is proposed, which suggested that borylcupration is probably the enantio-determining step while the proton-decupration is the diastereo-determining step.