Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Titanosiloxanes consisting of tetrahedrally coordinated Ti cores and branched siloxane cages†
Shohei
Saito‡
a,
Kenta
Kawamura‡
a,
Naoto
Sato
a,
Takamichi
Matsuno
 ab,
Hiroaki
Wada
a,
Kazuyuki
Kuroda
ab,
Hiroaki
Wada
a,
Kazuyuki
Kuroda
 ab and
Atsushi
Shimojima
ab and
Atsushi
Shimojima
 *ab
*ab
aDepartment of Applied Chemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan. E-mail: shimojima@waseda.jp
bKagami Memorial Research Institute for Materials Science and Technology, Waseda University, 2-8-26 Nishiwaseda, Shinjuku-ku, Tokyo, 169-0051, Japan
First published on 8th November 2024
Abstract
Well-defined titanosiloxane molecules with tetrahedrally coordinated Ti centers were synthesized by using monosilanol-functionalized siloxane cages as building blocks. The Ti sites are modified with –OSi(OSi)3 units, which is analogous to Ti-containing zeolites. Such titanosiloxane molecules are important as models for Ti-containing silica-based catalysts.
The bottom-up synthesis of siloxane-based nanomaterials using well-defined building blocks facilitates fine control over their physical properties and the emergence of new functions by molecular design.1–5 Polyhedral oligomeric silsesquioxanes (POSSs) with a general formula of (RSiO3/2)n, where n ≥ 6 and R = H or organic groups, are widely used as molecular building blocks owing to their rigid frameworks and facile functionalizability.6–9 Covalent linking of POSSs can afford various siloxane-based materials, such as discrete oligomers10–17 and porous networks.18,19 Regioselective functionalization of cage corners is a promising approach for controlling the linked geometries of POSSs.5–7 Mono-functionalized cubic POSSs (R′R7Si8O12, R′ = functional groups) are most commonly used as building blocks because they are easily synthesized via silylation of incompletely condensed trisilanol POSSs (R7(HO)3Si7O9).6,7,20 Mono-functionalized cubic POSSs have recently been connected via organic linkers to produce giant molecules with well-defined radial10,11 and dendritic structures.14,15
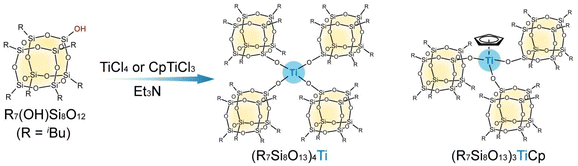
The formation of metallasiloxanes with Si–O–M (M = metal atoms) bonds using POSSs is of significant interest owing to their potential applications as molecular catalysts for a variety of organic reactions.21 The molecularly defined structures of POSS-based metallasiloxanes also provide important models for heterogeneous catalysts, such as metal-containing zeolites. Metal–POSS complexes, in which one silicon atom at the vertex of the cubic POSS is substituted with a metal atom (e.g., Zr,22–24 Sn,23 V,25 or Ti24,26–28), have been well studied because they are easily synthesized by reacting the corresponding trisilanol POSS with a metal source. Previous reports have revealed that the properties of POSS-based metallasiloxanes depend on their coordination numbers as well as steric and electronic effects associated with the ligand.21,29,30 We recently reported the synthesis of a nanoporous titanosiloxane by three-dimensional (3D) cross-linking of an octasilanol-functionalized cage siloxane (Si8O12(OSiMe2OH)8) with titanium tetraethoxide.31 The Ti sites surrounded by siloxane cages were found to be catalytically active toward cyclohexene oxidation; however, their detailed structures have not been elucidated, which is mainly ascribable to the structural ambiguity associated with the amorphous 3D network.
Herein, we report the synthesis and isolation of well-defined titanosiloxane molecules comprising Ti cores and branched siloxane cages by reacting monosilanol POSSs (iBu7(HO)Si8O12) with a titanium source (titanium(IV) chloride (TiCl4) or cyclopentadienyltitanium(IV) trichloride (CpTiCl3)) (Scheme 1). Although a dumbbell-shaped Ti–POSS compound, in which two siloxane cages are bridged by Ti species, has been reported in the literature,32 the synthesis of branched titanosiloxane molecules comprising three or four cage units is unprecedented. The precise syntheses of such large metallasiloxane compounds containing multiple POSSs pave the way for the modular synthesis of zeolite catalysts containing cage siloxanes as secondary building units.
