Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient synthesis of an unsupported aryl substituted iminoborane chain (HNArBH)2NAr (Ar = [1,1′-biphenyl]-2-yl)†
Rita
Stauder
a,
Markus
Ströbele
 b and
Holger F.
Bettinger
b and
Holger F.
Bettinger
 *a
*a
aInstitut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: holger.bettinger@uni-tuebingen.de
bInstitut für Anorganische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany
First published on 1st October 2024
Abstract
The reaction of 2-aminobiphenyl ArNH2 with triethylamine borane (Et3NBH3) at 165 °C, about 40 °C lower in temperature than that used for borazine synthesis, gives a linear HArN-BH-NAr-BH-NArH (Ar = [1,1'-biphenyl]-2-yl) chain in good yield (57%). This BN oligomer could be characterized by single crystal X-ray crystallography.
Electronic properties of polycyclic aromatic hydrocarbons (PAHs) can be fundamentally changed when single CC units are substituted by isosteric and isoelectronic BN units.1–6 This concept was transferred from two-dimensional networks, graphene and hexagonal boron-nitride being the prime examples, to one-dimensional polymers.7 How do properties change when incorporating BN units into 1D polyacetylene (PA) and turn it into poly(iminoborane) (PIB)? For compounds with para-phenylene linked NBN units it could be observed that π conjugation is extended over the NBN unit.7 The calculated band structures suggested a larger band gap for PIB compared to PA.8
While the synthesis of poly(aminoboranes) has seen significant advance over the years,9–13 synthesis of PIBs has been a rocky road so far.14 Polymerization by thermal dehydrogenation of ammonia borane or aminoborane compounds has often led to the cyclic trimer borazine, the favored product under most circumstances, instead of a polymer chain.15–18 In some reports formation of PIBs is proposed,15,19–21 but they could not be satisfyingly characterized. Their existence, therefore, could not be doubtlessly proven due to lack of modern analysis methods, lack of solubility or due to low yields.18
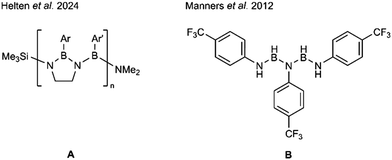
A significant step forward in this field could be achieved by Helten and coworkers when they forced the BN-backbone in a linear shape by applying Neilson's method of stabilizing the backbone with an alkyl corset22,23 to yield “cyclolinear” oligo- and poly(iminoborane)s A (Fig. 1).24–26 By this procedure formation of borazines could be prevented completely, and quite large monodisperse oligo(iminoborane)s could be synthesized and structurally characterized.26
The longest iminoborane oligomer that is unsupported by an alkyl corset and that could be structurally characterized contains three nitrogen and two boron atoms, ArNH-BH-N(Ar)-BH-NH(Ar), Ar = p-C6H4CF3B (Fig. 1).18 This chain was obtained by Manners et al. from the dehydrocoupling of ArNH2·BH3 at room temperature as one of the five reaction products over a reaction time of months. The linear oligomer could be obtained as “small crop of crystals”18 from the mixture, but could not be characterized by NMR. Köster et al. observed that the reaction of primary aromatic amines and triethylamine borane results in N,N′,N′′-trisubstituted borazines (Scheme 1a).16,17 This group reported in 1968 the formation of an oligomer, PhN(BH-NHPh)2, in the reaction of BH(NHPh)2 with Et3N·BH3 at 100 °C as a low yield by-product (7%) of borazine formation.17 Not unexpectedly, the characterization possible in 1968 falls short of present standards.
