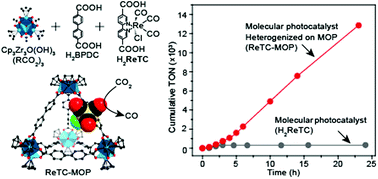
The heterogenization of photocatalytic molecules typically enhances stability at the expense of activity. Therefore, a new approach to stabilizing molecular catalysts without compromising their original catalytic features is highly desired. In this study, we found that Zr-based metal–organic polyhedra (MOP) stabilized the photocatalytic compound ReTC [ReI(CO)3(BPYDC)(Cl), BPYDC = 2,2′-bipyridine-5,5′-dicarboxylate] without degrading its catalytic activity. ReTC was chemically bound to discrete cages of the MOP and was found to maintain its maximum CO2-to-CO conversion activity (660 h−1 turnover frequency (TOF)) for at least 24 h under visible light irradiation. The free molecular form of the same compound (H2ReTC) initially showed an activity of 131 h−1 TOF, which was lost within 2 h. The cumulative turnover number of ReTC-MOP after a 24 h reaction was 12 847, which was 42.0 times the value of 306 for molecular ReTC. The high catalytic activity and stability of ReTC-MOP are attributed to the fact that this MOP material provides an extremely small framework for chemical binding of ReTC, such that the catalyst has a high degree of motional freedom and enhanced light absorption while being protected in the reaction solution.