i
Bu7(HO)Si8O12 was synthesized from a mono-hydride POSS (iBu7HSi8O12), which was obtained by reacting an incompletely condensed POSS (iBu7(HO)3Si7O9) with trichlorosilane (HSiCl3) according to a previously reported procedure.33 A titanosiloxane molecule consisting of a Ti core and four branched siloxane cages (iBu7Si8O13)4Ti was synthesized by reacting iBu7(HO)Si8O12 with TiCl4 in the presence of Et3N (Scheme 1). The molar ratio was iBu7(HO)Si8O12/TiCl4/Et3N = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8. After the reaction at 0 °C for 6 h, the precipitates of Et3N·HCl were removed by filtration and the filtrate was evaporated under reduced pressure. Needle-shaped crystals of (iBu7Si8O13)4Ti were obtained by recrystallization using a binary acetonitrile–toluene solvent (see the ESI† for details).
4.8. After the reaction at 0 °C for 6 h, the precipitates of Et3N·HCl were removed by filtration and the filtrate was evaporated under reduced pressure. Needle-shaped crystals of (iBu7Si8O13)4Ti were obtained by recrystallization using a binary acetonitrile–toluene solvent (see the ESI† for details).
The 29Si NMR spectrum of a CDCl3 solution of the crystals (Fig. 1a and Fig. S1, ESI†) exhibits T3 signals ((SiO)3SiiBu; −66.97, −67.89, and −68.01 ppm) and a higher-field signal attributable to the (SiO)3Si(OTi) site (−115.15 ppm)32 with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 intensity ratio, consistent with the four inequivalent Si atoms in (iBu7Si8O13)4Ti. The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum (Fig. 1b) shows a signal corresponding to the Na+ adduct of (iBu7Si8O13)4Ti (m/z = 3401.9) (calcd for C112H252O52Si32TiNa+ [M + Na]+: 3400.8). These results indicate that (iBu7Si8O13)4Ti had been successfully synthesized and isolated.
1 intensity ratio, consistent with the four inequivalent Si atoms in (iBu7Si8O13)4Ti. The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum (Fig. 1b) shows a signal corresponding to the Na+ adduct of (iBu7Si8O13)4Ti (m/z = 3401.9) (calcd for C112H252O52Si32TiNa+ [M + Na]+: 3400.8). These results indicate that (iBu7Si8O13)4Ti had been successfully synthesized and isolated.
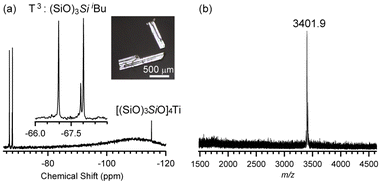 | ||
| Fig. 1 (a) 29Si NMR and (b) MALDI-TOF MS spectra of (iBu7Si8O13)4Ti after recrystallization. Inset: optical microscopy image of the crystals of (iBu7Si8O13)4Ti. | ||
(iBu7Si8O13)4Ti was subjected to single-crystal X-ray diffractometry (Fig. 2a), which revealed that its crystal is monoclinic and in the P21/c space group (crystal data are presented in Table S1, ESI†).34 In addition, four cage-siloxane units were attached to a Ti atom with a tetrahedral geometry (Ti–O lengths and O–Ti–O and Ti–O–Si angles are listed in Tables S2–S4, ESI†). It should be noted that one of the six O–Ti–O angles is 107.3(3)°, which is slightly different from the others (109–111°) (Fig. 2b and Table S3, ESI†). Moreover, one of the four Ti–O–Si angles is 162.7(4)°, which is also slightly different from the others (172–178°) (Fig. 2b and Table S4, ESI†). These results indicate that the tetrahedral structure of (iBu7Si8O13)4Ti is slightly distorted in the crystal, in contrast to the 29Si NMR results that suggest that the four (SiO)3Si(OTi) sites are chemically equivalent in solution. Distortion is likely to occur in the crystal in order to form a more densely packed crystal structure than can be achieved by an undistorted tetrahedral arrangement. From the perspective of the molecular arrangement in the crystal structure, the molecules are densely packed, with cages arranged to form a wavy structure in the bc-plane (Fig. 2c, and Fig. S2 and S3, ESI†). Note that the large amount of disorder is mainly associated with the iBu groups in the (iBu7Si8O13)4Ti crystal. Because the position of the metal atom in the siloxane framework is an important factor for metal-containing silica catalysts,35,36 this well-defined molecular arrangement will be a valuable model of silica-based catalysts.