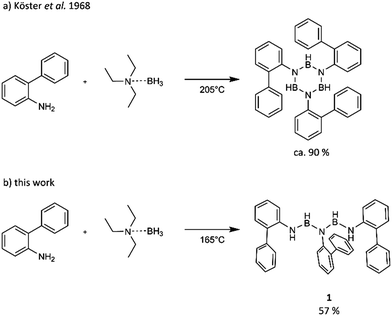 | ||
| Scheme 1 Scheme of previously known reaction between 2-aminobiphenyl and triethylamine borane and of our reaction at lower temperature. | ||
Here we show that a slight modification of Köster's borazine synthesis method gives a five-membered BN oligomer without use of stabilizing cyclic alkyl moieties (Scheme 1b). The iminoborane oligomer was obtained in good yields in a single reaction step, and could be fully characterized.
Heating an equimolar mixture of 2-aminobiphenyl and Et3N·BH3 at 165 °C in the absence of a solvent for 5.5 hours, gives iminoborane oligomer 1. The pure product can be obtained after recrystallization from acetonitrile in a yield of 57%.
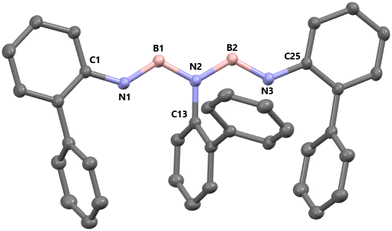
The product crystallizes from acetonitrile in the monoclinic space group P21/n with eight molecules in the unit cell. A single molecule consists of a five-membered iminoborane-chain with biphenylyl units at each nitrogen atom (Fig. 2). The outer N–B bond distances N1–B1 (1.413(2) Å) and N3–B2 (1.417(2) Å) are slightly shorter than the inner N–B bond distances N2–B1 (1.441(2) Å) and N2–B2 (1.440(2) Å), which is in line with the findings in compound B reported by Manners et al.18 in 2012. The bond distances between the terminal aryl units and the nitrogen atoms, N1–C1 (1.409(2) Å) and N3–C25 (1.404(1) Å), are also diminished compared to the central bond N2–C13 (1.437(1) Å). The rings of the terminal biphenylyl units directly connected to the chain are only slightly tilted to the NBN-plane, 9.6° for the unit at N1 and 1.4° for the unit at N3. The other rings of the terminal biphenylyl groups and the ring of the central biphenylyl group connected to N2 are all inclined to the NBN-plane in the same way but to different extents. The phenyl ring of the N1-unit is inclined by 77.2°, the ring of the N2-unit by 75.3° and the one of the N3-unit by 71.2°.
NMR spectroscopy supports the molecular structure crystallographically found. The 1H NMR spectrum shows the required 27 aromatic protons, two protons bound to nitrogen, and two protons bound to boron represented by a very broad singlet at 4.88 ppm. In the 1H NMR spectrum no coupling to boron could be detected due to the signal shape. However, from a simulation (see ESI, Fig. S6†) using the software WSolids127 with a 11B spin–lattice relaxation time T1 of 11B fixed at 0.88 ms (obtained from the peak width at half-height in the 11B NMR spectrum) a coupling constant of 1J(11B,1H) = 100 Hz could be deduced.
In conclusion, it was possible to synthesize an iminoborane oligomer by a simple and convenient procedure in a fairly good yield. The molecular composition and structure could be doubtlessly proven. This building block could be useful for constructing larger iminoborane oligomers or even poly(iminoborane).
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for compound 1 have been deposited at the Cambridge Crystallographic Data Centre under 2307985.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Klaus Eichele at the Institut für Anorganische Chemie (Universität Tübingen) for the analysis of the boron-hydrogen coupling.References
- Z. Liu and T. B. Marder, Angew. Chem., Int. Ed., 2008, 47, 242–244 CrossRef CAS PubMed.
- M. Hirai, N. Tanaka, M. Sakai and S. Yamaguchi, Chem. Rev., 2019, 119, 8291–8331 CrossRef CAS PubMed.
- M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed.
- X. Y. Wang, J. Y. Wang and J. Pei, Chem. – Eur. J., 2015, 21, 3528–3539 CrossRef CAS PubMed.
