Principles and theories of green chemistry for corrosion science and engineering: design and application
Chandrabhan
Verma
 *a,
Dheeraj Singh
Chauhan
*a,
Dheeraj Singh
Chauhan
 b,
Ruby
Aslam
c,
Priyabrata
Banerjee
b,
Ruby
Aslam
c,
Priyabrata
Banerjee
 d,
Jeenat
Aslam
e,
Taiwo W.
Quadri
d,
Jeenat
Aslam
e,
Taiwo W.
Quadri
 fg,
Saman
Zehra
fg,
Saman
Zehra
 h,
Dakeshwar Kumar
Verma
h,
Dakeshwar Kumar
Verma
 i,
Mumtaz A.
Quraishi
i,
Mumtaz A.
Quraishi
 b,
Shikha
Dubey
k,
Akram
AlFantazi
a and
Tahir
Rasheed
j
b,
Shikha
Dubey
k,
Akram
AlFantazi
a and
Tahir
Rasheed
j
aDepartment of Chemical Engineering, Khalifa University of Science and Technology, P.O. Box 127788, Abu Dhabi, United Arab Emirates. E-mail: chandraverma.rs.apc@itbhu.ac.in
bDepartment of Chemistry, Faculty of Basic Science, AKS University, Satna 485001, Madhya Pradesh, India. E-mail: dheeraj.chauhan.rs.apc@itbhu.ac.in; maquraishi.apc@itbhu.ac.in
cSchool of Civil Engineering and Architecture, Chongqing University of Science and Technology, Chongqing, China. E-mail: drrubyaslam@gmail.com
dElectric Mobility and Tribology Research Group, CSIR-Central Mechanical Engineering Research Institute, Mahatma Gandhi Avenue, Durgapur 713209, India. E-mail: pr_banerjee@cmeri.res.in
eDepartment of Chemistry, College of Science, Taibah University, Yanbu 30799, Al-Madina, Saudi Arabia. E-mail: drjeenataslam@outlook.com
fCentre for Materials Science, College of Science, Engineering and Technology, University of South Africa, Johannesburg 1710, South Africa. E-mail: taiwoquadri27@gmail.com
gInstitute for Nanotechnology and Water Sustainability, College of Science, Engineering and Technology, University of South Africa, Johannesburg 1710, South Africa. E-mail: taiwoquadri27@gmail.com
hCorrosion Research Laboratory, Department of Applied Chemistry, Faculty of Engineering and Technology, Aligarh Muslim University, Aligarh 202002, India. E-mail: samanzehra2050@gmail.com
iDepartment of Chemistry, Government Digvijay Autonomous Postgraduate College, Rajnandgaon, Chhattisgarh 491441, India. E-mail: dakeshwarverma@gmail.com
jInterdisciplinary Research Center for Advanced Materials, King Fahd University of Petroleum and Minerals, Dhahran 31261, Saudi Arabia. E-mail: tahir.rasheed@hotmail.com
kDepartment of Chemistry, School of Sciences, Hemvati Nandan Bahuguna Garhwal University, Srinagar 246174, Garhwal, India. E-mail: dubey.shikha.bhu@gmail.com
First published on 20th February 2024
Abstract
Given the high toxicity of inorganic inhibitors, organic substances, primarily heterocycles, have been proven to be one of the most efficient, cost-effective, and practical alternatives. Severe limitations in the application of organic corrosion inhibitors, particularly their environmental toxicity, have greatly accelerated the investigation of eco-friendly and sustainable alternatives. Corrosion control has made significant use of green chemistry ideas in recent years. This involves using different sustainable materials, techniques and strategies for corrosion control. Bio-based materials, including plant extracts, natural polymers, gums, waste, amino acids, and carbohydrates, are widely employed as sustainable materials. They are considered the best eco-friendly substitutes owing to their natural origin, biodegradability, and non-accumulation. Recently, several green synthetic techniques have been used to create green synthetic inhibitors, including microwave (MW) and ultrasonic (US) irradiation, particularly in conjunction with one-step multicomponent reactions (MCRs). Besides being green and sustainable, compounds derived from MW and US irradiation are more effective inhibitors than those obtained via traditional synthesis. Synthetic inhibitors derived using sustainable chemicals, solvents, and catalysts are also regarded as green alternatives. Inhibitors synthesized using natural substrates such as AAs and carbohydrates are semisynthetic alternatives. Recently, self-healing and synergism have emerged as additional environmentally friendly corrosion prevention methods. Computational modeling and simulations such as density functional theory (DFT), molecular dynamics (MD), and Monte Carlo (MC) simulations save money and resources by minimizing the number of experimental trials. Herein, we discuss the current research on using various eco-friendly and sustainable materials, technologies, and strategies for corrosion prevention together with their challenges and opportunities.
1. Introduction
1.1. Corrosion and corrosion inhibition: a shift from inorganic (toxic) to green corrosion inhibitors
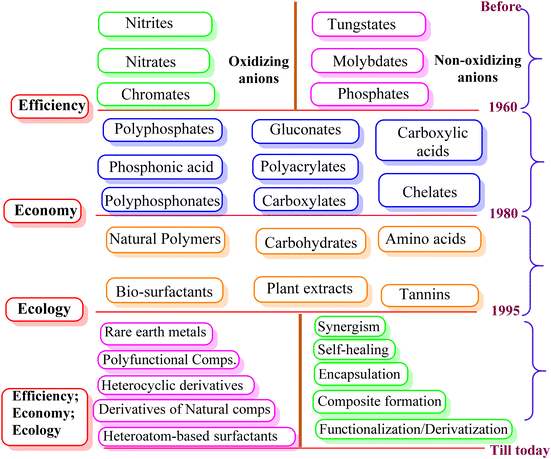
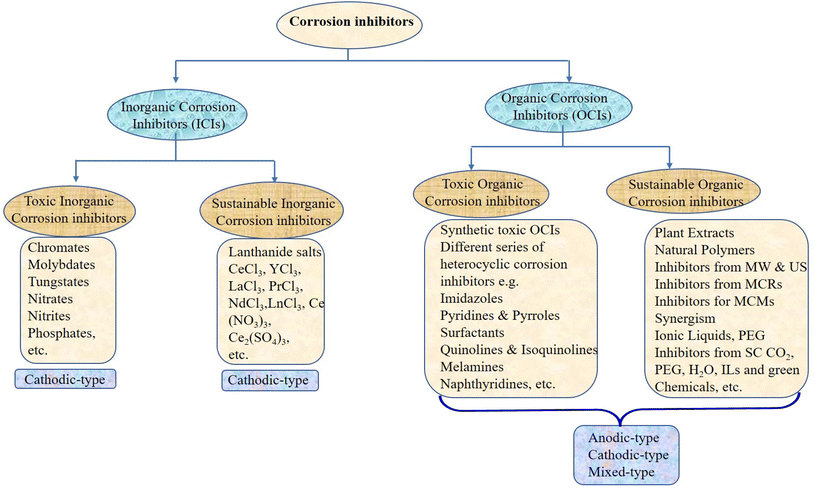
Metallic materials are extensively employed in various fields, including building supplies, particularly in the petroleum, oil, and gas industries.1 Unfortunately, most metals rapidly succumb to corrosive degradation due to the reactivity of environmental elements as they are thermodynamically unstable in their pure form. Corrosion is a natural phenomenon that impacts the economy, public safety, and environment. Numerous industrial processes employ extremely aggressive electrolytes, which disintegrate metallic components and undesirable surface contaminants. The National Association of Corrosion Engineers (NACE) estimation predicts that the global cost of corrosion is about 3.4% (US $2.5 trillion) of the world's gross domestic product (GDP).2,3 Fortunately, existing techniques can reduce the expense of corrosion by 15% (US $375 billion) to 35% (US $875 billion).4,5 Before 1965, the efficiency of corrosion inhibitors was the primary factor in their selection, regardless of their effect on the environment. The first line of protection was utilizing inorganic species such as chromates, nitrates, nitrites, phosphates, molybdates, and tungstates because of their great potential at relatively low concentrations. These species are called passivators because they shield metal surfaces from corrosion by generating a passive covering. Chromates, nitrates, and nitrites are oxidizing anions or oxygen-free passivators as they passivate metal surfaces without oxygen.6,7 In contrast, phosphates, molybdates, and tungstates passivate metal surfaces only in the presence of oxygen; therefore, they are referred to as oxygen-dependent passivators or non-oxidizing anions.8 However, because of their toxicity and bioaccumulative nature, less expensive and more practical substitutes gradually replaced them (Fig. 1). Economic factors came into play between 1965 and 1978, and corrosion engineers and scientists created and used some affordable substitutes. Following that (1980–1995), rising ecological consciousness compelled scientists and engineers to employ comparatively eco-friendly substitutes. However, recent (from 1995) corrosion science and engineering studies have cast doubt on the creation of reasonably priced ecologically suitable substitutes. Among them, organic compounds remain the top options given that they are well-established as one of the most efficient, cost-effective, and profitable means of corrosion protection.9–11The use of numerous materials with natural and manmade origins in green corrosion protection has recently increased.9–11 Natural green corrosion inhibitors, including plant extracts, have been extensively researched and tested. Because of their plant-based origin, they are the best environmentally friendly, commercially viable, bio-degradable, bio-tolerable, and non-bioaccumulative substitutes for hazardous corrosion inhibitors.12–14 Each extract contains a variety of phytochemicals, ranging from simple to complex, which are joined by frequent conjugation to form polar functional groups and aromatic rings. In addition to other natural substances, such as carbohydrates, bio-surfactants, biopolymers, amino acids, and pharmaceuticals (chemical medications), green corrosion inhibition has attracted significant attention.15–19 The term “green substitute” can also be used to describe synthetic inhibitors made utilizing green starting materials (e.g. carbohydrates, amino acids, and natural resources), green solvents (e.g. water, supercritical carbon dioxide and deep eutectic solvent), and green (bio-based) catalysts. Additionally, multicomponent reactions (MCRs) have several advantages over traditional synthetic processes, making them “green”.20Fig. 2 illustrates common examples of toxic and sustainable organic and inorganic corrosion inhibitors. Accordingly, corrosion inhibitors prepared via MCRs, particularly applying microwave and ultrasound irradiation, can also be referred to as green alternatives.21,22 Herein, we offer a thorough and historical overview of the developments in green corrosion inhibition. Green chemistry principles and theories are emphasized in the design, development, and use of green corrosion inhibitors and green inhibition. The advantages, challenges, and prospects of each series of green alternatives have been explored using the action and behavior of industrial-based electrolytes.
Corrosion science and engineering have made significant strides in recent years, focusing on the design, synthesis, and implementation of environmentally friendly alternatives to conventional toxic inhibitors in response to growing public awareness of environmental issues and strict ecological policies.23,24 Examining the bioaccumulation potential, biodegradability, and environmental toxicity of a chemical species is a straightforward way to ascertain whether it is environmentally friendly as a corrosion inhibitor.25,26 These factors are evaluated by international organizations such as the Oslo and Paris Commission (OSPAR) and the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH). REACH, a regulation of the European Union (EU), was adopted on December 18, 2006, and became effective on June 7, 2007.27 The 849-page EU regulation discusses the design, development, and use of chemicals and their effects on the natural world and human health. The Oslo Convention against Dumping of Wastes at Sea, signed in 1972, and the Paris Convention on Marine Pollution from Land-Based Sources, signed in 1974, were combined and updated to become OSPAR on September 22, 1992.28–30 The bioaccumulation, biodegradability and toxicity of chemical compounds and their effects on the environment and human health can all be evaluated using the recommendations and indices established by these commissions. The effective and lethal concentrations of a chemical compound, which are denoted as EC50 and LC50, respectively, can be used to evaluate the toxicity of the compound before it is utilized as a corrosion inhibitor.31,32 EC50 indicates the chemical concentration that negatively impacts the growth of the living population, while LC50 suggests the concentration of a substance that results in the death of 50% of the population. A lower LC50/EC50 ratio indicates more significant sensitive toxicity and vice versa. Alternatively, a substance is considered harmless if its LC50/EC50 value exceeds 10 mg kg−1.33,34
Microorganisms known as decomposers spontaneously break down most chemicals, albeit the process is quite sluggish and can take days, months, or even years.35 If a chemical breaks down by 60% or more in 28 days, it can be considered environmentally benign.36 The ability of a chemical to accumulate in a living thing when its only supply is water is known as bioaccumulation.37,38 Usually, the partition coefficient, which is abbreviated as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW or DOW, is used to measure it. In general, KOW or DOW denotes the ratio of a concentration of a compound in a mixture of two immiscible solvents at equilibrium. The measurement in an octanol and water mixture is called bioaccumulation. An environmentally friendly compound should have a KOW or DOW value of three or less.39 Sustainable technology development depends on addressing the problem of toxicological data (i.e. toxicity (LC50/EC50), biodegradability and bioaccumulation (KOW or DOW) and the environment and human health) in corrosion inhibition investigations.40,41 The utilization of toxicology data is essential to guaranteeing that the corrosion inhibitors employed in different industries are appropriate for human health, the environment, and corrosion prevention.42,43 To reduce any possible impact on ecosystems and human health, researchers and developers can make well-informed decisions by evaluating the toxicity of corrosion inhibitors.44 This proactive strategy helps ensure that the market accepts these innovations and they remain viable over the long run, while also in agreement with ethical and environmental concerns. The foundation for responsible innovation is laid by highlighting the significance of toxicity data in the early phases of corrosion inhibitor research.40,41 It represents a dedication to developing technology that addresses the direct problems associated with corrosion, while also advancing ecological responsibility and sustainability in the long run.
KOW or DOW, is used to measure it. In general, KOW or DOW denotes the ratio of a concentration of a compound in a mixture of two immiscible solvents at equilibrium. The measurement in an octanol and water mixture is called bioaccumulation. An environmentally friendly compound should have a KOW or DOW value of three or less.39 Sustainable technology development depends on addressing the problem of toxicological data (i.e. toxicity (LC50/EC50), biodegradability and bioaccumulation (KOW or DOW) and the environment and human health) in corrosion inhibition investigations.40,41 The utilization of toxicology data is essential to guaranteeing that the corrosion inhibitors employed in different industries are appropriate for human health, the environment, and corrosion prevention.42,43 To reduce any possible impact on ecosystems and human health, researchers and developers can make well-informed decisions by evaluating the toxicity of corrosion inhibitors.44 This proactive strategy helps ensure that the market accepts these innovations and they remain viable over the long run, while also in agreement with ethical and environmental concerns. The foundation for responsible innovation is laid by highlighting the significance of toxicity data in the early phases of corrosion inhibitor research.40,41 It represents a dedication to developing technology that addresses the direct problems associated with corrosion, while also advancing ecological responsibility and sustainability in the long run.