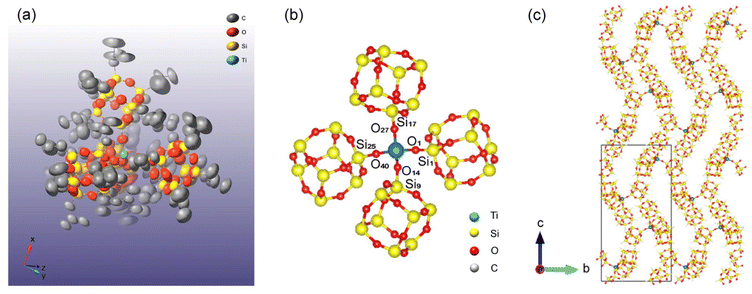 | ||
| Fig. 2 (a and b) Molecular structure of (iBu7Si8O13)4Ti obtained by single-crystal X-ray diffractometry. Hydrogen atoms are omitted in (a) and both hydrogen and carbon atoms are omitted in (b) for clarity. Color code: yellow, Si; gray, C; red, O. Thermal ellipsoids are drawn at the 30% probability level in (a). (c) Crystal structure of (iBu7Si8O13)4Ti viewed along the bc plane. Hydrogen and carbon atoms are omitted for clarity. Color code: blue, Ti; yellow, Si; red, O. CrystalMaker and VESTA (version 3)40 were used to visualize the structural models. | ||
The diffuse reflectance (DR) ultraviolet–visible (UV–Vis) spectrum of (iBu7Si8O13)4Ti (Fig. S4, ESI†) shows a sharp band centered at 210 nm with a broad band at 230–330 nm. The former band is assigned to the ligand-to-metal charge-transfer transition (LMCT) of tetrahedrally coordinated Ti,28,37,38 while the latter is likely due to the LMCT of octahedrally coordinated Ti,28,37,38 which is probably formed via the partial cleavage of the Si–O–Ti bonds in (iBu7Si8O13)4Ti owing to their instability against moisture39 and/or pulverization. While we confirmed that pristine (iBu7Si8O13)4Ti crystals are relatively stable in air, a certain degree of deterioration was observed as the crystals were pulverized for DR UV–Vis spectroscopy. The 29Si NMR spectrum of the powdered (iBu7Si8O13)4Ti left to stand in air at room temperature and 40% humidity for 1 d exhibited a signal for iBu7(HO)Si8O12, suggesting that some of the Si–O–Ti bonds had cleaved (Fig. S5, ESI†). Although improving stability is an issue that remains to be addressed, (iBu7Si8O13)4Ti is promising as a model of heterogeneous catalysts because tetrahedrally coordinated Ti is known to exhibit effective catalytic activity for a variety of reactions, including olefin epoxidation and polymerization.
We examined varying the number of cage units to further design branched titanosiloxanes. A titanosiloxane molecule consisting of a Ti core and three branched siloxane cages ((iBu7Si8O13)3TiCp) was synthesized by reacting iBu7(HO)Si8O12 with CpTiCl3 in the presence of Et3N at the molar ratio of iBu7(HO)Si8O12/CpTiCl3/Et3N = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2 (see the ESI† for details).
3.2 (see the ESI† for details).
A powder product composed of (iBu7Si8O13)3TiCp and iBu7(HO)Si8O12 was obtained after the removal of Et3N·HCl and the solvent. Unfortunately, unlike (iBu7Si8O13)4Ti, crystals of (iBu7Si8O13)3TiCp could not be obtained by recrystallization, and it was isolated using gel permeation chromatography (GPC). The 29Si NMR spectrum (Fig. S6, ESI†) of the product after GPC separation showed a signal at −111.54 ppm, indicative of Si–O–Ti bond formation,32 and three T3 signals (−67.06, −67.78, and −67.85 ppm) assignable to the (SiO)3SiiBu sites in (iBu7Si8O13)3TiCp. The small Q3 signal is probably due to the hydrolysis of the Si–O–Ti bonds during the GPC separation. MALDI-TOF MS (Fig. S7, ESI†) detected the Na+ adduct of (iBu7Si8O13)3TiCp (m/z = 2634.5) (calcd for C89H194O39Si24TiNa+ [M + Na]+: 2633.4). These results confirm the formation of (iBu7Si8O13)3TiCp. Compared to the four cage units in (iBu7Si8O13)4Ti, a molecular structure comprising three siloxane cage units is expected to provide superior accessibility of reactant molecules to the Ti site. Although further molecular design is required to improve the hydrolytic stability of both (iBu7Si8O13)4Ti and (iBu7Si8O13)3TiCp, these molecules are potentially important titanosiloxane-based catalysts.
