Experimental unified pH scale in 1,2-dichloroethane
Abstract
Experimental potentiometric unified pH (pHabs) scale is presented in 1,2-dichloroethane (1,2-DCE). The scale was compiled using differential potentiometric measurements, carried out by pair-wise comparisons between solutions. Aqueous standard buffer solutions were used as anchor points, so that the obtained pH values are linked to (i.e., are traceable to) the conventional aqueous pH scale and are expressed as 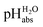 values. They are directly comparable to pH values in water in terms of the chemical potential of the solvated proton. The “ladder” approach was used for data analysis and
values. They are directly comparable to pH values in water in terms of the chemical potential of the solvated proton. The “ladder” approach was used for data analysis and 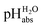 values were assigned to 19 solutions in 1,2-DCE, leading to a
values were assigned to 19 solutions in 1,2-DCE, leading to a 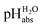 scale spanning from −2.9 to 11.0. This is the first time that successful potentiometric
scale spanning from −2.9 to 11.0. This is the first time that successful potentiometric 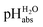 measurements have been carried out in a solvent of as low polarity as 1,2-DCE. The whole set of measurements, comprising a total of 85 ΔpHabs values, has a consistency standard deviation of 0.17 pH units. This is higher compared to the consistency standard deviations reported in the literature for similar measurements in other solvents and reflects the experimental difficulties with potentiometric measurements in a low-polarity solvent. This result means that potentiometric measurement of
measurements have been carried out in a solvent of as low polarity as 1,2-DCE. The whole set of measurements, comprising a total of 85 ΔpHabs values, has a consistency standard deviation of 0.17 pH units. This is higher compared to the consistency standard deviations reported in the literature for similar measurements in other solvents and reflects the experimental difficulties with potentiometric measurements in a low-polarity solvent. This result means that potentiometric measurement of 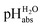 values is possible in low-polarity solvents, and it is thus expected that potentiometric
values is possible in low-polarity solvents, and it is thus expected that potentiometric 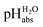 measurement is possible in most organic solvents, opening the possibility of experimentally connecting many solvents into the unified
measurement is possible in most organic solvents, opening the possibility of experimentally connecting many solvents into the unified 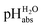 scale.
scale.



 Please wait while we load your content...
Please wait while we load your content...