- Z. X. Giustra and S. Y. Liu, J. Am. Chem. Soc., 2018, 140, 1184–1194 CrossRef CAS PubMed.
- A. Borissov, Y. K. Maurya, L. Moshniaha, W. S. Wong, M. Żyła-Karwowska and M. StÈ©pień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed.
- H. Helten, Chem. – Eur. J., 2016, 22, 12972–12982 CrossRef CAS PubMed.
- A. Abdurahman, M. Albrecht, A. Shukla and M. Dolg, J. Chem. Phys., 1999, 110, 8819–8824 CrossRef CAS.
- O. J. Metters, A. M. Chapman, A. P. M. Robertson, C. H. Woodall, P. J. Gates, D. F. Wass and I. Manners, Chem. Commun., 2014, 50, 12146–12149 RSC.
- X. M. Chen, S. C. Liu, C. Q. Xu, Y. Jing, D. Wei, J. Li and X. Chen, Chem. Commun., 2019, 55, 12239–12242 RSC.
- C. Zhang, X. Jiang, Y. Jing, N. Zhang, P. Wang, H. Wang, K. Jiang, X. M. Chen and X. Chen, Inorg. Chem., 2024, 63, 10519–10526 CrossRef CAS PubMed.
- A. Staubitz, A. P. Soto and I. Manners, Angew. Chem., Int. Ed., 2008, 47, 6212–6215 CrossRef CAS PubMed.
- C. A. De Albuquerque Pinheiro, C. Roiland, P. Jehan and G. Alcaraz, Angew. Chem., Int. Ed., 2018, 57, 1519–1522 CrossRef CAS PubMed.
- T. Beweries and H. Helten, in Encyclopedia of Inorganic and Bioinorganic Chemistry, 2020, pp. 1–25 Search PubMed.
- J. E. Burch, W. Gerrard and E. F. Mooney, J. Chem. Soc., 1962, 2200–2203 RSC.
- R. Köster, S. Hattori and Y. Morita, Angew. Chem., Int. Ed. Engl., 1965, 77, 719–720 CrossRef.
- R. Köster, H. Bellut and S. Hattori, Justus Liebigs Ann. Chem., 1968, 720, 1–22 CrossRef.
- H. Helten, A. P. M. Robertson, A. Staubitz, J. R. Vance, M. F. Haddow and I. Manners, Chem. – Eur. J., 2012, 18, 4665–4680 CrossRef CAS PubMed.
- M. G. Hu, R. A. Geanangel and W. W. Wendlandt, Thermochim. Acta, 1978, 23, 249–255 CrossRef CAS.
- H.-U. Meier, P. Paetzold and E. Schröder, Chem. Ber., 1984, 117, 1954–1964 CrossRef CAS.
- P. Paetzold, Adv. Inorg. Chem., 1987, 31, 123–169 CrossRef CAS.
- S. Y. Shaw, D. A. DuBois, W. H. Watson and R. H. Neilson, Inorg. Chem., 1988, 974–976 CrossRef CAS.
- S. Y. Shaw and H. Neilson, Inorg. Chem., 1994, 3239–3245 CrossRef CAS.
- O. Ayhan, T. Eckert, F. A. Plamper and H. Helten, Angew. Chem., Int. Ed., 2016, 55, 13321–13325 CrossRef CAS PubMed.
- O. Ayhan, N. A. Riensch, C. Glasmacher and H. Helten, Chem. – Eur. J., 2018, 24, 5883–5894 CrossRef CAS PubMed.
- M. Maier, A. Friedrich, J. S. Schneider, J. A. P. Sprenger, J. Klopf, L. Fritze, M. Finze and H. Helten, ChemistryEurope, 2024, 2, 1–8 CrossRef.
- K. Eichele, WSolids1, version 1.21.7, Universität Tübingen, 2021 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2307985. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02280g |
| This journal is © The Royal Society of Chemistry 2024 |