1.2. Green corrosion inhibition: practices and applications
Green corrosion inhibition has emerged as a sustainable and environmentally conscious method to safeguard metal structures against the detrimental impacts of corrosion.45 These techniques aim to mitigate the ecological footprint associated with conventional corrosion inhibitors by utilizing inhibitors derived from renewable sources. These inhibitors create a protective layer on the metal surface, preventing corrosive substances from infiltrating and deteriorating the metal through film formation, adsorption, and passivation. Numerous alternatives of natural and synthetic origins have been developed to protect against metallic corrosion in industrial environments (Fig. 3). Among them, corrosion inhibitors made from plants and animals, such as biological extracts, biopolymers (such as polysaccharides), and amino acids, are common. These alternatives undoubtedly contain a variety of molecules known as biochemicals and phytochemicals, which are obtained from animals and plants, respectively (Fig. 3), aiding in their absorption and function as efficient inhibitors.46 However, the degradation of the inhibitors obtained from biological systems at high temperatures limits their utility. Thus, synthetic alternatives are still the best option because they are considerably more stable, efficient, and cost-effective, particularly at high solution temperatures and with aggressive electrolytes. The use of green corrosion inhibitors offers several advantages. Firstly, minimizing reliance on non-renewable resources and restricting the release of dangerous substances into the environment help preserve the ecosystem. Green inhibitors are also frequently biodegradable, ensuring their safe disposal without endangering the ecosystem in the long term.47,48 Additionally, they exhibit lower toxicity levels than their traditional counterparts, enhancing the safety of those involved in their application and handling. Another notable advantage of green inhibitors is their cost-effectiveness, given that they can be produced at competitive prices due to the availability of renewable resources and scalable manufacturing processes.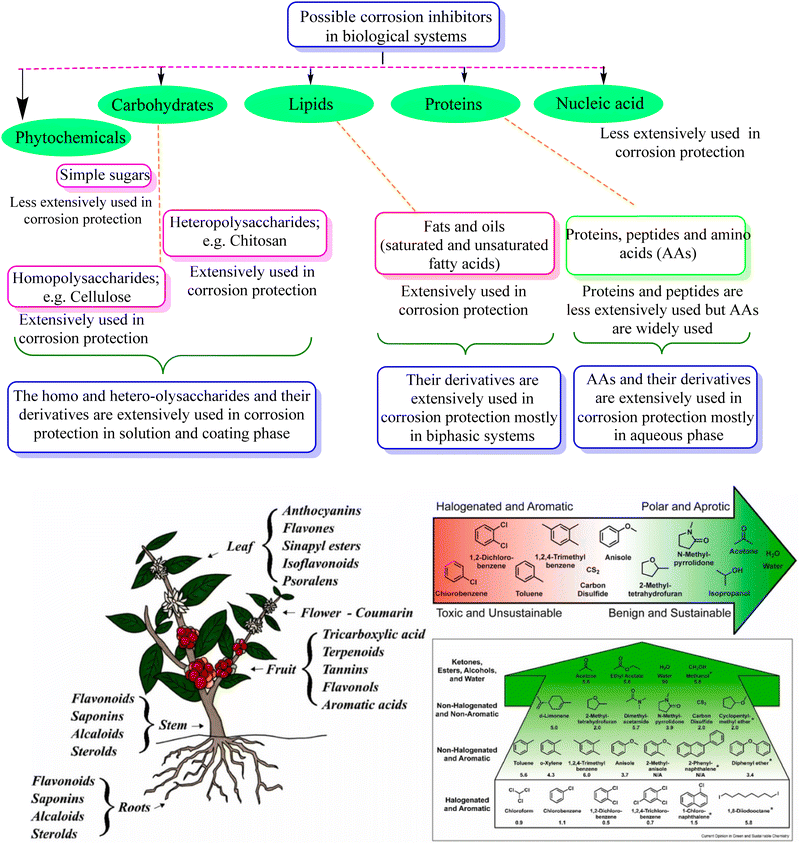 | ||
| Fig. 3 Schematic illustration of some major classes of natural (animal and plant derived) corrosion inhibitors and their peculiar examples (upper) and different phytochemicals or constituents present in extracts that can serve as corrosion inhibitors (lower)46 [the lower portion was reproduced from ref. 46 and appropriate permission has been obtained, Copyright, Elsevier, 2017]. | ||
Various industries including oil and gas refineries, power generation, pharmaceutical utilities, and metal and mining, have adopted green corrosion inhibition practices.49 The demand and market size for corrosion inhibitors that are oil-based, solvent-based, and water-based are rapidly increasing due to the rising industrialization. During the period 2022–2029, the corrosion inhibitor business is projected to grow at an astounding compound annual growth rate (CAGR) of 4.7%. The worldwide market for corrosion inhibitors is expected to grow from US $7.5 billion in 2021 to US $11.33 billion by 2029. Because of its large reserves of petroleum and natural gas and increased R&D efforts, Asia–Pacific now holds a monopoly on the corrosion inhibitors market. Green inhibitors are employed in bridges, buildings, and pipelines in the construction and infrastructure sector to prevent corrosion, extend their lifespan, and reduce maintenance costs.50 The automotive and transportation industries enhance the corrosion resistance of their vehicles and prolong their service life by incorporating green inhibitors into coatings, fuel systems, and cooling systems. Furthermore, energy and power generation systems, such as power plants, wind turbines, and solar panels, benefit from green inhibitors to ensure the efficiency and durability of these renewable energy sources. Green corrosion inhibitors play a pivotal role in safeguarding ships, offshore platforms, and underwater structures from corrosion, given that they are constantly exposed to harsh marine and offshore environments, which can be detrimental to these systems.51 In manufacturing and industrial processes such as metalworking, oil and gas refining, and chemical processing, green inhibitors are essential for preventing equipment and component corrosion, thereby maintaining operational efficiency and minimizing downtime. The successful implementation of green corrosion inhibition practices necessitates the optimization of formulations, appropriate surface preparation, and practical application methods. Real-world examples underscore the effectiveness and cost-efficiency of green inhibitors across various industries, showcasing their potential to revolutionize corrosion prevention, while promoting environmentally responsible practices.52
1.3. Green chemistry and green chemistry principles in service of corrosion science
The term “green chemistry”, which is also referred to as clean chemistry or benign and sustainable chemistry, encompasses the fabrication of chemicals and the development of processes aimed at reducing risks to human health and minimizing environmental pollution. The fundamental objective of green chemistry solutions is mitigating or eradicating the harmful impacts of chemicals throughout their life cycle. The inception of green chemistry dates back to 1991, which was marked by the launch of the Alternative Synthetic Pathways for Pollution Prevention research program by the U.S. Environmental Protection Agency (EPA). This program emerged under the umbrella of the Pollution Prevention Act of 1990. It substantially shifted from previous EPA endeavours by emphasizing reducing or eliminating hazardous substance production. This approach is different from the conventional management of chemicals after manufacturing and their release into the environment. Over time, this research initiative expanded to encompass the exploration of more environmentally friendly solvents and safer chemicals. In 1996, “green chemistry” was officially embraced to include these principles.53,54 Since then, green chemistry has emerged as a promising avenue of exploration regarding metallic material degradation. Specifically, employing inhibitors is a strategic approach for pre-empting, managing, or retarding metal corrosion. Traditional corrosion inhibitors often involve toxic or environmentally harmful substances, leading to unintended consequences. In contrast, green chemistry techniques seek to design effective inhibitors against corrosion and are environmentally friendly throughout their life cycle. Green chemistry presents a promising approach for preventing, managing, and mitigating corrosion. In recent years, the focus of green chemistry has shifted towards safeguarding both the environment and human well-being through economically advantageous means, aiming to eliminate toxins and curtail waste. In the realm of metallic material degradation, which is an area often confronted with the use of harmful substances, “green chemistry” has emerged as a thriving domain of research. The use of inhibitors is a well-established strategy when combatting metal corrosion through prevention, control, or deceleration. Conventional corrosion inhibitors have been demonstrated to be effective but are associated with significant drawbacks, including environmental ramifications, toxicity concerns, and adherence to regulatory stipulations.However, as the emphasis on environmental impact grows, the demand for more sustainable alternatives is increasing accordingly. In this case, “green chemicals” or environmentally friendly compounds offer a novel and promising avenue for addressing corrosion issues. Within the realm of corrosion science, green chemistry frequently involves the deployment of inhibitors, which are substances designed to reduce the corrosion rate. These inhibitors are meticulously engineered to exhibit biodegradability, renewability, and reduced toxicity. They can be derived from natural sources such as plants, biopolymers, and amino acids, which contain organic compounds capable of forming protective films on metal surfaces. Additionally, embracing green methodologies for corrosion resistance may involve substituting solvent-based coatings with water-based counterparts. The endeavours in green chemistry all share a common goal, i.e., to diminish the environmental footprint, while effectively safeguarding materials from corrosion. This contribution to corrosion inhibition stems from the commitment of green chemistry to developing environmentally friendly and sustainable inhibitors. These inhibitors are meticulously designed to reduce the reliance on toxic chemicals and minimize waste generation.
The theories and concepts of green chemistry offer a fresh perspective on corrosion science and engineering by emphasizing the creation of environmentally friendly materials and processes with the fewest detrimental effects on the environment.20,55 Essentially, green chemistry encourages the development of intrinsically safe protective coatings and corrosion inhibitors by reducing or eliminating the hazardous chemicals commonly employed in corrosion control practices. Green chemistry connects corrosion research with broader sustainability goals by highlighting the creation of environmentally friendly solutions, such as bio-based inhibitors derived from renewable resources and using energy-efficient electrochemical processes that produce fewer toxic byproducts. The lifetime and integrity of materials are guaranteed by this integration, which also promotes a comprehensive approach where environmental stewardship and material preservation principles come together to drive innovation and responsible practices in corrosion research and engineering. The twelve principles of green chemistry reinforce creating and using environmentally friendly materials and processes.56–58 Regarding green corrosion inhibitors, these principles direct the creation and application of materials that meet standards such as waste avoidance, safer chemical design, and the utilization of renewable feedstocks. Green corrosion inhibitors reduce environmental pollution by ensuring that their application produces few or no harmful by-products, which is consistent with waste prevention principles.59,60 Furthermore, these inhibitors place a high priority on creating non-toxic compounds and protecting ecosystems. The synthesis of these materials using renewable resources is consistent with the concepts of sustainability and resource efficiency. In addition, adding these inhibitors lessens operations that consume a large amount of energy, which is an example of energy efficiency. Generally, green corrosion inhibitors promote safe, effective, and sustainable corrosion prevention methods, while reducing harmful environmental effects, embodying the twelve principles.
Reducing toxicity, limiting environmental effects, and utilizing plant extracts, amino acids, and carbohydrates as corrosion inhibitors are examples of various green chemistry principles.3,14,15 This is because they provide non-toxic and biodegradable solutions, being composed of natural substances and can be considered environmentally benign substitutes for synthetic inhibitors. Furthermore, applying plant extracts, amino acids, and carbohydrates can lessen the requirement for energy-intensive procedures to create synthetic inhibitors, which is consistent with the energy efficiency principle. More environmentally friendly compounds that inhibit corrosion with less adverse effects on human health and the environment include biodegradable polymers, polyethylene glycol (PEG), and ionic liquids (ILs).3,20 These materials are prime examples of green chemistry. These substances adhere to the synthesis of less dangerous chemicals by reducing their volatility and toxicity, while offering efficient corrosion prevention. Biodegradable polymers fulfil the crucial requirement of designing for breakdown by guaranteeing that the inhibitors decompose into innocuous chemicals.
The solvent-free strategy adheres to the waste prevention concept by minimizing environmental damage related to solvent use by avoiding the requirement for solvents.61,62 Additionally, the solvent-free approach promotes atom economy by combining all reactants in the finished product and maximizing the resource efficiency. It lowers health and safety hazards, which is consistent with creating safer chemicals. By giving preference to environmentally friendly solutions, corrosion inhibitors developed from green solvents exemplify multiple green chemistry concepts.63–65 The successful application of green chemistry principles to environmental responsibility and corrosion avoidance is exemplified by corrosion inhibitors derived from green solvents. Green chemistry principles are best shown by MW (microwave)- and ultrasonic-derived corrosion inhibitors, which promote efficient synthesis methods that reduce energy consumption and waste production.22 Faster reaction times, energy savings, and improved process efficiency are common outcomes of MW and US irradiation.66 Furthermore, consistent with the idea of creating cleaner chemicals, these techniques frequently require little or no harmful solvents. The environmental factor, sometimes known as the E-factor, is a metric to assess how a chemical process affects the environment. Eqn (1) can be used to determine the E-factor by dividing the entire amount of waste generated during a procedure by the mass of the intended product.67 A method with a lower E-factor produces less waste, and therefore is more environmentally friendly. The environmental impact of a process is influenced by several variables, such as raw material selection, energy use, and waste production. However, information regarding the E-factor of corrosion inhibitors is scarce. Our research team synthesized three glucose derivatives, namely ethylenediamine-modified glucose (EMG), tetramethylenediamine-modified glucose (TMG), and hexamethylenediamine-modified glucose (HMG), and investigated their ability to inhibit mild steel corrosion in 1 M HCl.68 The results showed that for every kg of product (EMG, TMG, and HMG), 1–2 kg of waste was produced, which is acceptable considering the application of corrosion inhibition.69,70 A subsequent investigation indicated that the E-factors of zwitterionic corrosion inhibitors derived from amino acids were 2.592 and 4.854, respectively.71
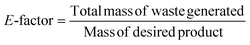 | (1) |
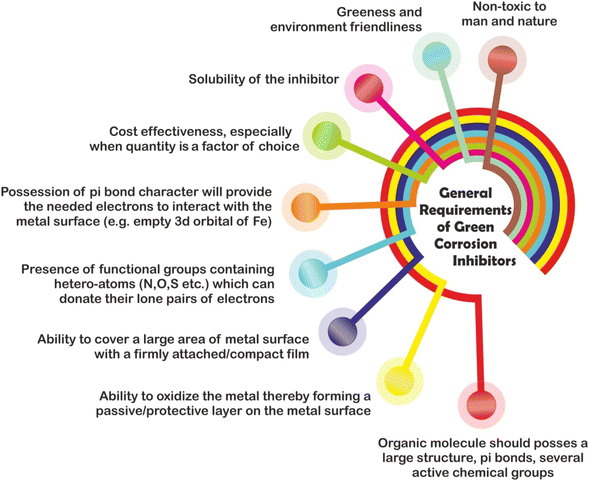 | ||
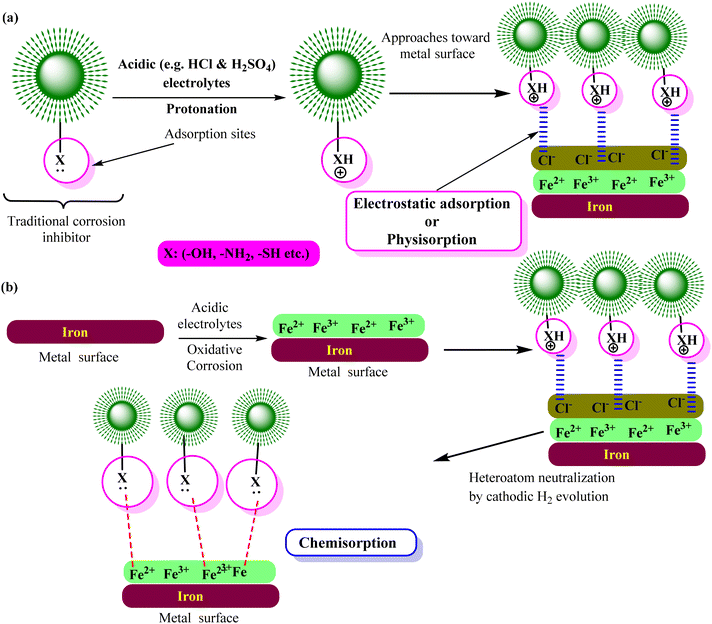
| Fig. 4 Schematic illustration of the general requirements of a compound to be used as a corrosion inhibitor [self-illustration, copyright permission not required]. | ||
Similar to traditional inhibitors, sustainable corrosion inhibitors of natural and biological origin become effective by forming a corrosion inhibitive film through their adsorption. The adsorption may be purely chemical, physical or physiochemical type. Generally, the adsorption of the inhibitor begins with physisorption and is established as chemisorption. Physisorption involves weak electrostatic interactions, while chemisorption involves stronger chemical bonds. Fig. 5 represents the physisorption and chemisorption of an organic inhibitor in an acidic electrolyte. Certain green corrosion inhibitors participate in redox reactions, donating or accepting electrons from the metal surface. This electron transfer process alters the electrochemical reactions during corrosion, reducing its rate.77,78 The combined action of multiple inhibitors can lead to a more significant reduction in the corrosion rate. The enhanced inhibitive effect of using two or more corrosion inhibitors in combination, where their combined effect is more efficient than the sum of their individual effects, is known as the synergistic mechanism of corrosion inhibition.79,80 It can result in corrosion protection, which is more effective and economical. In organic inhibitors, halide ions (I−, Br− and Cl−) frequently work in concert with corrosion inhibitors.81,82 Increasing the adsorption of organic inhibitors on metal surfaces can strengthen the corrosion prevention. Furthermore, Zn2+, Cu2+, and Sn2+ are the primary metal cations that can work together to form a more stable protective film on the metal surface. For instance, zinc ions are frequently employed in conjunction with organic inhibitors as synergists.79,83–85
Surfactants and polymeric compounds are also used in corrosion protection as synergists. Some inorganic anions (NO3−, SO42− and PO43−) can influence the electrochemical processes at the metal interface, thereby acting as synergists.86,87 They can alter the kinetics of corrosion and enhance the overall efficacy of the process in inhibiting corrosion. The type of metal being protected, the particular corrosion environment and the type of corrosion inhibitor used all influence the choice of synergists. Synergistic pairings are frequently customized to meet the unique requirements of a specific corrosion situation. Noticeably, the inhibition potential of inhibitors depends on many factors, including their electronic structure, nature of the metal and electrolytes, operating temperature and presence of additives.88–90 After getting adsorbed, green corrosion inhibitors retard both anodic and cathodic reactions, although in some cases, slight cathodic or anodic predominance has also been reported.
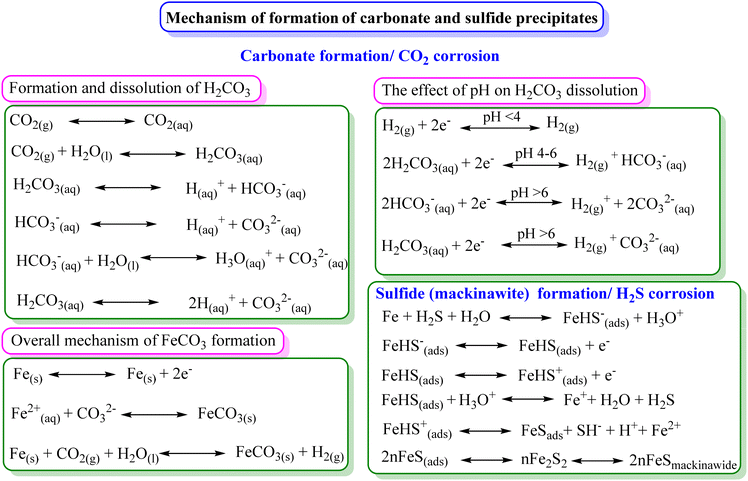
The corrosion protective film of inhibitors acts as a barrier between the metal and the corrosive environment.91,92 The inhibitor film may be monolayered or polylayered depending on the nature of the metal, electrolyte and inhibitors. Its specific functional groups can strongly bond with the metal surface, creating a well-organized and tightly packed film. In some circumstances, it can undergo chemical reactions on the metal surface, forming protective precipitates. Also, corrosion products provide some protection from further dissolution by serving as a barrier between the metal and the environment. The formation of carbonates and sulfides in the presence of CO2 and H2S is a common example of corrosion protection via precipitates.93,94 The formation of precipitates occurs under certain conditions, e.g., the formation of FeCO3 occurs preferably below pH 6 and temperature of 90 °C.95 The dissolution of CO2 in H2O and the formation of FeCO3 are presented in Fig. 6.
