Cu/CeO2 catalysts for reverse water gas shift reactions: the effect of the preparation method†
Abstract
The reverse water gas shift reaction is one of the most prospective CO2 utilization approaches. Cu has excellent selectivity for CO and CeO2 is rich in surface oxygen vacancies for CO2 activation. These unique properties are often used to develop efficient Cu/CeO2 catalysts in RWGS. In this paper, Cu/CeO2 is prepared by plasma-induced micro-combustion. The effect of the subsequent calcination after micro-combustion on the structure and catalytic property is systemically studied. Because of the mild temperature of micro-combustion, highly dispersed Cu species load on the surface of CeO2 for the catalyst without calcination (Cu/CeO2-mc). During calcination, the highly dispersed Cu species form two kinds of species, Cu–Ce solid solution structure and small CuO clusters (Cu/CeO2-mcc). The Cu–Ce solid solution effectively enhances the generation of oxygen vacancies, which improves the adsorption and activation of CO2. The catalytic performance of Cu/CeO2-mcc thereby is superior to Cu/CeO2-mc in RWGS. In situ diffuse reflectance infrared fourier transform spectroscopy analysis demonstrates that the formate pathway is the main mechanism of RWGS. CO2 adsorbed on the surface of Cu/CeO2-mcc mainly forms bidentate 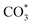 species. While monodentate
species. While monodentate 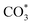 generates on the surface of Cu/CeO2-mc. And
generates on the surface of Cu/CeO2-mc. And 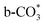 decomposes to CO easier than
decomposes to CO easier than 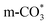 , thus Cu/CeO2-mcc exhibits excellent catalytic properties. This work provides a new approach for structural modulation of catalysts with excellent catalytic performance in RWGS.
, thus Cu/CeO2-mcc exhibits excellent catalytic properties. This work provides a new approach for structural modulation of catalysts with excellent catalytic performance in RWGS.



 Please wait while we load your content...
Please wait while we load your content...