Mechanisms and selectivity of methanol oxidation on PtRuM3/C-MWCNT (M = Fe and Co) electrocatalysts†
Abstract
Methanol oxidation efficiency and resistance to CO poisoning are the most challenging issues associated with direct methanol fuel cells. Much experimental effort has been undertaken, such as generating Pt-based binary and ternary nanoparticles, creating composite substrates, and fabricating nanoparticles with special shapes, to overcome these drawbacks. Our previous experiment showed that ternary PtRuM3/C-MWCNT (M = Fe and Co; C-MWCNT = carbon Vulcan-multiwalled carbon nanotube) electrocatalysts exhibited high methanol oxidation activity and tolerance to CO poisoning. However, reaction mechanisms on ternary PtRuM3/C-MWCNT (M = Fe and Co) electrocatalysts remain unknown. Therefore, this work is devoted to elucidating the problem using density functional theory calculations and thermodynamic models. Our present study showed that methanol oxidation proceeds via four possible reaction pathways on the surface of PtRuM3/C-MWCNTs, where the most favourable one follows a series of steps converting 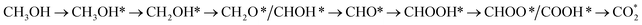 with a thermodynamic barrier of 0.513 eV for applied potentials of U = 0 V and 1.005 V on PtRuFe3/C-MWCNTs and 0.404 eV for U = 0 V and 0.167 eV for U = 1.005 V on PtRuCo3/C-MWCNTs. We also provide physical insights into the interaction between methanol oxidation intermediates and substrates' surface by analysing electronic properties. Our findings support the results of our previous experiment. The results of this study can be useful for rationally designing the anode for fuel cells.
with a thermodynamic barrier of 0.513 eV for applied potentials of U = 0 V and 1.005 V on PtRuFe3/C-MWCNTs and 0.404 eV for U = 0 V and 0.167 eV for U = 1.005 V on PtRuCo3/C-MWCNTs. We also provide physical insights into the interaction between methanol oxidation intermediates and substrates' surface by analysing electronic properties. Our findings support the results of our previous experiment. The results of this study can be useful for rationally designing the anode for fuel cells.



 Please wait while we load your content...
Please wait while we load your content...