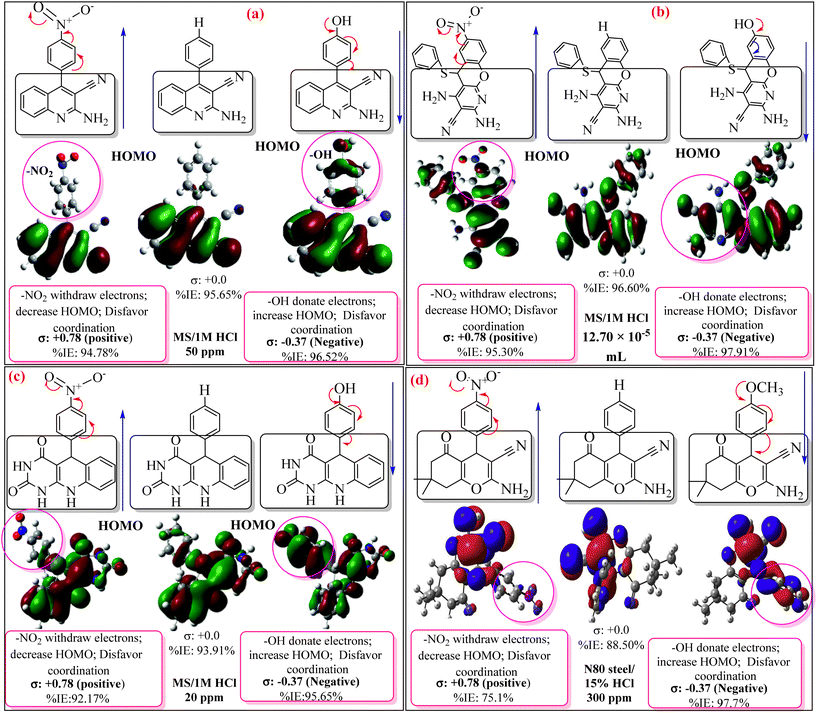
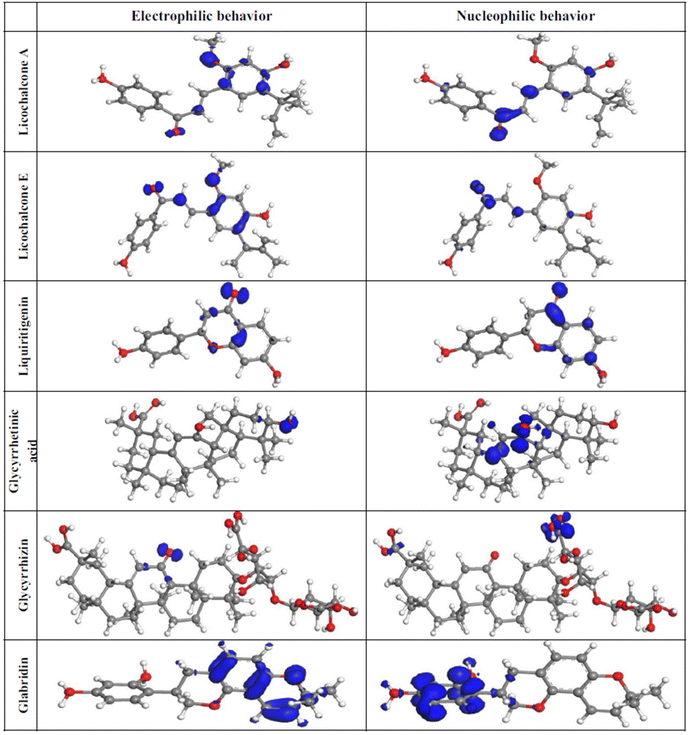
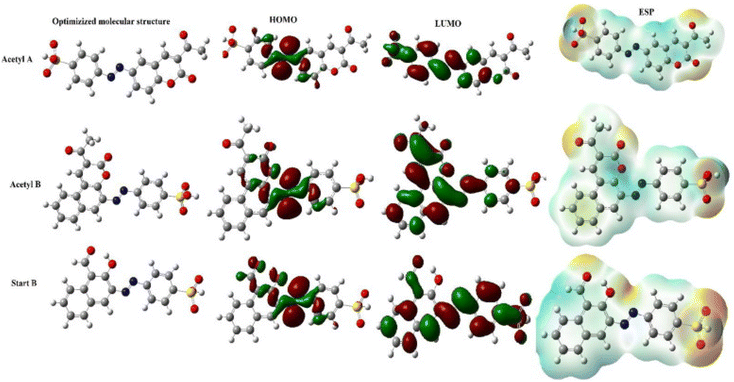
The most efficient organic inhibitors have polar functional groups in their chemical structures.9–11,96 The substituents substantially impact the coordination proficiency of organic inhibitors by altering the electron density at the donor sites, which are also known as adsorption or coordination sites. The polar substituents at the p-position that produce resonance effects include –OCH3, –OH, –NMe2, –NH2, –NO2, –CN, and –COOH. The corrosion inhibition efficiency (%IE) may occasionally be increased by e-withdrawing polar substituents at the o-position due to the potential chelation and substituent effects of polar substituents at the o-position.11 Due to their −R-effect, e-withdrawing substituents such as –NO2, –CN, and –COOH, often harm the inhibition potential. Alternatively, macromolecules (polymers) may be beneficial for the %IE by increasing the solubility of the inhibitor.97–99 These substituents affect the electron density at donor sites due to their ±R-effect (Taft substituent constant) and ±R-effect (Hammett substituent constant). The effect of some common substituents on the inhibition potential of some heterocyclic inhibitors is schematically presented in Fig. 7.
1.4. Greenness of corrosion inhibitors: toxicity, solubility control, and degradation aspects
Corrosion inhibitors are classified as “green” based on three main criteria, i.e., toxicity, solubility control, and degradation. These factors are critical when determining and guaranteeing the environmental friendliness of corrosion inhibitors. The utilization of green corrosion inhibitors exemplifies how the reduction of toxicity can significantly enhance the principles of sustainable development. This multifaceted approach can be elucidated through various key facets. Firstly, addressing human health and safety is pivotal. Conventional corrosion inhibitors frequently incorporate toxic elements such as heavy metals and hazardous organic compounds.100 These substances pose substantial threats to the well-being of humans throughout their lifecycle, from production to disposal. Those engaged in the manufacturing and application of these inhibitors encounter potential exposure to perilous chemicals. Thus, the shift towards green corrosion inhibitors, distinguished by lower toxicity levels, effectively mitigates these health risks, fostering safer working environments. Furthermore, environmental protection becomes paramount, given that the harmful inherent components in conventional corrosion inhibitors have the potential to infiltrate the environment during both their application and disposal phases.101 This pollution, with far-reaching consequences for soil, water, and air quality, threatens ecosystems and potentially contaminates the food chain.Green corrosion inhibitors are formulated with a keen awareness of minimizing environmental harm. By curtailing the discharge of toxic substances, these alternatives play a pivotal role in shielding natural resources and maintaining the ecological equilibrium.102 Sustainable manufacturing, as a pivotal tenet, is equally underscored. Green corrosion inhibitors are frequently composed of natural extracts,103,104 biopolymers,16 and ecologically-friendly compounds. These materials are marked by their renewability and sustainable attributes compared to the less sustainable toxic alternatives. This shift towards greener materials harmonizes the manufacturing process with sustainable paradigms, thereby curbing resource depletion and energy usage. The significance of waste reduction is also accentuated. The reduced toxicity of green corrosion inhibitors makes managing waste generated from their production and use considerably less complex. In contrast, toxic waste disposal requires specialized handling and treatment, inevitably exacerbating the environmental consequences. Adopting green inhibitors eliminates the need for specialized waste management, thus easing the burden on waste management systems.59 The pivotal aspect of regulatory compliance has to be considered. Given the stringent regulations governing the employment and disposal of toxic substances in various regions, integrating green corrosion inhibitors inherently facilitates adherence to these mandates. This proactive approach not only averts potential penalties but also averts legal entanglements associated with the use of toxic materials. Ultimately, the pursuit of long-term sustainability takes center stage. As industries shift towards sustainable practices, incorporating green corrosion inhibitors merges seamlessly with overarching sustainable development objectives. The abatement of toxicity curtails adverse impacts on both human society and the environment and contributes to the realization of a more harmonious and sustainable future.
Solubility control is a fundamental concept that is crucial in advancing sustainable development, particularly in green corrosion inhibitors. This principle is based on the intricate balance between two critical aspects, i.e., the solubility of the inhibitor and its effectiveness in preventing corrosion. Within this complex interplay, the solubility characteristics of an inhibitor can significantly impact both its performance and environmental impact.20 Corrosion inhibitors with high solubility carry the inherent risk of potentially leaching into the surrounding environment. When these highly soluble inhibitors are employed, they are more likely to extend beyond their designated application areas. This can result in unintended environmental consequences, such as the contamination of soil, water bodies, and ecosystems. Furthermore, the release of these inhibitors into the environment can disrupt the delicate ecological balance and adversely affect the flora, fauna, and human health. Thus, solubility control is pivotal in mitigating these undesirable outcomes. Alternatively, inhibitors with low solubility may face challenges in providing adequate corrosion protection. If an inhibitor has limited solubility, it may struggle to disperse and interact with the metal surface to form a protective layer. This can compromise the ability of the inhibitor to prevent corrosion effectively. Consequently, there should be a trade-off between the solubility of an inhibitor and its capacity to deliver reliable corrosion resistance. The essence of green corrosion inhibitors lies in their endeavor to strike an optimal balance in solubility. The goal is to identify and formulate inhibitors that achieve an ideal solubility threshold. This equilibrium ensures that the inhibitor dissolves sufficiently, allowing it to interact with the metal surface and inhibit corrosion. Simultaneously, the solubility of the inhibitor is controlled to prevent its excessive leaching into the environment. By attaining this equilibrium, green corrosion inhibitors simultaneously address corrosion prevention and environmental impact. The controlled solubility ensures that the inhibitor effectively serves its intended purpose, while minimizing the potential for harm to the ecosystem and human health.105 This harmonious alignment of effectiveness and environmental consideration exemplifies a fundamental principle of sustainable development, namely the capability to harmonize technological progress with the responsible management of natural resources.
Degradation represents a pivotal and multifaceted aspect that significantly contributes to the advancement of sustainable development, especially within the domain of green corrosion inhibitors. This principle is based on the inherent capacity of these inhibitors to undergo breakdown or degradation over time. The underlying objective of degradation is to ensure that inhibitors do not persist in the environment, curtailing their potential for long-term and adverse ecological effects. By integrating degradation as a central consideration, green corrosion inhibitors underscore their commitment to effective corrosion prevention and environmental responsibility.106 Biodegradability is a cornerstone of green corrosion inhibitors and pivotal in orchestrating their compatibility with sustainable principles.13 This intrinsic quality empowers these inhibitors to be naturally dismantled by microorganisms or other environmental processes, ultimately forming non-toxic byproducts. This natural degradation pathway serves as a counterbalance to the persistence of traditional inhibitors that can endure and accumulate in the environment, potentially inducing negative ramifications. The propensity of green corrosion inhibitors to biodegrade enhances their sustainability quotient by preventing the undue accumulation of inhibitors in the ecosystem and natural systems.59 Incorporating biodegradable materials takes precedence to effectively facilitate degradation and curtail the ecological footprint of green corrosion inhibitors. Bio-based polymers107 and natural compounds108 are prime examples of materials that align with the principles of sustainable development. These materials, marked by their capacity to undergo natural degradation processes, further amplify the environmental compatibility of green inhibitors. Integrating these materials not only fosters the breakdown of inhibitors but also minimizes their potential to disrupt the ecological balance, thus bolstering their credentials as environmentally friendly solutions.
The holistic perspective on green corrosion inhibitors reveals that their sustainability exceeds their mere efficacy in corrosion prevention. Their foundational principles include low toxicity, solubility control, and crucially, degradation. This comprehensive approach underscores the overarching commitment to curbing the adverse impacts on human well-being and the environment throughout their lifecycle. By adhering to these principles, green inhibitors seamlessly align with the core tenets of sustainable chemistry, effectively advocating for the adoption of corrosion prevention practices that are effective and environmentally conscientious.109 It is important to recognize that the evolution of green corrosion inhibitors is an ongoing process marked by continuous research and development endeavors. The perpetual quest to refine these inhibitors and enhance their green credentials remains imperative. As science and technology progress, the endeavors to further optimize the degradation characteristics of these inhibitors remain paramount, bolstering the efficacy of sustainable approaches in corrosion control. This commitment to innovation resonates strongly with the overarching goals of sustainable development, i.e., harmonious coexistence between technological advancement and environmental stewardship.
In conclusion, transitioning from traditional hazardous corrosion inhibitors to their green alternatives offers numerous advantages. This paradigm shift protects the environment and human health. It significantly contributes to sustainable development by promoting safer workplaces, reducing environmental pollution, supporting sustainable manufacturing, and ensuring compliance with legal requirements. Controlling solubility is pivotal in sustainable development, especially regarding eco-friendly corrosion inhibitors. Striking the right balance between functional efficacy and environmental responsibility is essential, given that the solubility and ability of an inhibitor to prevent corrosion are intricately linked. Pursuing optimal solubility thresholds is at the heart of green corrosion inhibitors, embodying their commitment to aligning with the principles of sustainable development. Sustainable progress in green corrosion inhibitors significantly hinges on the aspect of degradation. These inhibitors prioritize minimal environmental impact and limited persistence in ecosystems by emphasizing their inherent capacity to break down over time. Achieving this objective involves integrating biodegradable materials and practices, which aligns seamlessly with the principles of sustainable development. The amalgamation of degradation, alongside other environmentally conscious attributes, signifies the evolution of green corrosion inhibitors towards more responsible and effective corrosion prevention methodologies.110
1.5. Green metallic materials: pursuit of sustainable corrosion inhibition
Metallic materials, especially steel alloys are the most commonly used metallic alloys for building materials across various industries, including infrastructure development, because of their high mechanical strength, extended lifespan, versatility, and low cost.111,112 The annual steel output already exceeds 2 billion tons; by 2050, it is projected to increase by 33%.113,114 In the oil–gas and petroleum sectors, these alloys have also been used widely for building transport pipes, storage tanks, distillation and purification towers, etc. Iron is recovered from two types of ore, i.e., magnetite (Fe3O4) and hematite (Fe2O3), using a blast furnace and a significant amount of coke or coal. However, steel production releases considerable CO2, a greenhouse gas (GHG) contributing 7–11% of global GHG emissions.115,116 To maintain global warming (GW) at 1.5 °C, the United Nations (UN) advises that industry GHG emissions must be drastically decreased.117,118 To accomplish this goal, the steel industry and other sectors must reduce their GHG emissions by 93% by 2050 (IEA; International Energy Agency). The steel industry is one of the largest CO2 producers. In the UK, 25% of all industrial GHG emissions are related to the manufacturing of steel.119Fig. 8 depicts the amount of energy used by significant UK industries together with their greenhouse gas emissions.119,120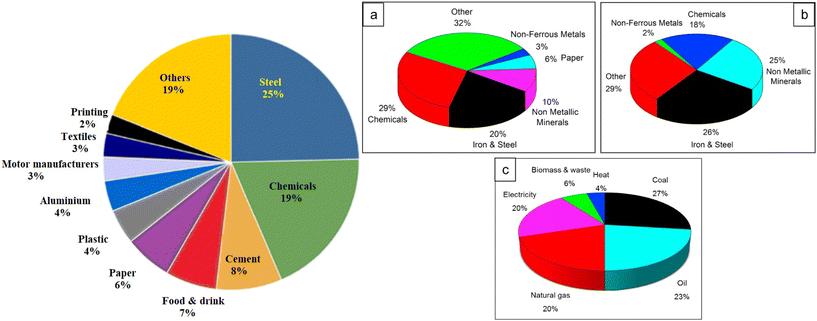 | ||
| Fig. 8 (Left) Greenhouse gas (GHG) emissions from the UK industry.119 [Reproduced from ref. 119 with permission, Copyright, Elsevier, 2021.] (Right) (a) Share of energy consumption in the industrial sector; (b) CO2 emissions and (c) final energy use in the industrial sector by fuel type.120 [Reproduced from ref. 120 with permission, Copyright, Elsevier, 2021.] | ||
A literature examination revealed that a ton of steel ore heated in a blast furnace emits 1710–1714 kg CO2.119,120 Coke production and sintering are responsible for almost 90% of the CO2 emissions from blast furnaces. Alternatively, renewable energy sources, electrification, and hydrogen are often used as fuel to produce green carbon steel. Recently, to reduce CO2 emissions and limit local warming, many European countries, including the UK, have started restricting steel production using conventional methods. The two most practical and efficient strategies to minimize GHG emissions are electrification and hydrogen as a fuel, but they are also likely to be expensive. The cost of steel produced through direct reduction utilizing electricity and hydrogen would reportedly be 20–30% costlier than steel generated through conventional steel building. The fact that hydrogen is a low- or zero-carbon energy source should be emphasized. However, roughly 70% of steel is created using blast furnace heating. Alternatively, in Sweden, the United States, and Europe in particular, where GHG emissions have decreased by 95%, 20%, and 40%, respectively, hydrogen-based steelmaking has made significant achievements.
Techniques and procedures to address socioeconomic and environmental problems are in great demand due to the increased ecological concerns about green chemistry and sustainable development. Due to urbanization and industrialization, carbon steel is one of the most often used metal-based construction materials, and its use and production are both predicted to increase. Thus, steel industry experts and authorities are searching for ways to develop efficient and sustainable steelmaking processes that have the potential to reduce the global CO2 emissions to almost zero to lessen the impact of GHG emissions. Compared to conventional blast furnace steelmaking, it is thought that the global CO2 emissions from the production of green carbon steel can be reduced by 95%. Notably, the release of about two tons of CO2 into the environment is correlated with the production of one ton of steel. Currently, there is a two billion-ton annual demand for steel worldwide. Thus, the production and marketing of green carbon are necessary for its advancement. Since 1975, up to a 50% reduction in GHG emissions has been achieved due to efforts such as carbon capture and storage (CCS), using bioenergy instead of coal for heating, electrification, and using hydrogen as an energy carrier. However, despite the substantial improvements in the creation of green carbon steel, around 75% of steel manufacturing is still heated using fossil fuels. Nevertheless, many nations, notably those in Europe, aim to address this issue, where Sweden is currently leading globally.121 Projections show that 31% of global steelmaking companies heat their facilities without the use of fossil fuels.
