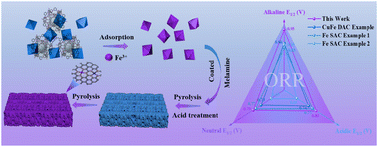
The oxygen reduction reaction (ORR) serves as a pivotal process in the operation of zinc–air batteries and fuel cells, playing a decisive role in their energy conversion efficiency. However, the scarcity of high-performance ORR electrocatalysts has emerged as a crucial bottleneck that restricts the enhancement of energy conversion efficiency in these sustainable electrochemical systems. In this study, we employ a self-template strategy for the precise preparation of copper and iron bimetallic dual-atom active sites anchored on metal–organic framework (MOF)-derived highly poriferous carbon nanosheets. This bimetallic diatomic catalyst (CuFe–NCN) possesses highly homogeneous and atomically precisely dispersed active sites, which significantly enhances its catalytic activity for the ORR throughout an extensive pH range. It exhibits outstanding electrocatalytic activity for the ORR in alkaline, neutral, and acidic solutions, outperforming commercial Pt/C catalysts. Specifically, the half-wave potentials are 0.95 V in alkaline solution, 0.79 V in neutral solution, and 0.80 V in acidic solution. To explore the bifunctional properties of the electrocatalyst, its oxygen evolution reaction (OER) performance was studied. For CuFe–NCN, the potential is 310 mV at a current density of 10 mA cm−2, which is superior to that of commercial RuO2 (440 mV). When utilized in the assembly of zinc–air battery (ZAB) devices, it functions steadily at 5 mA cm−2 for a period of 910 h and reaches a power density peak of 281 mW cm−2. Furthermore, the assembled flexible zinc–air battery maintains stable operation for 200, 150, and 150 h at 0.5, 1, and 2 mA cm−2, respectively.