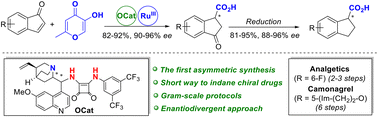
The first practical enantiodivergent synthesis of bioactive indane-1-carboxylic acid derivatives is developed. A key step of the synthesis is highly enantioselective organocatalytic Michael addition of allomaltol, an available natural kojic acid derivative, to 1H-inden-1-ones. Subsequent oxidative fragmentation of the reaction products under the action of RuCl3/NaIO4 followed by chemoselective reduction of the resulting ketoacids with zinc amalgam afforded indane-1-carboxylic acids of high enantiomeric purity unavailable so far. The synthetic potential of the method was demonstrated by a gram-scale synthesis of (S)- and (R)-enantiomers of anticoagulant camonagrel.