A Swedish company estimated that the cost of green carbon steel will be 20–30% higher than carbon steel made from fossil fuels. This method requires 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of hydrogen to produce the two million tons of green steel. Fortunately, the use of green carbon steel may not impact the overall cost of the finished goods. For instance, the value of a middle-size vehicle would only increase by 0.3% to 0.7%, or not more than €250, if traditional carbon steel was converted to green carbon steel at a rate of 100%. Engine cars contribute roughly 23% of GHG emissions. Similarly, steel contributes approximately 25% of appliance carbon emissions. However, switching entirely from traditional to green carbon steel would only add about 2–4% to the price of the appliance, or about €12. Notably, the use of fossil-free heating reduces GHG emissions and coal pollutants. Nevertheless, heavy metal and chemical impurities related to iron ores remain, and thus may need particular treatments before discharge.
000 tons of hydrogen to produce the two million tons of green steel. Fortunately, the use of green carbon steel may not impact the overall cost of the finished goods. For instance, the value of a middle-size vehicle would only increase by 0.3% to 0.7%, or not more than €250, if traditional carbon steel was converted to green carbon steel at a rate of 100%. Engine cars contribute roughly 23% of GHG emissions. Similarly, steel contributes approximately 25% of appliance carbon emissions. However, switching entirely from traditional to green carbon steel would only add about 2–4% to the price of the appliance, or about €12. Notably, the use of fossil-free heating reduces GHG emissions and coal pollutants. Nevertheless, heavy metal and chemical impurities related to iron ores remain, and thus may need particular treatments before discharge.
2. Green corrosion inhibition: current advancements and future directions
2.1. Green corrosion inhibition by selection: replacement of toxic alternatives
Sustainable chemistry or “green chemistry” is a relatively new and expanding field of chemistry and chemical engineering that focuses on developing goods and procedures that utilize less harmful materials and produce less waste. Recently, the manufacture and use of risky conventional volatile corrosion-inhibiting substances have been prohibited by regulations related to the environment and the global growing ecological consciousness. Therefore, improving synthetic and engineering chemistry using environmentally benign starting materials or carefully planning synthesis employing non-classical energy sources such as ultrasound and microwave heating is imperative. One of the best alternative synthetic methodologies for “green synthesis” is the use of MCRs in conjunction with ultrasonic (sonochemical) and microwave irradiation. Green corrosion inhibition refers to the use of environmentally friendly and non-toxic substances to prevent or reduce the corrosion of metals. The goal is to replace traditional corrosion inhibitors that may be toxic or harmful to the environment and human health. This approach is aligned with sustainable practices and is gaining increasing attention as industries seek to reduce their environmental impact.122,123To achieve green corrosion inhibition, researchers and industries are focused on finding alternative corrosion inhibitors that meet the following criteria:
a. Non-toxic: The selected inhibitors should not pose risks to human health or the environment during production, application, or disposal.
b. Biodegradable: The inhibitors should be easily broken down by natural processes, reducing their environmental persistence.
c. Renewable: Preferably, the inhibitors should be derived from renewable resources, which ensures a continuous supply without depleting finite resources.
d. High performance: The selected inhibitors should effectively protect metals from corrosion, maintaining or surpassing the performance of traditional toxic inhibitors.
e. Cost-effective: The inhibitors should be economically viable and comparable in cost to conventional inhibitors to encourage their widespread adoption.
f. Minimal side effects: The inhibitors should not adversely affect the properties or performance of the metal substrate.
g. Compatibility: The chosen inhibitors should be compatible with existing corrosion protection methods and coatings.124,125
The selection of a suitable green corrosion inhibitor depends on various factors, such as the metal to be protected, the environment in which it will be used, and the specific corrosion challenges it needs to address. Extensive research and testing are necessary to identify and optimize the most effective and environmentally friendly option for a particular application. Replacing toxic alternatives with green corrosion inhibitors is a step toward sustainable and responsible corrosion protection practices, contributing to preserving human health and the environment.126 Researchers are exploring various green corrosion inhibition strategies, such as using plant extracts, naturally occurring compounds, green synthesis of nanoparticles, and bio-based polymers. These substances have shown promising results in laboratory studies as corrosion inhibitors for different metals. However, although progress is being made in this field, it is important to note that developing effective green corrosion inhibitors may require further research and testing before they can be widely adopted in industrial applications. The choice of the most suitable inhibitor may also vary depending on the specific metal, environment, and intended application. The movement towards green corrosion inhibition reflects a broader trend in seeking sustainable and environmentally responsible solutions across various industries. In this regard, Goni et al. (2019), Shehata et al. (2018) and Verma et al. (2018) demonstrated the application of green corrosion inhibitors such as plant extracts, ionic liquids, heterocyclic organic compounds, nanomaterials, drug molecules, and carbon allotropes, and their advantages and future outlook.102 Promoting the replacement of toxic alternatives contributes to the overall goal of reducing the impact of human activities on the planet.127,128
There are several other approaches to achieving green corrosion inhibition, as follows:
Bio-based inhibitors: These are derived from natural sources, such as plant extracts, essential oils, and biodegradable polymers. They offer adequate corrosion protection, while being non-toxic and eco-friendly. Green synthesized inhibitors: These are compounds synthesized using green chemistry principles, which aim to minimize the use of hazardous substances and reduce waste generation. Nanotechnology-based inhibitors: Nanostructured materials can be corrosion inhibitors due to their unique properties. Some nanomaterials have shown excellent corrosion inhibition performance, while being relatively environmentally safe. Passivation techniques: Instead of chemical inhibitors, passivation methods can form a protective layer on the metal surface, preventing corrosion. Passivation often involves the use of non-toxic substances. Hybrid inhibitors: These are combinations of different non-toxic substances or methods to provide enhanced corrosion protection.
Researchers and industries are actively exploring green corrosion inhibitors to promote sustainability and reduce their environmental impact. These inhibitors are designed to be as effective as their traditional counterparts but without the negative environmental consequences. The selection and replacement of toxic alternatives involve finding suitable green corrosion inhibitors to replace those currently in use. This process involves the following: Identifying non-toxic compounds: Researchers are searching for compounds that have inhibitive properties but are non-toxic to humans and the environment. Natural compounds, plant extracts, and biodegradable materials are often considered for this purpose. Screening and testing: Once potential green corrosion inhibitors are identified, they undergo rigorous screening and testing. Various parameters, such as corrosion rate, inhibition efficiency, and compatibility with the metal surface, are evaluated to determine their effectiveness. Performance comparison: The selected green corrosion inhibitors are compared with traditional toxic ones to ensure they provide similar levels of corrosion protection. Application feasibility: Besides corrosion inhibition efficiency, practical aspects such as cost-effectiveness, ease of application, and long-term stability are considered to ensure that green inhibitors are viable alternatives. Implementing and promoting green practices: After successful identification and testing, industries and end-users are encouraged to adopt green corrosion inhibitors and replace toxic alternatives. Xhanari et al. reviewed green corrosion inhibitors for aluminium and its alloys. In the same vein, different literature reviews have shown that phytochemicals can act as green corrosion inhibitors in various corrosive conditions.129–132 By adopting green corrosion inhibitors, industries can reduce their environmental footprint and contribute to sustainable practices. Additionally, choosing non-toxic alternatives can lead to safer working conditions for employees and improved environmental protection. Thus, the transition to green corrosion inhibition is essential to a more sustainable and eco-friendly future.
Gravimetric analysis and electrochemical investigations are performed to learn about the basic properties of corrosion inhibitors, for example, adsorption nature, corrosion potential, inhibition efficiency, and adsorption types on the surface of metals by the inhibitor. In general, this can be performed in the lab via weight loss (WL) measurement, which does not require complicated or expensive tools. Electrochemical systems are typically three-electrode systems in which the metal to be inhibited is employed as the working electrode. Electrochemical tests often involve open circuit potential (OCP), linear polarization resistance (LPR), electrochemical impedance spectroscopy (EIS) and potentiodynamic polariation (PDP) studies. El Ibrahimi (2020) used experimental methodologies in which WL and EIS demonstrated that the correct sequence of %IE follows the order of MPBD > PBD > DMBD, which can be attributed to the presence of electron-donating groups.133 Alaoui (2018) extensively examined triazepine carboxylate derivatives such as Cl–Me–CO2Et, Me–CN, and Cl–Me–CN, with Cl–Me–CN having a %IE of 99% at a concentration of 10−3 M.134 Mo (2017) used a Schiff base, N-isonicotinamido-3-methoxy-4-hydroxybenzalaldimine (IM), to create vanillin and isoniazid green corrosion inhibitors, which demonstrated outstanding inhibition effectiveness according to the EIS analysis.135 Abdallah (2016) utilized phenyl sulphonylethanone derivatives (PSED) compound-1 and compound-2 with reported values of 85.5% and 83.5% at 5 × 104 M, respectively.136 According to Yadav et al. (2016), EIS analysis indicated %IE values of 95.7% for BIHT and 91.9% for MIHT. Their increased value is related to the presence of heteroatoms (N, O, S) and aromatic rings from the inhibitors, which significantly interact with the surface of Fe (110).137
Experimental approaches help determine corrosion mitigation on metals and alloys in harsh media. Therefore, many experimental techniques such as gravimetric and electrochemical analyses are widely used. These approaches are also used for determining the primary effectiveness of inhibitors in corrosion inhibition. 2-mercaptobenzothiazole (MBT)-functionalized ILs are used to prevent corrosion. EIS and PDP studies demonstrated that MBT-functionalized inhibitors inhibited corrosion in bronze significantly more than the standard IL [BMIM][BF4].138 The two new green ILs [VAIM][PF6] and [VAIM][BF4] displayed effective corrosion inhibition for carbon steel in 1.0 M HCl solution, with [VAIM][PF6] performing better than [VAIM][BF4].139 Two ammonium-derived ILs, N-trioctyl-N-methyl ammonium (TMA) methyl sulphate and N-tetradecyl-N-trimethyl ammonium (TTA) methylsulfate, were applied as corrosion inhibitors for API-X52 steel (1 M HCl), in which TMA showed 85% inhibition efficiency. In contrast, TTA exhibited 68% %IE according to the electrochemical analysis at the optimum concentration of 0.209 mM and 0.272 mM, respectively.140 Three pyrazine derivatives, HMIMI, BMIMI, and MPIMI, were used as corrosion inhibitors for mild steel (1 M HCl), and their inhibition efficiencies were determined to be 93.1%, 87.8%, and 80.4%, respectively, according to EIS measurements at their optimum concentration of 1 × 10−3 M.141 At low temperatures, [BsMIM][HSO4] and [BsMIM][BF4] showed strong corrosion inhibition characteristics in hostile medium (1 M H2SO4 solution). The inhibitory efficiency of [BsMIM][BF4] and [BsMIM][HSO4] is quite comparable.
Traditional approaches are widely used in experiments to assess corrosion and its process in metallic materials such as metals and alloys. Cao et al. (2020) investigated the effect of N-doped carbon dots on carbon steel corrosion in sulphuric acid media. They evaluated N-doped CDs as corrosion inhibitors for carbon steel using X-ray diffraction spectroscopy (XRD), Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), and electrochemical methods. According to the PDP study, they reported 97.8% percentage inhibition efficiency at a concentration of 30 mg L−1 N-CDs. They also observed that as the concentration of inhibitor increased, the values of anodic Tafel slope (βa) and cathodic Tafel slope (βc) decreased gradually. The PDP results demonstrated their good corrosion inhibition ability.142 Saraswat and Yadav (2020) published similar results, in which they employed S, N co-doped CDs as a possible corrosion inhibitor for mild steel in 1 M HCl. The WL and electrochemical techniques were used in their investigation. CD1 and CD2 had inhibitory efficiencies of 94.4% and 90.8%, respectively, at their optimal concentrations, according to the EIS measurements.143 Surface characterization methods, such as FTIR, XRD, scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS), were used to determine their inhibitory efficiency,yu adsorptive property, and interaction with metal surfaces. The morphological changes in the metal surface in the presence of an acidic solution were examined using these techniques before and after using the inhibitors. The chemistry of the metal surface was investigated using EDS. The AFM and XPS methods were used to describe the changes in the bonding energy between the 3D metal surface and inhibitor.
Yuwei et al. (2020) also investigated the inhibitory performance of N-doped citric acid-based CDs on the surface of mild steel in 1 M HCl electrolyte.144 They described a variety of methodologies for determining the inhibitory performance of the CD materials, including electrochemical measurement, FTIR, XPS, SEM, and AFM. The results of the surface characterization demonstrated great agreement with the other applied techniques.144 Organic compounds containing heteroatoms (O, P, N, S) have been widely explored as mild steel corrosion inhibitors. Jia et al. (2011) studied the inhibitory characteristics of L-cysteine derivatives such as NASHCYS, NACYS, NASBCYS, and CYS in mild steel corrosive solutions containing 1 M HCl. Their study included the density functional theory (DFT), molecular dynamics (MD) simulation, PDP, and WL techniques. NASBCYS was shown to be the most effective corrosion inhibitor among the inhibitors tested.145 Muthukrishna et al. (2014) used AC impedance, PDP, and WL methods to assess the %IE of CDHBAP against mild steel in a corrosive solution of 1 M H2SO4.146 The results showed that CDHBAP has 99% %IE at 100 ppm concentration. The creation of a protective barrier over the metal surface was verified by SEM and FTIR.146 Ousslim et al. (2009) evaluated the mild steel corrosion inhibition efficiency of two piperazine derivatives in an aggressive solution of 3.9 M HCl through the PDP and WL methods.147 Mohamed (2006) investigated quinine as a corrosion inhibitor for low carbon steel in HCl solution using the PDP and EIS methodologies, reporting an inhibition efficacy of 96% at a concentration of 0.48 mM.148 Rajeswarie et al. (2013) investigated the inhibition activity of three Schiff bases on cast iron using aqueous solutions of NaCl, NaOH, NH4Cl, and HCl.149 The additive impact of KI synergism was investigated. Electrochemical measurements and the WL technique were employed for this aim. The results showed that at low temperatures, the inhibitory efficiency was reduced with an increase in concentration. Also, it was discovered that the adsorption of Schiff base obeyed the Langmuir adsorption isotherm with and without KI.149 At the optimal concentration (303 K), Inh III (R = –OCH3) demonstrated an inhibition efficacy of 90.5%.150 At high concentrations, Gemini surfactant had an %IE of 94.2%.151
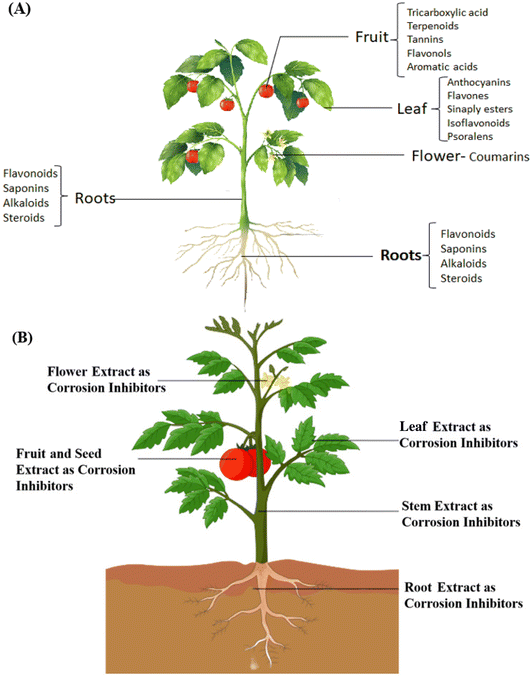
2.2. Green corrosion inhibition using natural products (plant extracts, amino acids, carbohydrates, etc.)
The use of green corrosion inhibitors generated from natural sources presents a possible route towards a more sustainable and corrosion-resistant future for metal-based infrastructure and applications, as society increasingly values sustainability and environmental responsibility. Recent years have seen a substantial increase in interest in using natural ingredients as green corrosion inhibitors as part of a larger commitment to environmentally benign and long-lasting corrosion prevention techniques. The concepts, processes, and use of these eco-friendly inhibitors are highlighted in this section, which examines the developing topic of “green corrosion inhibition using natural products”. The intrinsic corrosion inhibition capabilities of natural products, including plant extracts, essential oils, and bio-based compounds, are thoroughly examined. Compared to conventional chemical inhibitors, these inhibitors have a lower environmental effect due to their emphasis on eco-friendliness and biodegradability. Natural products have the potential to change corrosion protection tactics in a variety of sectors, while also shedding light on current research initiatives and difficulties in the industry.Substances originating from plants, animals, or other naturally occurring sources are referred to as natural products. They are often employed in several fields, including medicine, cosmetics, and even corrosion inhibition. Natural goods are prized for their possible medicinal benefits, low environmental impact, and environmentally friendly manufacturing processes.152 Plant extracts, essential oils, proteins, polysaccharides, and other substances can all be included. These organic components are more environmentally friendly than synthetic compounds and are well-known for their wide range of uses.153 Although several synthetic chemicals have shown effective anticorrosive properties, the majority are very harmful to both humans and the environment. Industry use of corrosion inhibitors has long raised safety and environmental concerns on a worldwide scale. These inhibitors may harm organ systems, such as the kidneys and liver, either temporarily or permanently, or they may interfere with biochemical processes or enzyme systems at a specific location inside the body.154 The toxicity may manifest either during the synthesis of the compound or its applications. Thus, natural anticorrosion products, which are safe for the environment and non-toxic, are now often used as a result of these hazardous consequences. Fig. 9 depicts how natural products can be used to inhibit green corrosion. The suitability of several natural compounds that have been investigated as corrosion inhibitors is described in the following sections.