Conclusions
We synthesized (iBu7Si8O13)4Ti and (iBu7Si8O13)3TiCp as a new class of titanosiloxanes consisting of Ti cores and branched siloxane cages by reacting monosilanol POSSs with Ti sources. (iBu7Si8O13)4Ti was determined to have a slightly distorted tetrahedral structure by X-ray crystallography, with cage units arranged in a wave-like structure in the crystal. Because the performance of metal-containing silica catalysts depends on various parameters, including the coordination number of the metal atom, bond length, and bond angles, the precise synthesis of well-defined titanosiloxanes in this study will advance the design of siloxane-based catalysts.Data availability
Crystallographic data reported in this manuscript have been deposited in the Cambridge Crystallographic Data Centre under supplementary publication no. CCDC 2388983 and can be obtained from https://www.ccdc.cam.ac.uk/. The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Mr S. Sato, Dr H. Sato (Rigaku Corporation) and Mr T. Goto (Materials Characterization Central Lab., Waseda Univ.) for the X-ray diffraction analysis. We are grateful to the Materials Characterization Central Lab., Waseda Univ. for supporting the NMR and MS measurements. This work was supported in part by Grant-in-Aid for the Strategic International Collaborative Research Program (SICORP) “France-Japan Joint Call on Molecular Technology” from the Japan Science and Technology Agency (JST). This work was also supported in part by JSPS KAKENHI (grant no. 23H02051). S. Saito is thankful to the JX-Waseda Research Fund for Young Researchers.References
- C. Sanchez, G. Soler-Illia, F. Ribot, T. Lalot, C. R. Mayer and V. Cabuil, Chem. Mater., 2001, 13, 3061 CrossRef CAS.
- X. Yu, K. Yue, I.-F. Hsieh, Y. Li, X.-H. Dong, C. Liu, Y. Xin, H.-F. Wang, A.-C. Shi, G. R. Newkome, R.-M. Ho, E.-Q. Chen, W.-B. Zhang and S. Z. D. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 10078 CrossRef CAS PubMed.
- W.-B. Zhang, X. Yu, C.-L. Wang, H.-J. Sun, I.-F. Hsieh, Y. Li, X.-H. Dong, K. Yue, R. Van Horn and S. Z. D. Cheng, Macromolecules, 2014, 47, 1221 CrossRef CAS.
- Y. Chujo and K. Tanaka, Bull. Chem. Soc. Jpn., 2015, 88, 633 CrossRef CAS.
- A. Shimojima and K. Kuroda, Molecules, 2020, 25, 524 CrossRef CAS PubMed.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081 CrossRef CAS PubMed.
- G. Li, L. Wang, H. Ni and C. U. Pittman Jr., J. Inorg. Organomet. Polym. Mater., 2001, 11, 123 CrossRef CAS.
- K. Naka and Y. Irie, Polym. Int., 2017, 66, 187 CrossRef CAS.
- Y. Du and H. Liu, Dalton Trans., 2020, 49, 5396 RSC.
- H. Araki and K. Naka, Polym. J., 2012, 44, 340 CrossRef CAS.
- K. Yamamoto, D. Kawaguchi, T. Abe, T. Komino, M. Mamada, T. Kabe, C. Adachi, K. Naka and K. Tanaka, Langmuir, 2020, 36, 9960 CrossRef CAS PubMed.
- S. E. Anderson, C. Mitchell, T. S. Haddad, A. Vij, J. J. Schwab and M. T. Bowers, Chem. Mater., 2006, 18, 1490 CrossRef CAS.
- S. Gießmann, A. Fischer and F. T. Edelmann, Z. Anorg. Allg. Chem., 2004, 630, 1982 CrossRef.
- X. Wang, Y. Yang, P. Gao, D. Li, F. Yang, H. Shen, H. Guo, F. Xu and D. Wu, Chem. Commun., 2014, 50, 6126 RSC.
- M. Huang, C.-H. Hsu, J. Wang, S. Mei, X. Dong, Y. Li, M. Li, H. Liu, W. Zhang, T. Aida, W.-B. Zhang, K. Yue and S. Z. D. Cheng, Science, 2015, 348, 424 CrossRef CAS PubMed.
- N. Rey, S. Carenco, C. Carcel, A. Ouali, D. Portehault, M. Wong Chi Man and C. Sanchez, Eur. J. Inorg. Chem., 2019, 27, 3148 CrossRef.
