Medium-temperature calcination and acid pressure leaching extract the Al2O3 from coal gangue: activation mechanism and kinetic analysis
Abstract
Bauxite is an important strategic resource, and it is facing with the problem of balance between high demand of bauxite ore and low resource of bauxite reserves in China. This research takes the Fuxin coal gangue as the object and extracts Al2O3 by medium-temperature calcination and acid pressure leaching process. The results show that at a calcination temperature of 650 °C, calcination time of 2 h, acid pressure leaching temperature of 160 °C and acid pressure leaching time of 6 h, the extraction ratio of Al2O3 reaches 80.19%. Furthermore, the research finding that the complete activation temperatures of kaolinite and muscovite are 650 °C and 850 °C, respectively, and the decomposition reactions of active Si, active Al, and metakaolinite occur above 800 °C, which leads to a low extraction ratio of Al2O3. The acid pressure leaching process can directly destroy the muscovite structure at a calcination temperature of 650 °C. The acid pressure leaching kinetic equations are studied by three kinetic models, and the apparent activation energies of the reactions are calculated by the Arrhenius formula. The results show that acid pressure leaching is subject to solid residue in-layer diffusion control, and the kinetic equation is “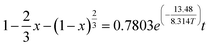 ”. The apparent activation energy is 13.48 kJ mol−1.
”. The apparent activation energy is 13.48 kJ mol−1.



 Please wait while we load your content...
Please wait while we load your content...