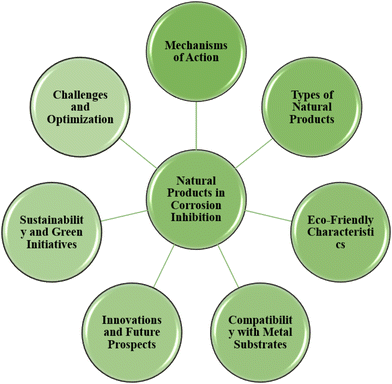 | ||
| Fig. 9 Schematic presentation of different aspects of using natural products in corrosion protection [self-illustration, copyright permission not required]. | ||
The electronic structures of phytochemicals frequently resemble conventional synthetic organic corrosion inhibitors. Consequently, it is understood that different compounds can provide corrosion resistance under varying corrosive circumstances.13 Since the majority of plants and their extracts are non-toxic, they are a great alternative to toxic organic inhibitors. Fig. 10B displays several inhibitors obtained from various plant sections. They are especially appealing because of how quickly they can be disposed of due to their biodegradable nature. Additionally, their extraction process is very simple and inexpensive. Extracting ecologically friendly corrosion inhibitors from a range of plant parts, such as fruit, leaves, bark, roots, seeds, or peels, is conceivable. The inhibitory effects of a plant extract are frequently caused by a combination of phytochemicals with various functional groups capable of sticking to the surface of metals. However, the intricate makeup of biomass extracts has prevented a thorough understanding of their mechanism for inhibiting corrosion. Analyzing each of the distinct and/or combined interactions of organic species on the metal surface is difficult without first isolating the active elements of any biomass extract. Plant extracts can be seen as eco-friendly and sustainable resources as inhibitors for metals and alloy corrosion in hostile media, such as HCl, H2SO4, H3PO4, and HNO3.128 This is due to their biological and natural origins, as well as their eco-friendly extraction. Plant extracts were obtained using a variety of techniques in the literature. However, the scope of the current review does not allow a thorough discussion of these techniques. Fig. 11 illustrates an outline of the extraction procedure.20
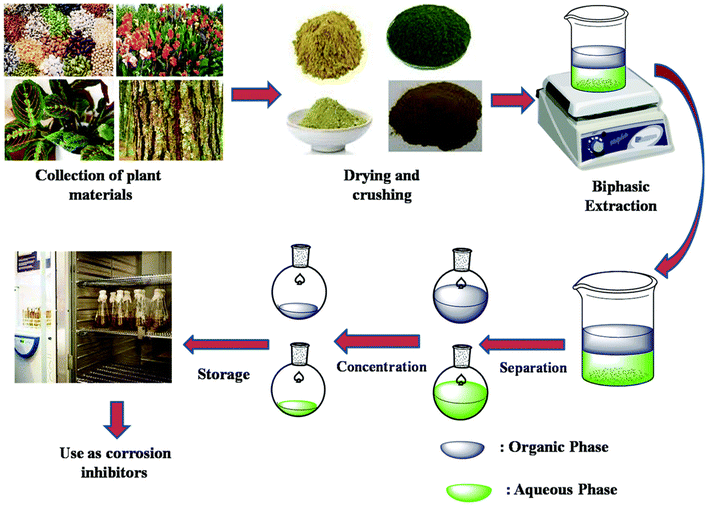 | ||
| Fig. 11 Schematic diagram of extraction procedure of phytochemicals.20 [Reproduced from ref. 20, RSC publication, copyright permission is required.] | ||
Celandine (Chelidonium majus) and the dried stems, leaves, and seeds of other plants were employed as plant extracts in the 1930s in H2SO4 pickling baths. A corrosion-controlling additive called ZH-1 was created, which was made of finely divided oil cake, a by-product in the production of phytin. According to Saleh et al.,156 steel is well protected by Opuntia extract, Aloe eru leaves, orange peels and mango peels in 5% and 10% HCl at 25 °C and 40 °C, respectively. According to Srivastav,157 lignin, castor oil seeds, acacia gum, black pepper, tobacco, and black pepper all work well as steel inhibitors in an acidic environment. The effectiveness of caffeine158 and nicotine159 in preventing metallic corrosion was investigated. The use of herbs (such as anise, hibiscus, garden cress, coriander, and black cumin) as a new form of green inhibitors for the acidic corrosion of steel was demonstrated by Khamis et al.160 El-etre161 researched the use of natural honey as a copper aqueous solution corrosion inhibitor. Carbon steel has also been the subject of a similar investigation.162 Sathiyanathan et al.163 investigated the effects of an ethanolic extract of Ricinus communis leaves on the prevention of mild steel from corroding in acid medium. The anticorrosion activity of henna164 and thyme165 extracts was examined. Pomegranate peel166–169 and beet root170 were investigated as corrosion inhibitors in different corrosive environments. Emblica officinalis,171Terminalia chebula,172–174Terminalia bellirica,175 and Acacia concinna176 have all been studied for their anticorrosion properties.
For steel in acid medium, corrosion prevention studies have also been conducted using extracts of Eucalyptus leaves, Carica papaya, Annona squamosa, Swertia angustifolia, Pongamia glabra, Azadirachta indica, Eugenia jambolans, Accacia Arabica, and Vernonia amygdalina.177 Tea wastes178 and Andrographis paniculata179 have both been found to have anticorrosive properties. Rosmarinus officinalis aqueous extract was examined by Kliškić et al.180 as an aluminium alloy corrosion inhibitor in a chloride solution. Abdallah et al.181 examined the anticorrosion properties of guar gum. Martinez and Štern182 conducted research on the Mimosa tannin-induced inhibition of low carbon steel corrosion in H2SO4 medium. In both HCl and H2SO4 medium, Oguzie looked into the effectiveness of Telfairia occidentalis extract as a corrosion inhibitor.183 Avwiri and colleagues investigated the ability of Vernonia amygdalina to prevent the corrosion of aluminium alloys in HCl and HNO3.184 Gunasekaran et al.185 investigated the inhibition of mild steel corrosion by Zanthoxylum alatum extract in aqueous orthophosphonic acid. The leaves of Nypa fruticans wurmb186 were investigated for their ability to prevent mild steel from corroding in HCl solution. The impact of saccharides, specifically reducing sugars like mannose and fructose, on the dissolution of zinc and aluminum in alkaline conditions was studied by Müller.187
Hammouti et al. investigated the extracts of ginger,188 jojoba oil,189,190 eugenol, and acetyl-eugenol191 for the purpose of preventing the corrosion of steel in acidic environments. Oguzie investigated the inhibitory effects of Sansevieria trifasciata extract192 and Ocimum viridis extract193 on the acid and alkaline corrosion of aluminium alloy. El-Etre et al.194 investigated the effects of Lawsonia extract against the acid-induced corrosion of metals, khillah extract195 for the inhibition of SX 316 steel corrosion in acid media, Opuntia extract196 for the inhibition of aluminium corrosion in acid medium, and vanillin197 for the inhibition of mild steel corrosion in acid media. Li et al.198 investigated the anticorrosion properties of berberine, an alkaloid isolated from Coptis, for mild steel corrosion in H2SO4 medium. Cassia occidentalis, Carica papaya, Delonix regia, and Datura stramonium as well as Azadiracta indica, Calotropis procera, and Auforpio turkiale sap, have all been reported to be effective as inhibitors of acid corrosion by Zucchi and Omar.199 Awad200 investigated the anticorrosive properties of quinine on carbon steel in 1 M HCl.
Numerous of these plant extracts exhibit corrosion inhibitory action, which may be caused by the presence of heterocyclic components such alkaloids and flavonoids. Even the presence of cellulose, tannins, or polycyclic chemicals tends to increase the number of layers that form over the metal surface, which promotes corrosion inhibition. Thus, according to the current debate, it may be inferred that plant extracts are the best candidates to replace the conventionally used, costly, and hazardous inorganic and synthetic organic corrosion inhibitors. Numerous phytochemicals and other compounds found in plant extracts have excellent adsorption and corrosion-inhibiting properties. As efficient corrosion-control agents for aluminium, steel, and other metallic materials in acidic (HNO3, H2SO4, H3PO4 and HCl), basic (NaOH), and neutral (NaCl) conditions, a wide variety of extracts, including leaves, roots, stems, and other extracts, have been investigated. It has been noted that the leaf is the part of the plant that contains the most phytochemicals, which is where they are produced.
The effective use of a variety of essential oils to prevent metal corrosion in both acidic and alkaline environments has been covered in numerous reports. Essential oils, including jojoba oil,189,190 rosemary oil,202–204Artemisia oil,205 and Eucalyptus oil,206 have been evaluated as effective inhibitors against corrosion. Additionally, it has been discovered that essential oils obtained from various sources work well as anticorrosion agents for a variety of metals in a variety of hostile conditions (e.g., HCl, H2SO4, and NaCl). According to the results, oil adheres to the metal surface and slows corrosion. Znini et al.207–209 observed that essential oils such as Mentha spicata and Warionia sahara prevent the corrosion of steel in acidic environments. Also, the use of Moroccan Ammodaucus leucotrichus fruit-derived essential oils, Limbarda crithmoides,210 and Verbena officinalis211 to prevent mild steel corrosion in HCl medium has been studied.
The possible use of green inhibitors based on essential oils has attracted increasing interest in recent years. Numerous investigations have been conducted to define a variety of essential oils and assess how well they prevent metal corrosion brought on by chemical assault. However, there are few published findings on the effectiveness of essential oils for inhibiting microbial-induced corrosion.212 Additionally, to date, there are no published studies on the anticorrosion properties of commercially available ultra-pure essential oils such as cinnamon, clove, basil, and oregano against harsh environments. This may be due to the fact that although they show significant technological promise, ultra-pure commercial essential oils are very expensive, resulting in a lack of interest in employing them as corrosion inhibitors. Alternatively, essential oils have strong antibacterial properties.213 López et al.214 assessed the antimicrobial activity of essential oils of Rosmarinus officinalis, Syzygium aromaticum, basillicum, Anethum graveolens, Origanum vulgare, Zingiber officinalis Thymus vulgaris and Cinnamon zeylanicum against a group of food-borne bacteria (e.g., yeast, A. flavus, P. islandicum, and C. albicans). Ağaoğlu et al.215 reported that a few dietary additives, such as essential oils (red crushed pepper, anise, cumin, cloves, poppy, cardamom, ginger, fennel, and cinnamon), exhibited antibacterial action against certain microorganisms (M. luteus, P. aeruginosa, K. pneumoniae, E. faecalis, E. coli, S. aureus, and C. albicans). With the exception of M. luteus, cinnamon was determined to be the most effective antibacterial agent. The essential oils of cloves, cinnamon, cumin, and fennel had less antibacterial action against these bacteria.
A literature survey also revealed that various other essential oils such as Artemisia herba-alba,216–220Artemisia mesatlantica,221,222Artemisia abrotanum,223,224Rosmarinus officinalis,225–227Eucalyptus globulus,228–231Foeniculum vulgare,232Syzygium aromaticum (Clove),233Carum carvi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234 have been applied for the protection of various metals and alloys in different corrosive environments. As corrosion inhibitors for different metals, essential oils have demonstrated remarkable promise. They are a desirable replacement for conventional chemical inhibitors due to their natural and environmentally benign qualities as well as their capacity to create protective barriers on metal surfaces. However, to completely comprehend their operations and maximize their efficiency in various metal corrosion conditions, more studies are necessary. The continued study of essential oils as corrosion inhibitors highlights their significance as ecologically benign and sustainable solutions in the field of metal corrosion prevention.
234 have been applied for the protection of various metals and alloys in different corrosive environments. As corrosion inhibitors for different metals, essential oils have demonstrated remarkable promise. They are a desirable replacement for conventional chemical inhibitors due to their natural and environmentally benign qualities as well as their capacity to create protective barriers on metal surfaces. However, to completely comprehend their operations and maximize their efficiency in various metal corrosion conditions, more studies are necessary. The continued study of essential oils as corrosion inhibitors highlights their significance as ecologically benign and sustainable solutions in the field of metal corrosion prevention.
Given the environmental concerns associated with conventional corrosion inhibitors, the use of amino acids as corrosion inhibitors provides a sustainable alternative. Amino acids are non-toxic and readily available compounds that can replace biohazardous inhibitors such as nitrites, benzoates, phosphonates, and quaternary ammonium salts. Their easy production and non-toxic nature make amino acids an attractive choice for corrosion inhibition in various industries. Furthermore, amino acids exhibit an adsorption mechanism on metal surfaces, which allows them to act as natural corrosion inhibitors. This adsorption mechanism involves binding to the metal surface via the nitrogen atom of the amino acid molecule. This adsorption process facilitates the formation of a protective layer on the metal surface, effectively slowing down the corrosion rate. In addition to their effectiveness as corrosion inhibitors, amino acids also meet the increasing requirements of environmental regulations.
The biodegradability of amino acids ensures that they do not contribute to environmental pollution, aligning with the principles of green chemistry and sustainable development. Amino acids have emerged as promising corrosion inhibitors due to their ability to prevent or slow down the corrosion process. The use of amino acids as corrosion inhibitors is of great importance in various industries. For example, in the oil and gas industry, where corrosion poses a significant threat to equipment and infrastructure, the use of amino acids as corrosion inhibitors can significantly reduce the economic and environmental costs associated with corrosion. Furthermore, amino acids have been found to be particularly effective in acidic media, where the corrosion rates are typically higher.
Zhang et al.18 tested amino acids such as glycine, cysteine, glutamic acid and their derivative glutathione as copper corrosion inhibitors. In aerated 0.5 M HCl solution, Zhang et al.237 conducted a comparative study among benzotriazole (BTA), alanine and cysteine by performing WL, PDP and EIS. de Matos et al.238 observed that cysteine exhibited inhibitory effects at higher concentrations (10−3 and 10−2 M) in de-aerated sulfuric media. This was attributed to the formation of a Cu(I)–cysteine complex and the subsequent film formation on the copper surface. The influence of pH of a 0.5 M Na2SO4 solution on the corrosion protection properties of cysteine for copper was investigated by Petrović et al.239 and Simonović et al.240 A summary of some of the work reported in this field is presented in Table 1. According to the available data, it can be concluded that in a variety of industrial applications, amino acids have demonstrated great promise as corrosion inhibitors. They are a feasible substitute for conventional chemical inhibitors due to their capacity to create protective layers on metal surfaces and the fact that they are sustainable and environmentally friendly. However, to maximize their efficiency, comprehend their processes of action in more detail, and investigate their suitability with various metals and corrosive conditions, additional research and development are required. Amino acids show significant promise for influencing the future of corrosion inhibition technology as we continue to search for greener and more effective corrosion prevention solutions. However, despite the numerous benefits of amino acids as corrosion inhibitors, such as their eco-friendliness and film-forming ability, when determining whether they are appropriate for a given corrosion protection application, it is important to consider their limitations in terms of effectiveness, compatibility, and cost. Thus, scientists and businesses are devoting their efforts to overcoming these restrictions and realizing the full potential of amino acids in corrosion prevention.