- R. Kajiya, S. Sakakibara, H. Ikawa, K. Higashiguchi, K. Matsuda, H. Wada, K. Kuroda and A. Shimojima, Chem. Mater., 2019, 31, 9372 CrossRef CAS.
- W. Chaikittisilp, A. Sugawara, A. Shimojima and T. Okubo, Chem. Mater., 2010, 22, 4841 CrossRef CAS.
- Y. Kim, K. Koh, M. F. Roll, R. M. Laine and A. J. Matzger, Macromolecules, 2010, 43, 6995 CrossRef CAS.
- H. Liu, S. Kondo, R. Tanaka, H. Oku and M. Unno, J. Organomet. Chem., 2008, 693, 1301 CrossRef CAS.
- R. Murugavel, A. Voigt, M. G. Walawalkar and H. W. Roesky, Chem. Rev., 1996, 96, 2205 CrossRef CAS PubMed.
- F. J. Feher, J. Am. Chem. Soc., 1986, 108, 3850 CrossRef CAS.
- F. J. Feher, D. A. Newman and J. F. Walzer, J. Am. Chem. Soc., 1989, 111, 1741 CrossRef CAS.
- K. Wada, N. Itayama, N. Watanabe, M. Bundo, T. Kondo and T. Mitsudo, Organometallics, 2004, 23, 5824 CrossRef CAS.
- F. J. Feher and J. F. Walzer, Inorg. Chem., 1991, 30, 1689 CrossRef CAS.
- F. J. Feher, T. A. Budzichowski, K. Rahimian and J. W. Ziller, J. Am. Chem. Soc., 1992, 114, 3859 CrossRef CAS.
- M. Crocker, R. H. M. Herold and A. G. Orpen, Chem. Commun., 1997, 2411 RSC.
- M. Crocker, R. H. M. Herold, A. G. Orpen and M. T. A. Overgaag, J. Chem. Soc., Dalton Trans., 1999, 3791 RSC.
- M. C. Kung, M. V. Riofski, M. N. Missaghi and H. H. Kung, Chem. Commun., 2014, 50, 3262 RSC.
- M. Kejik, J. Brus, L. Jeremias, L. Simonikova, Z. Moravec, L. Kobera, A. Styskalik, C. E. Barnes and J. Pinkas, Inorg. Chem., 2024, 63, 2679–2694 CrossRef CAS PubMed.
- T. Hikino, K. Fujino, N. Sato, H. Wada, K. Kuroda and A. Shimojima, Chem. Lett., 2021, 50, 1643 CrossRef CAS.
- R. Duchateau, U. Cremer, R. J. Harmsen, S. I. Mohamud, H. C. L. Abbenhuis, R. A. van Santen, A. Meetsma, S. K.-H. Thiele, M. F. H. van Tol and M. Kranenburg, Organometallics, 1999, 18, 5447 CrossRef CAS.
- C.-H. Lu, C.-H. Tsai, F.-C. Chang, K.-U. Jeong and S.-W. Kuo, J. Colloid Interface Sci., 2011, 358, 93 CrossRef CAS PubMed.
- C112H252O52Si32Ti, M = 3377.78, monoclinic, space group P21/c, (#14), a = 20.4538(7), b = 21.7114(6), c = 42.2709(12) Å, α = 90°, β = 90.158(3)°, γ = 90°, V = 18771.6(10) Å3, T = 93 K, Z = 4, Dcalc = 1.195 g cm−3, F000 = 5328, R1 = 0.1399, wR2 = 0.4126, 133642 reflections collected, 33639 unique.
- J. Přech, Catal. Rev. Sci. Eng., 2018, 60, 71 CrossRef.
- N. Kosinov, C. Liu, E. J. M. Hensen and E. A. Pidko, Chem. Mater., 2018, 30, 3177 CrossRef CAS PubMed.
- M. Crocker, R. H. M. Herold, B. G. Roosenbrand, K. A. Emeis and A. E. Wilson, Colloids Surf., A, 1998, 139, 351 CrossRef CAS.
- V. A. D. O'Shea, M. Capel-Sanchez, G. Blanco-Brieva, J. M. Campos-Martin and J. L. G. Fierro, Angew. Chem., Int. Ed., 2003, 42, 5851 CrossRef PubMed.
- S. Marcinko and A. Y. Fadeev, Langmuir, 2004, 20, 2270 CrossRef CAS PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, spectral data (29Si NMR, MALDI-TOF MS, and UV–Vis), and crystal data. CCDC 2388983. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02848a |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |