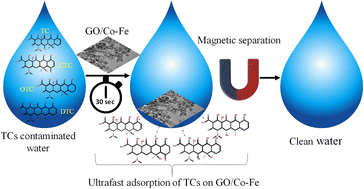
In this work, a graphene oxide-supported cobalt–iron oxide (GO/Co–Fe) magnetic nanocomposite was successfully synthesized using waste dry cells for the efficient and simultaneous removal of tetracycline (TC), chlortetracycline (CTC), oxytetracycline (OTC), and doxycycline (DTC) from aqueous solutions. The GO/Co–Fe nanocomposite was thoroughly characterized using Fourier transform infrared spectroscopy, vibrating sample magnetometry, X-ray diffraction, field emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and zeta potential analysis. This multi-faceted characterization provided clean insights into the composition and properties of the synthesized nanocomposite. The adsorption of tetracyclines (TCs) was systematically investigated by assessing the influence of critical factors, such as adsorbent dosage, contact duration, initial pH of the solution, initial concentration, and temperature. The GO/Co–Fe adsorbent showed high removal efficiencies of 94.1% TC, 94.32% CTC, 94.22% OTC, and 96.94% DTC within 30 s contact period. The maximum removal efficiency of TCs was found at a low adsorbent dose of 0.15 g L−1. Notably, this superior removal efficiency was achieved at neutral pH and room temperature, demonstrating the adsorbent's efficacy under environmentally viable conditions. The kinetic studies demonstrated that the adsorption process was fitted satisfactorily with the pseudo-second-order model. Additionally, the adsorption behaviour of TCs on the GO/Co–Fe adsorbent was assessed by isotherm models, Langmuir and Freundlich. The experimental data followed the Langmuir isotherm, signifying a monolayer adsorption mechanism on the surface of the adsorbent. The adsorption capacities (qm) of GO/Co–Fe for TC, CTC, OTC and DTC were determined to be 64.10, 71.43, 72.46 and 99.01 mg g−1, respectively. Importantly, the GO/Co–Fe adsorbent showed reusability capabilities. The super magnetic properties of GO/Co–Fe made it easy to use for several cycles. These results clearly establish GO/Co–Fe as an exceptionally effective adsorbent for the removal of TCs from aqueous systems, highlighting its great potentiality in water treatment applications.