| S. no. | Metal/medium | Amino acids | Findings | Ref. |
|---|---|---|---|---|
| 1 | Iron/1 M HCl | Cysteine, cystine, methionine, valine, omithine, glutamine, serine, threonine, glutamic acid, aspartic acid, leucine, asparagine, alanine, arginine, glycine | All tested amino acids behaved as cathodic inhibitors. Cystine, methionine and cysteine gave the highest surface protection due to the presence of sulfur atoms in their molecular structure | 241 |
| 2 | Iron/0.1 M H2SO4 | Glutamic acid derivatives | N-Phthaloyl-L-glutamic acid (66.89%) < N-benzoyl-L-glutamic acid (64.03%) N-(1-oxooctadecyl)-L-glutamic acid (60.04%). Formation of protective layers | 242 |
| 3 | Iron/9 g L−1 NaCl | Methionine and some amino esters: methionine ethyl ester and methionine methyl ester | %IE: B (80%) > A (40%) > methionine (28%) at 10−2 M. The sulfur atom improves the adsorption, whose electronegativity is improved by the ethyl moiety, an electron donor in the structure of B. The adherence of B aligns with the Frumkin isotherm | 243 |
| 4 | Carbon steel/0.5 M H2SO4 | Polyaspartic acid | Polyaspartic acid acted as a predominant anodic inhibitor. %IE = 80% at 10 °C. Formation of shielding layer. Freundlich adsorption isotherm | 244 |
| 5 | Low alloy steel/0.2 M ammoniated | Glutamic acid | %IE: glutamic acid (81.5%). A stable chelate was formed on to aluminum surface. Acted as a mixed-type inhibitor. Hill-de Boer isotherm. Chemical adsorption | 245 |
| 6 | Al/0.1 M HCl | Glutamic acid | %IE: glutamic acid (66.67%). Physisorption. Frumkin, Langmuir, Flory-Huggins, El-Awady adsorption isotherms and kinetic and thermodynamic | 246 |
| 7 | Al/0.2–1 M HCl | Alanine, valine, methionine, proline, and tryptophan | %IE: alanine (56.3%), valine (58.2%), leucine (62.4%), proline (63.3%), methionine (81.0%), and tryptophan (90.0%). Acted as mixed-type inhibitors. Adherence of these inhibitors on the aluminum surface aligns with the Frumkin and Langmuir isotherms. The SEM images validate the surface protection of aluminum in mixed acid solutions by amino acids | 247 |
| 8 | Al/1 M HCl + 1 M H2SO4 | Glycine, glutamic acid, alanine, valine, aspartic acid, phenylalanine | Chemisorption and physisorption play a crucial role in the inhibition of pitting corrosion. | 248 |
| 9 | Aluminum alloy AA7075/0.05 M NaCl | Glycine | Behaved as a mixed-type inhibitor. %IE of 87.4% at 500 ppm Gly + 100 ppm KI. S2− ions increase the SCC of alloy: Gly successfully inhibitedCu10Ni alloy corrosion | 249 |
| 10 | Cu10Ni/3.5% NaCl + 20 ppm Na2S | Cysteine, N-acetylcysteine, and methionine | %IE: cysteine (96%) > N-acetylcysteine (88%) > methionine (77%) at 6.0 mM. Langmuir adsorption isotherm. Strong physisorption | 250 |
| 11 | Cu10Al 5Ni/neutral 3.5% NaCl | Methionine | Acted as a mixed-type inhibitor; %IE increases with decreasing MET concentration; physical adsorption | 251 |
| 12 | Cu/3.5% NaCl | Methionine, asparagine, serene, glutamine, cysteine, and arginine, | %IE: Arg > Gln > Asn > Met > Cys > Ser at 10−2 M; Arg was mainly a cathodic inhibitor. However, Cys, Met, Asn, Gln, Ser behaved as mixed-type inhibitors | 252 |
| 13 | Cu/0.1 M H2SO4 | Cysteine | Cysteine acted as a good inhibitor for Cu corrosion; Physical adsorption | 253 |
| 14 | Cu/3.5% NaCl | Phenylalanine | Phenylalanine with Ce4+ ions gave a robust synergistic effect by forming Phe/Ce4+ complex film on the Cu surface; IE = 72% for 5 mM Phe + 2 mM Ce4+ | 254 |
| 15 | Cu/0.5 M HCl | Proline, glycine, alanine, phenylalanine, histidine and cysteine | Physical adsorption. Phenylalanine, cysteine and histidine gave the highest %IE. The theoretical studies corroborated the experimental observations | 255 |
| 16 | Cu/8 M H3PO4 | Cysteine | Mixed-type inhibitor; mixed adsorption | 256 |
| 17 | Cu/2 M HNO3 | Cysteine, glutamic acid, glycine, and its derivative (glutathione) | Physical adsorption; %IE increases in the following order: glutathione > Cys > Cys + Glu-A + Gly > Glu-A > Gly | 257 |
| 18 | Cu/0.5 M HCl | Methionine | MET behaved as a cathodic inhibitor; the mixed CTAB/MET has a better synergistic influence compared with the mixed CPB/methionine; stronger electrostatic interaction | 258 |
| 19 | Cu/0.5 M HCl | Glycine, phenylalanine, threonine, and glutamic acid | Mainly cathodic inhibitors (except phenylalanine); Glutamic acid exhibited the strongest protective effect (%IE = 53.6%) | 259 |
| 20 | Cu/0.5 M HCl | Glycine, tyrosine, valine, and alanine, | Behaved as mixed-type inhibitors. %IE depends on the chemical structure of amino acids. %IE: valine = alanine ≪ glycine < tyrosine | 260 |
| 21 | Cu/0.5 M H2SO4 in O2−-saturated solution | Methionine | Methionine demonstrated limited surface protection properties; physisorption; synergic effect between methionine and Zn2+ ions | 261 |
| 22 | Cu/0.5 M HCl | Serine, threonine, and glutamic acid | Cathodic inhibitors; %IE: glutamic acid (90.4%) > threonine > serine (54.7%) at 1 mM; chemical adsorption | 237 |
| 23 | Cu/0.5 M HCl | Cysteine | Mainly cathodic inhibitor; physical adsorption. In the presence of Cu2+ ions, the %IE increases | 262 |
Some of the many metal substrates that biopolymers may be used to prevent their corrosion include iron, aluminium, copper, and their alloys. Studies have shown that biopolymers exhibit excellent performances in preventing both general and localized corrosion. For instance, xanthan gum, a polysaccharide produced by bacterial fermentation, demonstrated encouraging outcomes in preventing mild steel corrosion.270 Similarly, polysaccharides extracted from plant sources have exhibited corrosion inhibition on several metallic surfaces.271 The type of metal substrate, the surrounding environment, and the particular biopolymer utilized all have an impact on how well biopolymers work. To achieve the best corrosion protection, biopolymer formulations must be specifically matched to the characteristics of the metal substrate and the corrosive environment. Sustainability is one of the most important benefits of using biopolymers to reduce corrosion. Because biopolymers are made from renewable resources, they have a lower impact on the environment than synthetic corrosion inhibitors manufacture and disposal. Furthermore, this lessens our reliance on fossil fuels. Unlike certain synthetic equivalents, biopolymers do not remain in the environment because of their biodegradability. Utilizing biopolymers supports the development of ecologically friendly corrosion mitigation techniques and is consistent with the concepts of green chemistry. Their use can result in a decrease in the production of hazardous waste and the overall carbon footprint of corrosion control techniques. However, despite their great promise, several issues need to be resolved before biopolymers can be widely used to limit corrosion. Performance irregularities may result from variations in biopolymer composition, supply, and extraction techniques. Thus, to achieve accurate and repeatable findings, biopolymer formulations and testing procedures must be standardized. Furthermore, for the practical use of biopolymer-based coatings and inhibitors, it is essential to comprehend their long-term stability and endurance. Additional study is required to determine how well biopolymers operate in various environmental settings and how well they work with various coating techniques.
2.2.4.1. Chitosan. Through several processes, including its capacity to create barrier coatings on metal surfaces, chitosan shows corrosion prevention. The amino and hydroxyl groups in chitosan interact with metal ions to produce a film on the surface of metals. This adsorption of chitosan is responsible for the formation of a film. This adsorption slows the corrosion process by blocking electrochemical reactions and preventing the migration of corrosive substances.272 Furthermore, the inherent biocidal properties of chitosan can suppress microbial-induced corrosion (MIC) by inhibiting the growth of corrosive microorganisms on metal surfaces.273 Its multifaceted inhibitive mechanisms contribute to its effectiveness under different corrosive conditions. Also, the corrosion inhibition performance of chitosan has been investigated across a wide range of metal substrates, including carbon steel,274–283 aluminum alloys,284,285 and copper.286–288
Several variables, including pH, temperature, concentration, and exposure period, may affect the effectiveness of chitosan. Because of its amphoteric nature, studies have indicated that the inhibitory impact of chitosan is more evident under neutral to acidic environments.272 Because different metal substrates exhibit varying electrochemical behaviours and surface qualities, chitosan performs differently with each type of metal substrate. Thus, to realize the best corrosion protection, chitosan formulations must be specifically matched to the characteristics of each metal given that their interaction with the metal surface determines the creation of protective layers. To increase the robustness and endurance of corrosion protection, recent research has concentrated on creating coatings and inhibitors based on chitosan. The inhibitive qualities of chitosan can be enhanced by combining it with other substances, such as nanoparticles, to optimize its effectiveness for certain purposes. Green corrosion prevention is based on the same principles as chitosan, which is environmentally beneficial. Chitosan, which is derived from natural sources and has biodegradable properties, provides a sustainable substitute for synthetic corrosion inhibitors. Its ability to prevent microbial-induced corrosion as well as general and localised corrosion makes it a vital tool in the creation of environmentally friendly corrosion control methods.
2.2.4.2. Alginates. Due to its biodegradability and low toxicity, alginate, a natural biopolymer derived from seaweed, offers a sustainable and environmentally benign alternative for corrosion inhibition. Its importance in corrosion control techniques are highlighted by its mechanisms of inhibition, compatibility with different metal substrates, and alignment with green chemistry concepts.289 As research progresses, alginate-based corrosion inhibitors are poised to play a crucial role in promoting both environmental sustainability and corrosion protection. Alginate exhibits corrosion inhibition properties through several mechanisms,290 as follows: (a) Film Formation: Alginate molecules adhere to metal surfaces to form a shielding coating, which serves as a physical barrier against corrosive substances. Corrosive ions cannot spread as easily through this coating, which prevents them from reaching the metal surface. (b) Passivation: Alginate molecules adhere to metal surfaces to form a shielding coating, which serves as a physical barrier against corrosive substances. Corrosive ions cannot spread as easily through this coating, which prevents them from reaching the metal surface. (c) Complexation: Alginate can complex with metal cations, reducing their reactivity and preventing them from participating in corrosive processes.
The corrosion inhibition effectiveness of alginate has been investigated on various metal substrates, including steel, aluminum, and copper.15,289–295 The type of metal and the surrounding environment can have an impact on how well it performs. Alginate has demonstrated great promise in preventing corrosion on aluminium surfaces, making it applicable in fields such as aerospace and automotive where aluminium alloys are often utilized. The ability of alginate to inhibit corrosion can be affected by variables such as pH, temperature, concentration, and exposure duration. Thus, achieving the best corrosion protection requires optimizing these characteristics. However, although alginate has potential as a green corrosion inhibitor, it is still associated with certain difficulties. For example, inconsistent outcomes may be caused by variations in the alginate content, extraction techniques, and performance under various circumstances. Thus, to achieve accurate and repeatable results, the testing procedures and formulas must be standardized. Alginate-based coatings should be optimized for certain metal substrates in future studies, and its compatibility with other coating systems should also be investigated. Furthermore, the creation of alginate-based smart coatings that adapt to shifting environmental conditions may pave the way for cutting-edge methods of corrosion prevention.
2.2.4.3. Lignin. In recent years, researchers have explored the use of lignin, a complex natural polymer found in plant cell walls,296 as an eco-friendly corrosion inhibitor. Lignin offers corrosion inhibition through several mechanisms,297 as follows: (a) Adsorption: Lignin molecules can adhere to metal surfaces and provide a shielding layer. This coating serves as a physical barrier, preventing corrosive substances such as oxygen and moisture from coming into contact with the metal. (b) Complexation: Lignin has functional groups that can interact with metal ions during the corrosion process, such as phenolic hydroxyls. As a result of these interactions, fewer metal ions are available for corrosion processes, resulting in stable complexes. (c) Passivation: On metal surfaces, lignin can encourage the development of passive layers. These layers inhibit further corrosion by blocking electron transfer at the metal–electrolyte interface. The effectiveness of lignin as a corrosion inhibitor has been assessed on a variety of metal substrates, including steel, aluminium, and copper.297–301 The efficacy of lignin-based inhibitors may vary with environmental factors such as pH, temperature, and exposure time. Lignin-based corrosion inhibitors are positioned to play a crucial role in advancing environmental sustainability and efficient corrosion prevention as research continues to improve and overcome obstacles.
2.2.4.4. Polysaccharides. Among the natural substances investigated for corrosion inhibition, polysaccharides, which are composed of glucose units such as cellulose and starch, have demonstrated promise. Polysaccharides demonstrate the ability to prevent corrosion through several processes, as follows: (a) Barrier effect: When used as coatings or additives, cellulose and starch form a physical barrier on the metal surface. This barrier protects the metal from corrosive substances including ions, oxygen, and moisture. Thus, by limiting the direct interaction between the metal and these corrosive elements, the rate of corrosion is reduced significantly. (b) Adsorption: The surface of the metal can adsorb molecules of cellulose and starch. The interactions between the metal atoms and the hydroxyl groups found in these polysaccharides during this adsorption are both physical and chemical. Consequently, a shielding layer develops, further preventing corrosive species from reaching the metal substrate. (c) Complex formation: Starch and cellulose both have hydroxyl groups that can interact with the metal cations in the corrosive environment. Complexes are created as a result of this interaction and are less reactive than the free metal ions. Hence, the metal is vulnerable to corrosion.
Cellulose and starch have demonstrated corrosion inhibition efficacy on a variety of metal substrates such as steel, aluminum, and copper.302 A potential and environmentally acceptable option for corrosion inhibition is provided by cellulose and starch. Their importance in corrosion control techniques is highlighted by their numerous modes of inhibition, compatibility with a range of metal substrates, and agreement with green chemistry principles. Cellulose and starch-based corrosion inhibitors are positioned to play a crucial role in advancing environmental sustainability and efficient corrosion prevention as research advances and obstacles are overcome. However, although biopolymers-based corrosion inhibitors show promise, several challenges and areas for future research exist, as follows: Variability: Inconsistent outcomes may be caused by variations in composition, extraction techniques, and performance under various circumstances. Thus, to achieve accurate results, testing procedures and formulas must be standardized. Optimization: To improve formulations for certain metal substrates and ambient circumstances, more studies are required. Also, it is crucial to customize these inhibitors to meet certain requirements. Innovation: Corrosion prevention may be revolutionized by investigating novel strategies such as intelligent coatings and sophisticated materials that contain cellulose and starch, such as self-healing coatings and sensitive materials that adjust to shifting environmental circumstances.
2.3. Green corrosion inhibition using energy-efficient synthesis
Green chemistry, also known as sustainable chemistry, has emphasized the value of safeguarding the environment and human health over the last few decades. This fast-growing approach in the overall realm of corrosion inhibition-related science and technology entails reducing or eliminating the use and manufacturing of hazardous inhibitor compounds by appropriate target-specific innovation, aligned design, and user-friendly application. Additionally, the as-required materials should have energy-efficient synthetic routes.55 The mass level of environmental awareness and rigorous environmental regulations worldwide have strictly prohibited the synthesis and utilization of conventional hazardous corrosion inhibitors. In this perspective, designed engineering and subsequent development of synthetic inhibitors must be ameliorated by employing ecologically benign starting precursor materials or unconventional energy sources such as microwave and ultrasonic (i.e., sonochemical) heating in the contemporary domain. More specifically, microwave and ultrasonic heating have numerous advantages, including immediate interior heating, excellent temperature uniformity, and high selectivity, which are attributed to their benefits in the development of inhibitors via unconventional heating techniques. Green synthesis can be accomplished using multicomponent reactions, e.g., microwave and ultrasonic irradiation. It is worthwhile to mention that implementing multicomponent reactions has attracted significant attention because of their superior features such as easy operational protocols, less time consuming nature, less laborious, high reaction yield, and significantly lower quantities of waste production.303 Moreover, organic synthesis in the solid phase often correlates with a greater reaction yield, shorter reaction times, and significantly avoids the use of hazardous solvents and metal catalysts, providing another reason for green synthesis.The corrosion retardance effectiveness of six thiadiazole derivatives with various alkyl chain lengths, e.g., ethyl (for IC-2), n-propyl (for IC-3), n-pentyl (for IC-5), heptyl (for IC-7), undecyl (for IC-11) and tridecyl (for IC-13), was assessed for the steel surface in 1 M H2SO4.304 Both conventional and green microwave heating approaches were employed to develop the targeted inhibitors. As the reaction rate was expedited with marginally larger yields, the inhibitor molecules were produced more effectively by employing the microwave irradiation approach, as depicted in Fig. 12(a). The required acid, concentrated sulfuric acid, and thiosemicarbazide were mixed in a flask placed within a closed vessel. The mixture was magnetically stirred in a microwave reactor at 80–90 °C (20 W) for one hour. Once the reaction was finished, the mixture was chilled in cold water and neutralized with ammonia solution. EIS and SEM were utilized to comprehend the corrosion inhibition effectiveness of the six compounds. It was observed that all the as-synthesized inhibitors could reduce the double-layer capacitance compared to that of the blank owing to the variations in the thickness of the as-formed protective layer of the inhibitors. As more surface coverage was achieved, the inhibition efficiency increased from IC-2 to IC-11 with an increase in the alkyl chain length. Surprisingly, the inhibition efficiency decreased for IC-13, indicating that the alkyl chain of IC13 is no longer straight but twisted into a loop-like shape. Consequently, less surface coverage was associated with IC-13, although it possessed the longest chain length. The Langmuir adsorption isotherm further confirmed the chemisorption tendency of the inhibitors.
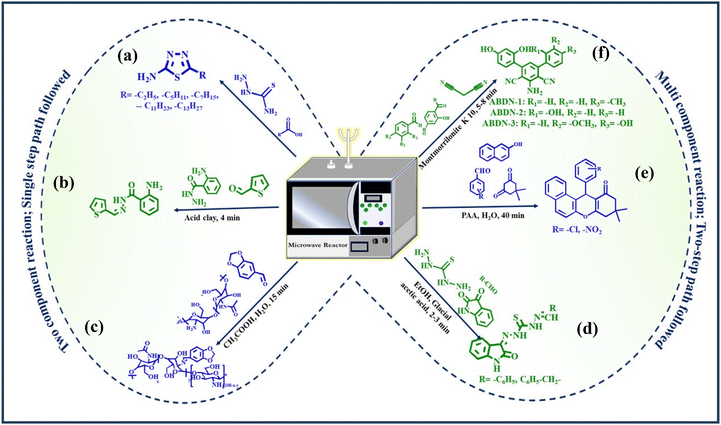 | ||
| Fig. 12 Schematic presentation of the green synthetic pathways for corrosion inhibitors via microwave irradiation for (a) thiadiazole derivatives304 [reproduced from ref. 304 with permission; Copyright, Elsevier, 2012], (b) ATMBH305 [reproduced from ref. 305 with permission; Copyright, RSC, 2018], (c) piperonal-chitosan (Pip-Cht)306 [reproduced from ref. 306 with permission; Copyright, ACS, 2014], (d) thiosemicarbazone derivative307 [reproduced from ref. 307 with permission; Copyright, Elsevier, 2015], (e) xanthone derivative308 [reproduced from ref. 308 with permission; Copyright, Elsevier, 2023], and (f) ABDN derivative309 [reproduced from ref. 309 with permission; Copyright, Elsevier, 2015]. | ||
In 2018, Singh et al. reported the use of 2-amino-N′-((thiophen-2-yl)methylene)benzohydrazide (ATMBH) as an excellent corrosion inhibitor for the protection of mild steel in the presence of 0.5 M H2SO4.305 ATMBH was synthesized via a solvent-free microwave-assisted methodology, as shown in Fig. 12(b). Thiophene-2-carboxaldehyde was first reacted with ortho-amino benzoylhydrazide in the presence of a catalytic amount of acid clay in a microwave reactor. The targeted product was formed in 91% yield within 4 min of reaction. The significance of this green synthesis protocol was highlighted by its added environmental benignity, high yield of the as-synthesized corrosion inhibitor, diminished energy consumption, and shorter reaction time than other conventional synthetic procedures. Gravimetric analysis and electrochemical investigations were used to investigate the corrosion inhibition efficiency of the inhibitors. It was observed that the corrosion inhibition efficiency of ATMBH increased with an increase in its concentration in the electrolyte solvent. SEM and AFM further validated these inhibition efficiency analyses results. The inhibition efficiency of ATMBH is attributed to the blockage of the active sites (specifically, the cathodic sites) through its physisorption and chemisorption on the mild steel surface, which followed the Langmuir adsorption isotherm. The contact angle measurement revealed the reduced hydrophilicity towards the aqueous acid solution in the presence of a higher concentration of inhibitor.
Similarly, this group reported the microwave-assisted synthesis of thiophene hydrazone derivatives, namely, acetyl thiophene benzohydrazide (ATBH), propanoyl thiophene amino benzohydrazide (PTABH), propanoyl thiophene benzohydrazide (PTBH) and acetyl thiophene amino benzohydrazide (ATABH).310 It was observed that the conventional reflux method in the presence of ethanol and acetic acid took 3–5 h, while the microwave-assisted technique helped in acquiring the as-required products within 3–5 min. Aoun et al. reported the use of 1-(3-bromopropyl)-4-(dimethylamino)pyridinium bromide (DPB) IL as a preferable contender for the corrosion mitigation of carbon steel in an HCl environment.311 EIS and LPR studies indicated an increase in the %IE with an increase in concentration. Alternatively, the thermodynamic studies revealed a discernible drop in %IE with an increase in temperature, indicating the physisorption of the inhibitor molecules under the current working circumstances. The Langmuir isotherm well fitted the physisorption nature of the inhibitor.
Piperonal-chitosan (Pip-Cht), a novel Schiff base of chitosan, was synthesized using microwave irradiation and characterized by spectroscopic methods. Gravimetric and electrochemical methods were used to analyze its corrosion-suppressing ability in carbon steel in 15% HCl media.312 Pip-Cht was synthesized by mixing chitosan with glacial acetic acid, distilled water and ethanolic piperonal solution, as shown in Fig. 12(c). The temperature of the microwave irradiation was ramped to 60 °C for 15 min. The effectiveness of the Pip-Cht was significantly increased by the addition of KI as a synergistic agent. Even at a high temperature of 65 °C, the inhibitor showed a substantial action that inhibited corrosion. The AFM study confirmed the adhesion of the inhibitor to the metallic surface and the development of a barrier film, which resulted in a reduction in the surface roughness of the metal. The presence of KI promoted the inhibitor adsorption, where the SEM study showed a smoother morphology of the steel surface in the presence of the inhibitor. DFT-based quantum chemical calculations showed that the Pip-Cht inhibitor exhibited improved adsorption behavior compared to the chitosan and piperonal parent molecules.
Three chitosan Schiff bases, namely, benzaldehyde (CSB-1), 4-(dimethylamino)benzaldehyde (CSB-2), and 4-hydroxy-3-methoxybenzaldehyde (CSB-3), as corrosion inhibitors were synthesized via the microwave-assisted pathway and their effective corrosion inhibition was analyzed on mild steel in the presence of 1 N HCl medium.313 After adding chitosan to distilled water, glacial acetic acid was mixed at room temperature. The as-required aldehydes were dissolved in ethanol. Subsequently, the aldehyde solution was added dropwise, while stirring continuously for 30 min at 303 K in the chitosan solution flask. The reaction mixture was subjected to 600 W microwave irradiation in a microwave-assisted reactor. Then, the sample temperature was raised gradually to 60 °C and maintained for 15–20 min until the completion of the reaction.
In another work, two isatin-thiosemicarbazone derivatives, namely, 1-benzylidene-5-(2-oxoindoline-3-ylidene) thiocarbohydrazone (TZ-1) and 1-(4-methylbenzylidene)-5-(2-oxoindolin-3-ylidene) thiocarbohydrazone (TZ-2), were synthesized. Their protective nature towards mitigating the corrosion of mild steel in the presence of 20% H2SO4 was assessed by employing experimental and computational analytical techniques.307 The synthetic procedure is schematically presented in Fig. 12(d). In the first step of the synthesis, thiocarbohydrazide was allowed to react with aldehyde in the presence of ethanol and glacial acetic acid. Herein, isatin was added to the mixture with the above-mentioned solvents under microwave irradiation for 2–3 min. The outcomes demonstrated that both inhibitors functioned as mixed-type inhibitors and that their adsorption on the metallic surface complied with the Langmuir isotherm model. Computational analyses were performed to validate the experimental findings. Later, Singh et al. reported the synthesis of two xanthone derivatives, namely 12-(4-chlorophenyl)-9,9-dimethyl-8,9,10,12-tetrahydro-11H-benzo[a]xanthen-11-one (BX-Cl) and 9,9-dimethyl-12-(4-nitrophenyl)-8,9,10,12-tetrahydro-11H-benzo[a]xanthen-11-one (BX-NO2), utilizing microwave-assisted technology, as presented in Fig. 12(e).308 An equimolar ratio of 2-napthol, dimidone, and 4-chloroaldehyde/4-nitroaldehyde was added to an aqueous solution of polyacrylic acid. Subsequently, the resultant combination was subjected to 40 min of microwave irradiation (560 W). These two derivatives were used to protect P110 steel in the presence of a 15% HCl solution. The incorporation of KI enhanced the corrosion inhibition and mitigation abilities of BX-Cl and BX-NO2. The WL studies showed that increasing the concentration of BX-Cl and BX-NO2 from 50 to 200 mg L−1 enhanced the corrosion inhibition efficiency from 62.10% to 92.21% and 47.36% to 84.21%, respectively. As calculated from the EIS data, the charge transfer resistance value reached 644.8 Ω cm2 for BX-Cl. This observation revealed the strong adsorption of the xanthone derivatives on the metal surface. However, the impedance decreased with an increase in temperature, which suggests that the increase in temperature led to the desorption of the adsorbed inhibitors from the metal surface.
Furthermore, the DFT and MD simulation results nicely corroborated the experimental findings. Three 2-aminobenzene-1,3-dicarbonitrile derivatives (ABDNs), namely, 5-amino-2,4-dihydroxy-4-methyl-1,1:3,1-terphenyl-4,6-dicarbonitrile (ABDN-1), 5-amino-2,2,4-trihydroxy-1,1:3,1-terphenyl4,6-dicarbonitrile (ABDN-2) and 5-amino-2,3,4-trihydroxy-1,3-methoxy,1:3,1-terphenyl-4,6-dicarbonitrile (ABDN-3), were synthesized and evaluated for their ability to suppress mild steel deterioration in 1 M HCl utilizing WLand electrochemical methods.309 The synthesis was performed using a microwave-assisted protocol involving a two-step process, as presented in Fig. 12(f). According to the electrochemical, morphological, and theoretical investigations, the corrosion inhibition effectiveness followed the order of ABDN-3 > ABDN-2 > ABDN-1, which was explained by the different substituents found in the aromatic ring. The electron-donation ability of –OH in ABDN-1 is somehow reduced by resonance, while the electron-donation ability of the –CH3 of ABDN-2 is facilitated by a positive inductive effect. Additionally, the strong electron-donating group, i.e., –OCH3 group, in ABDN-3 results in the better adsorption of ABDN-3. Accordingly, ABDN-3 acts as a better corrosion inhibitor than ABDN-2, followed by ABDN-1. The quantum chemical calculation investigation supported the WL, electrochemical, and surface analysis. This research group further utilized these inhibitors to analyze their corrosion inhibition effectivity on aluminum in the presence of 0.5 M NaOH solution.314
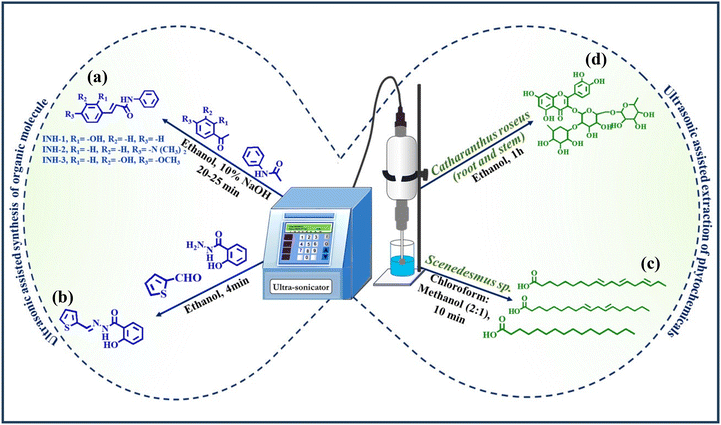 | ||
| Fig. 13 Schematic presentation of the green synthetic pathways for corrosion inhibitors via the ultrasound-assisted protocol for (a) INH-1, INH-2, and INH-3315 [reproduced from ref. 315 with permission; Copyright, Elsevier, 2014]; (b) HTMBH316 [reproduced from ref. 316 with permission; Copyright, ACS, 2018]; (c) Scenedesmus sp.extract317 [reproduced from ref. 317 with permission; Copyright, ACS, 2018]; and (d) Catharanthus roseus extract318 [reproduced from ref. 318 with permission; Copyright, RSC, 2020]. | ||
Recently, not only in chemical synthesis protocols but also several sonochemical-assisted extractions of phytochemicals have been reported in the literature. For example, Khanra et al. reported the utilization of Scenedesmus sp., a microalga, to extract unsaturated fatty acids for the protection purpose of mild steel in the presence of 1 M HCl solution.317 The cell pellets were centrifuged, dried, and then the fatty acids extracted using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of methanol and chloroform. After being ultrasonically treated for 10 min, the mixture was shaken for a whole night at 130 rpm in an orbital shaker (Fig. 13(c)). In the next step, the supernatant liquid was collected via further centrifugation. After phase separation, the lower organic phase was recovered and dried in a rotary evaporator. The Scenedesmus sp. fatty acid (SFA)-containing organic phase was used as an inhibitor. It was found that 9,12,15-octadecatrienoic acid (C18:3), 9,12-octadecadienoic acid (C18:2), and hexadecanoic acid (C16:0) constituted the majority of fatty acids, as analyzed by gas chromatography-mass spectrometry (GC-MS). The EIS study revealed that 95.1% corrosion inhibition efficiency was achieved for 36 ppm of inhibitor solution. The hydrogen gas evolution reaction (HER) also supported the decreased HER rate in the presence of SFA, i.e., there was less corrosion reaction in the presence of SFA. The ability of SFA to suppress the rate of corrosion followed the order of C18:3 > C18:2 > C16:0. The SEM, AFM and theoretical studies such as DFT nicely agreed with the electrochemical observations. Similarly, in 2020, ethanolic extracts of the roots and stems of Catharanthus roseus were obtained through ultrasonic energy, as schematically presented in Fig. 13(d).318 This extract was further used for the corrosion inhibition study of mild steel in a sea-water-like saline environment (i.e., 3.5 wt% NaCl). UV-vis spectroscopy (UV-vis), FTIR, Raman spectroscopy, gravimetric analysis, and electrochemical investigations (OCP, EIS and Tafel) were performed to investigate the corrosion inhibition efficiency. Around 70% corrosion inhibition efficacy was achieved from these phytochemicals. According to the outcomes of the quantum chemical analysis, the C. roseus extract was significantly adsorbed on the surface of mild steel.
2 mixture of methanol and chloroform. After being ultrasonically treated for 10 min, the mixture was shaken for a whole night at 130 rpm in an orbital shaker (Fig. 13(c)). In the next step, the supernatant liquid was collected via further centrifugation. After phase separation, the lower organic phase was recovered and dried in a rotary evaporator. The Scenedesmus sp. fatty acid (SFA)-containing organic phase was used as an inhibitor. It was found that 9,12,15-octadecatrienoic acid (C18:3), 9,12-octadecadienoic acid (C18:2), and hexadecanoic acid (C16:0) constituted the majority of fatty acids, as analyzed by gas chromatography-mass spectrometry (GC-MS). The EIS study revealed that 95.1% corrosion inhibition efficiency was achieved for 36 ppm of inhibitor solution. The hydrogen gas evolution reaction (HER) also supported the decreased HER rate in the presence of SFA, i.e., there was less corrosion reaction in the presence of SFA. The ability of SFA to suppress the rate of corrosion followed the order of C18:3 > C18:2 > C16:0. The SEM, AFM and theoretical studies such as DFT nicely agreed with the electrochemical observations. Similarly, in 2020, ethanolic extracts of the roots and stems of Catharanthus roseus were obtained through ultrasonic energy, as schematically presented in Fig. 13(d).318 This extract was further used for the corrosion inhibition study of mild steel in a sea-water-like saline environment (i.e., 3.5 wt% NaCl). UV-vis spectroscopy (UV-vis), FTIR, Raman spectroscopy, gravimetric analysis, and electrochemical investigations (OCP, EIS and Tafel) were performed to investigate the corrosion inhibition efficiency. Around 70% corrosion inhibition efficacy was achieved from these phytochemicals. According to the outcomes of the quantum chemical analysis, the C. roseus extract was significantly adsorbed on the surface of mild steel.
Similarly, Luo and coworkers demonstrated a highly effective enzyme-based ultrasound-assisted tannin extraction technique from acorns at a fixed pH of ∼5.0.319 The response surface methodology was implemented to further optimize the extraction conditions, including the four factors of temperature, ultrasonic power, ultrasonic time, and cellulose concentration. Firstly, the optimum ranges of the four significant factors were obtained through fundamental single-factor experiments. The experimental results were adequately explained using the second-order polynomial model. Consequently, the most suitable parameters used for extraction were as follows: 2.51 h, ultrasonic power of 97.92 W, temperature of 38 °C, and 3.44 g L−1 of cellulose.
2.4. Green corrosion inhibition using mechanochemical and one-step syntheses
The mechanical grinding of solids involves numerous processes, including:
(a) Breaking of particles to diminutive sizes.
(b) Creating larger, newly exposed surfaces.
(c) Forming dislocations and point defects in the crystalline structure.
(d) Inducing phase transformations in polymorphic materials.
(e) Chemical reactions including ionic exchange, decomposition, oxidation–reduction, complex and adduct formation.
The International Union of Pure and Applied Chemistry (IUPAC) defines a mechanochemical reaction as “a chemical reaction induced by the direct absorption of mechanical energy”.330 However, this area can be further divided into (i) mechanical activation of solids, (ii) mechanical alloying, and (iii) reactive milling of solids.331–333 The grinding of two solid substances leads to intricate series of transformations. The mechanical energy disrupts the order of the crystalline structure, creating cracks and exposing new surfaces. Deformation and potential melting occur at the edge collision points, forming hot spots where molecules experience high vibrational excitation, leading to bond breakage. Ultimately, the energy stored in the defects of the crystalline structure can facilitate slower chemical processes.Nowadays, milling under controlled temperature, light irradiation, sound agitation, or electrical impulses in newly developed experimental setups has led to reactions not achievable via conventional mechanochemical processing.326 Consequently, thermo-mechanochemistry, sono-mechanochemistry, electro-mechanochemistry and photo-mechanochemistry represent notable advances in modern mechanochemistry and herald a new level of solid-state reactivity of mechanochemistry 2.0 (Fig. 14).
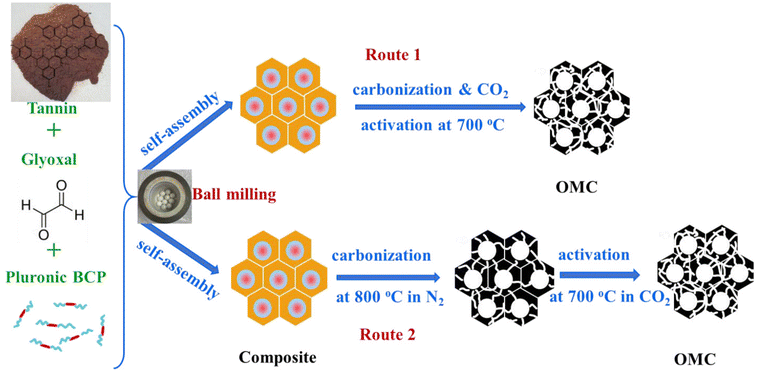 | ||
| Fig. 14 Diagrammatic representation of the two processes used in the mechanochemical synthesis of OMCs: carbonization under N2 and subsequent CO2 activation (route 2); one-step carbonization and CO2 activation (route 1) utilizing tannin, glyoxal, and block copolymer327 [reproduced from ref. 327; open access article, copyright permission is not required]. | ||
Mechanical milling using a mixer mill or planetary mill has been fruitfully utilized in organic synthesis under solvent-free conditions. In the literature, mechanical milling is sometimes called grinding, which may cause confusion. Thus, to differentiate these two mechanochemical techniques, it is strongly recommended that grinding is defined as a process in a mortar and pestle and the like, such as a Retsch RM100 mortar grinder. In contrast, milling should only refer to that performed in a mixer/shaker mill or a planetary mill.334,335 A mechanochemical process may involve carbon–carbon and carbon–heteroatom covalent bonds, metal–ligand coordination bonds, non-covalent interactions such as hydrogen bonds, halogen bonds or π–π arene stacking interactions. Alternatively, ball milling is a mechanical method broadly used to granulate minerals into very fine particles and prepare or alter inorganic solids. However, its use in organic synthesis is relatively uncommon.336,337
Milling can be conducted in various ways. The simplest method involves using a laboratory mortar and pestle. This manual milling process can induce many mechanochemical reactions that do not require overcoming a high energy barrier. Alternatively, ball mills are employed when higher energy levels are needed and when milling times extend to hours or even days. Laboratory vibrators, such as those of the Wiggle-Bug type, are highly efficient for milling small samples. For prolonged high-energy milling, such as in mechanical alloying or the amorphization of hard crystalline solids, very high energy vibrators like high-speed attritors or high-impact stainless steel ball mills (such as Spex type) are used.328,329 Ultrasonication can also be employed as a method of mechanochemical processing.338,339Fig. 15 shows a brief timeline of the development of mechanochemistry and direct mechanocatalysis. The solid-state synthesis can easily be monitored by measurement of the IR or UV spectrum as a Nujol mull. Solid-state NMR can be beneficial, and Mossbauer spectroscopy is most valuable with Fe and Sn compounds. Other techniques, such as high-resolution electron microscopy (HREM), electron cyclotron resonance (ECR) and extended X-ray absorption fine structure (EXAFS) spectroscopy, have been utilized to analyze new surfaces.328,340 Mass spectrometry (MS) can also be used to determine gaseous products, such as in the decomposition of bromates and nitrates.341
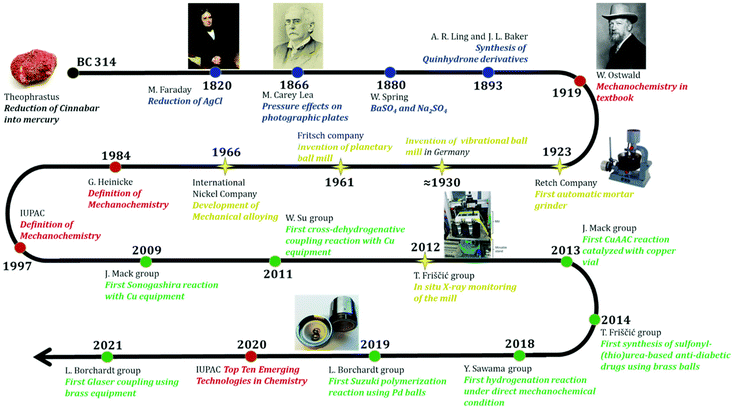 | ||
| Fig. 15 Timeline of mechanochemistry and “direct mechanocatalysis”342 [reproduced from ref. 342, RSC publication, copyright permission is not required]. | ||
Several corrosion inhibitors have been synthesized using solid-state or solvent-less synthesis procedures, as shown in Scheme 1. Some of the schemes are given below for the preparation of condensation products,343,344 pyrimidine derivatives,345 phosphonates,346 pyrazoles,324 indole derivatives,347,348etc. Considering the requirement for diverse industrial practices, the production of corrosion inhibitors is required (i) to be cost-effective, (ii) on a large scale, (iii) consume less time, and (iv) to be environmentally friendly. It can be shown in the given examples that the production of inhibitors, employing a solvent-free mechanochemical approach could result in their synthesis via simple stirring343,344 or grinding324 within the span of a few minutes and even at room temperature. In some cases, the synthesis also involves the use of greener catalysts such as nano-metal oxides348 and amino acids.347 Further, to enhance solvent-free synthesis, modern methods such as microwave irradiation-345 and ultrasonic irradiation-induced346 synthesis can be used, as presented in some examples. According to the above discussion, and the shared schemes, it is obvious that corrosion inhibitors can be produced using the mechanochemical approach in a simple synthetic procedure within significantly less time. This approach minimizes the production of waste and avoids the cumbersome isolation and purification steps required for conventional organic synthesis. This is incredibly convenient for the large-scale production of organic molecules to be used in various industries as corrosion inhibitors. Therefore, this methodology can prevent unnecessary waste, realize atom economy, lower-hazard chemical reactions due to the absence of toxic chemicals and reagents, and minimize the energy consumption. Considering the development of corrosion inhibitors using solid-phase preparation, this approach can be, in general, a cheaper, simple, cost-effective, and less time-consuming process, and therefore practically applicable for industrial purposes.
