Mechanism of methanol synthesis from CO2 on Cu/CeO2 and Cu/W-CeO2: a DFT investigation into the nature of W-doping†
Abstract
Cu/CeO2 catalysts have garnered great interest for efficient methanol synthesis from CO2, and a recent report found that doping W into CeO2 to construct Cu/W-CeO2 significantly improves methanol productivity and selectivity. The report indicated that W-doping increases and stabilizes the Ce3+ concentration on Cu/CeW0.25Ox for inhibiting the loss of lattice oxygen and creating redox-active oxygen vacancies on the CeO2 surface. However, the mechanistic function of the modified Cu/CeO2 has not been fully understood. In this work, starting from the determination of calculated models Cu8/CeO2-Ov and Cu8/W-CeO2-Ov, we theoretically investigated two possible reaction pathways, the formate pathway and the reverse water gas shift (RWGS) + CO hydrogenation pathway, aiming to have an insight into the nature of W-doping on methanol productivity and selectivity. The calculated results indicate that for Cu8/CeO2-Ov, the energy barrier for the rate-determining step (H2COOH* + H* → H2CO* + H2O*) in the formate pathway is higher than that of the rate-determining step (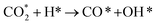 ) in the RWGS + CO hydrogenation pathway, both of which involve the cleavage of the C–O bond of
) in the RWGS + CO hydrogenation pathway, both of which involve the cleavage of the C–O bond of 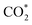 . However W-doping into Cu8/CeO2-Ov weakens the interaction of the Cu cluster with the Oup atom in
. However W-doping into Cu8/CeO2-Ov weakens the interaction of the Cu cluster with the Oup atom in 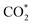 but enhances the C–Odown bonds, favoring the reaction towards the formate pathway and inhibiting the RWGS + CO hydrogenation pathway.
but enhances the C–Odown bonds, favoring the reaction towards the formate pathway and inhibiting the RWGS + CO hydrogenation pathway.



 Please wait while we load your content...
Please wait while we load your content...