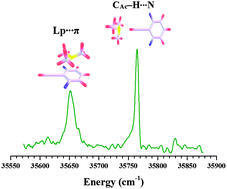
Binary complexes of 2,6-difluorophenylacetylene with methylamine, dimethylamine, trimethylamine and triethylamine were investigated using one colour resonant two photon ionization and infrared-optical double resonance spectroscopic techniques combined with high level ab initio calculations. All four amines form CAc–H⋯N hydrogen-bonded complexes. Additionally trimethylamine and triethylamine form complexes characterized by Lp⋯π interactions, due to the electron deficient nature of the phenyl ring of 2,6-difluorophenylacetylene. The Lp⋯π interacting structure of the 2,6-difluorophenylacetylene–trimethylamine complex is about 1.5 kJ mol−1 higher in energy than the CAc–H⋯N hydrogen-bonded structure, which is the global minimum. Energy decomposition analysis indicates that the electrostatics and dispersion interactions favour the formation of CAc–H⋯N and Lp⋯π complexes, respectively. Interestingly the CAc–H⋯N hydrogen-bonded complex of 2,6-difluorophenylacetylene–triethylamine showed a smaller shift in the acetylenic C–H stretching frequency than the 2,6-difluorophenylacetylene–trimethylamine complex. The observed fragmentation of the binary complexes of 2,6-difluorophenylacetylene with the four amines following resonant two-photon ionization can be explained on the basis of the intermolecular coulombic decay process.