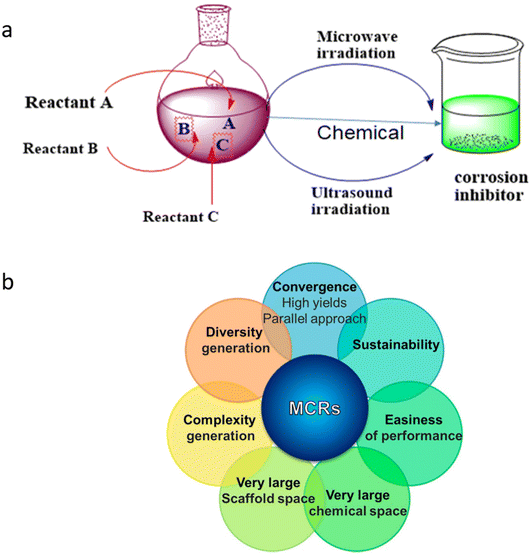 | ||
| Fig. 16 (a) Schematic of MCRs facilitated using microwave and ultrasound irradiation and (b) basic characteristics of MCRs356 [reproduced from ref. 356; open access article, copyright permission is not required]. | ||
The Strecker synthesis of α-amino cyanides developed in 1850 is considered the first documented MCR. Currently, metal-catalyzed MCRs, isocyanide-based MCRs, organoboron-based MCRs, and free radical-facilitated MCRs are the most reported one-step MCRs for developing novel organic molecules, including corrosion inhibitors. The synthesis of organic compounds having application in various industrial practices using one-step MCRs agrees with the principles of “green chemistry”. Thus, MCRs are frequently utilized for various chemical transformations, including Biginelli reaction, alkyne trimerization, Gewald reaction, Bucherer–Bergs reaction, Hantzsch pyridine synthesis, Kabachnik–Fields reaction, Grieco three-component coupling, Passerini reaction, Mannich reaction, Petasis reaction, Strecker amino acid synthesis, Ugi reaction, Asinger reaction, A3 coupling reaction and Pauson–Khand reaction.122,303,357–359 An increasing number of applications of MCRs has been reported in medicinal chemistry and drug discovery programs, combinatorial chemistry, natural product synthesis, agrochemistry, and polymer chemistry.303 A brief timeline of the development of MCRs is shown in Fig. 17.
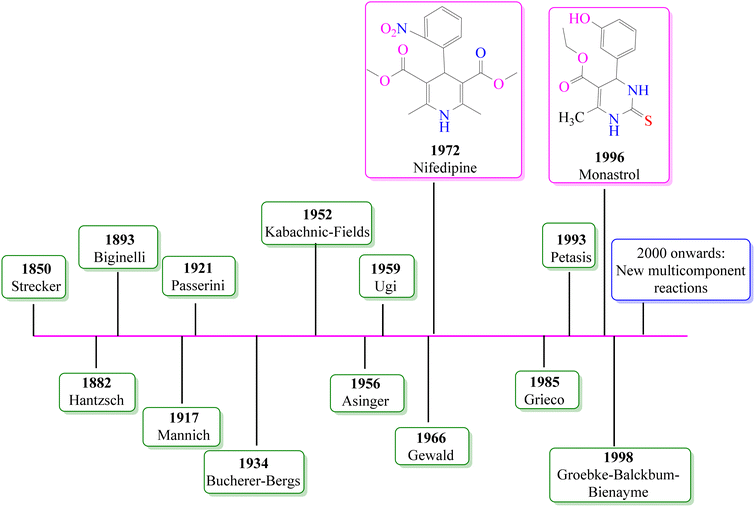 | ||
| Fig. 17 Timeline of multicomponent reactions (1850–2000) [self-illustration, copyright permission is not required]. | ||
In a green MCR, most of the atoms in the reagents should be converted to the product. Water is usually the major by-product of such green MCR reactions.349 Noticeably, compared to the conventional tedious synthesis of corrosion inhibitors, MCRs show synthetic advantages regarding simplicity, efficiency, selectivity, convergence, and atom economy. Ideally, all or most of the steps during multicomponent reactions (MCRs) would reach reversible equilibrium, with the last step being irreversible, leading to the final products. In this case, the products can precipitate from the reaction mixture, which enables purification by filtration and washing without chromatography or recrystallization. A survey of the literature showed that most of the research on corrosion inhibitors has been undertaken on the effect of electron-donating/withdrawing substituents and their influence on the corrosion inhibition efficiency.11,18,19 Hence, a thorough review of the literature is required to consider the crucial structural features of organic corrosion inhibitors such as (i) the carbon chain length, (ii) the nature of heteroatoms, (iii) the effect of stereochemistry, (iv) the effect of the ring size, and (v) influence of substituents on the corrosion inhibition performance. In this context, the MCR protocol has led to the development of a series of libraries consisting of heterocycles established for quantitative structure activity–relationship (QSAR) studies on corrosion inhibition. Thus, multicomponent reactions represent valuable tools in the repertoire of sustainable synthetic methods for developing environmentally benign corrosion inhibitors and their synergistic utilization with other green technologies, which can bring organic chemists closer to the ideal synthesis.
As discussed above, the requirement for efficient corrosion inhibitors based on organic molecules also attempts to address the ease of synthesis and cost-effectiveness of the synthesized products and overall environmentally safer procedures. MCRs have been recognized as one of the greenest alternatives for developing green chemicals with numerous industrial and biological applications. In this context, MCRs have several advantages such as ease of performance, lower number of steps, high selectively, facile automation, unique product, high atom economy, simple purification of the product, high synthetic efficiency, and low waste production because of the reduction in the number of work-up steps, thus saving time and resources.
Several corrosion inhibitors have been synthesized following the MCR approach, as shown in Scheme 2.360–365 Although corrosion inhibitors are derived using one-step MCRs, which deliver several synthetic benefits, MCRs suffer from several challenges. One of the biggest challenges of MCRs is the possibility of side reactions during the reaction progress, reducing the overall yield and efficiency of the synthesis. The side reactions not only decrease the result of the reaction but also cause the release of enormous amounts of environmentally harmful solvents and chemicals that adversely affect the surrounding environments. Also, the possibilities of bimolecular side reactions become greater under harsh reaction conditions. Therefore, it is recommended that the synthesis of corrosion inhibitors using MCRs should be carried out under comparatively milder conditions. Further, most of the corrosion inhibitors were synthesized using MCRs; therefore, exploring the synthesis of the corrosion inhibitors using three- and four-component reactions is also recommended. Generally, the corrosion inhibitors derived from MCRs are associated with high inhibition efficiency, which is attributed to several polar functional groups. The polar functional groups act as adsorption centers for metal–inhibitor interactions. The polar functional groups not only facilitate the adsorption of the corrosion inhibitors but also enhance their solubility in polar electrolytic media, such as H2O and HCl. The combination of MCRs with non-traditional irradiation further improves their synthetic efficiency. Therefore, it is recommended that MCRs be carried out in association with microwave and ultrasound irradiation. Insertion of polar functional groups should be enhanced as much as possible. The inhibition characteristics of the corrosion inhibitors derived from MCRs have been investigated mainly for ferrous alloys. Therefore, their use should be explored for non-ferrous metallic alloys. According to the ongoing discussion, it is also clear that corrosion inhibitors derived from MCRs are regarded as environmentally benign because of their association with several synthetic efficiencies, including higher reaction yield and lower reaction time. Thus, MCRs, in combination with non-conventional microwave and ultrasound irradiation, offer one of the greenest and most efficient protocols for developing and designing environmentally benign corrosion inhibitors.
2.5. Green corrosion inhibition using benign solvents, chemicals and catalysts
 | ||
| Scheme 3 Scheme for the synthesis of corrosion inhibitors based on (a) pyranopyrazoles,376 (b) pyrimidoquinolines,377 (c) pyrimidine derivatives,378 (d) tetrazoles,379 and (e) quinoline derivatives380 in an aqueous medium [the synthetic schemes were reproduced and suitable copyright permissions have been obtained]. | ||
Another aspect of analytical reagents for testing corrosion inhibitors is the application of acids and bases for digestion385 and as various auxiliary chemicals. Due to their different chemical properties, acids and bases require the application of slightly different parameters during their greenness assessment.386 The assessment systems are based on environmental, safety and health issues and problems with hazardous by-products or disposal problems. Fig. 18 lists some of the commonly used green solvents, including water.
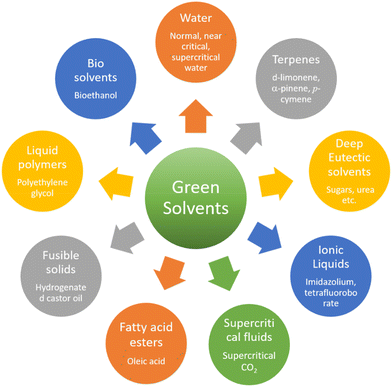 | ||
| Fig. 18 Different types of green solvents [self-illustration, copyright permission is not required]. | ||
In addition to water, several organic solvents have been recognized as green solvents for organic synthesis, including “fluorous” solvents such as perfluorinated alkanes, dialkyl ethers, and trialkyl amines.63 These perfluorinated liquids have useful and attractive properties for organic synthesis, such as chemical inertness, high thermal stability, nonflammability, extreme nonpolar character and small intermolecular attraction.387 ILs388–390 serve as solvents for reaction media for many separation or catalytic processes because there is a wide range of organic, inorganic and polymeric molecules that is well soluble in ionic liquids.391,392 The solvating properties of ionic liquids depend on their smaller anions and larger organic cations. Organic carbonates represent esters of carbonic acids and are a class of compounds with a broad field of application due to their unusual properties. They are readily available in large amounts, inexpensive, low (eco)toxic, and entirely biodegradable. Organic carbonates are widely used for extraction, pharmaceutical and medical applications, and batteries. At room temperature, carbon dioxide exists as a liquid with excellent wetting properties and very low viscosity. Above its critical temperature and pressure (31 °C and 73.8 bar, respectively) CO2 exists in the supercritical state,388,393 featuring gas-like viscosities and liquid-like densities. Also, carbon dioxide is renewable, nontoxic, nonflammable, readily evaporates and chemically inert towards many substances, and thus features outstanding characteristics for utilization in green chemistry. Biosolvents have been developed as an alternative to volatile organic compounds (VOC), which are usually harmful to the environment and human health. The most important chemical classes of biosolvents are esters of naturally occurring acids and fatty acids, bioethanol, terpenic compounds, isosorbide, glycerol, and glycerol derivatives.394,395 These compounds offer the advantage of being produced from renewable sources such as vegetable, animal or mineral raw materials by chemical and physical processes without consuming fossil resources. The objective of a green solvent is to minimize the environmental impact of the consumption of solvents in chemical production. Thus, the effective utilization of green solvents such as water, supercritical fluids, liquid polymers, and ILs can help reduce environmental pollution. The properties of novel green solvent or biosolvents include low toxicity, phase behavior, favourable chemical kinetics and thermodynamics, biodegradability, and non-flammability. Schemes for synthesizing corrosion inhibitors in greener solvents are shown in Scheme 4. The commonly used green solvents for preparing organic corrosion inhibitors include glycerol, methanol, and ethanol.396–400 This simplifies the synthesis protocol and reduces the overall cost of synthesis.
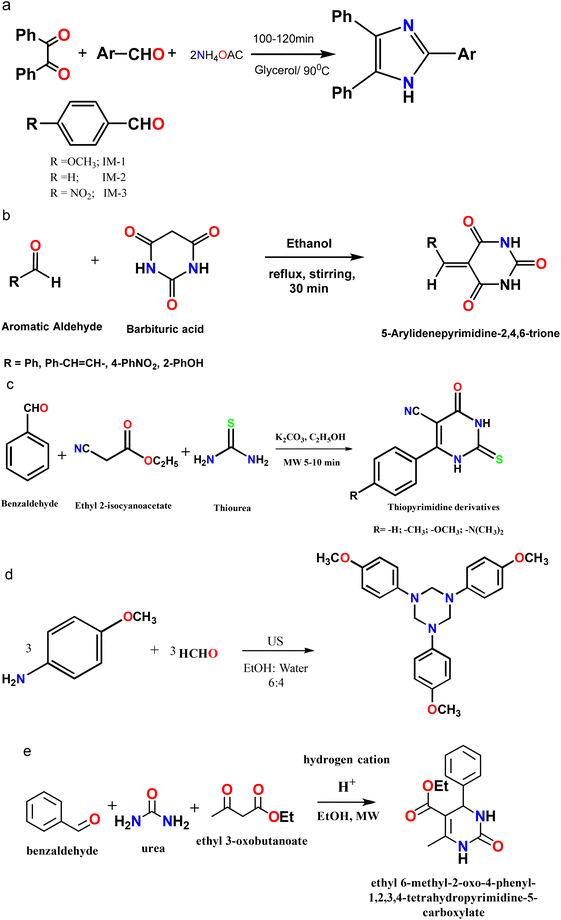 | ||
| Scheme 4 Scheme for the synthesis of corrosion inhibitors based on (a) imidazole derivatives396 [reproduced from ref. 396, open access publication, copyright permission is not required], (b) barbiturates397 [reproduced from ref. 397, open access publication, copyright permission is not required], (c) thiopyrimidines398 [reproduced from ref. 398 with permission, Copyright, Elsevier, 2016], (d) triazine derivatives399 [reproduced from ref. 399, copyright permission is not required], and (e) dihydropyrimidinones400 [reproduced from ref. 400 with permission, Copyright, Elsevier, 2020] in environmentally benign solvents. | ||
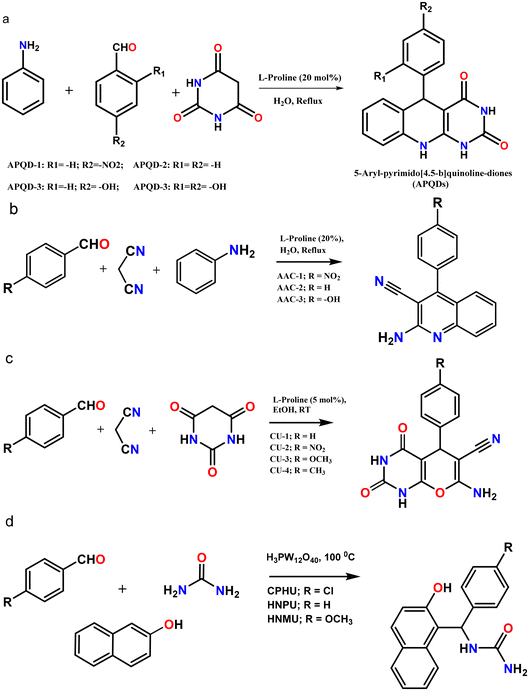 | ||
| Scheme 5 Schemes for the synthesis of corrosion inhibitors based on (a) pyrimidoquinolines,403,404 (b) quinoline derivatives,405 (c) condensed uracils,406 and (d) urea derivatives407 using environmentally safer catalysts [the synthetic schemes were reproduced and suitable copyright permissions have been obtained]. | ||
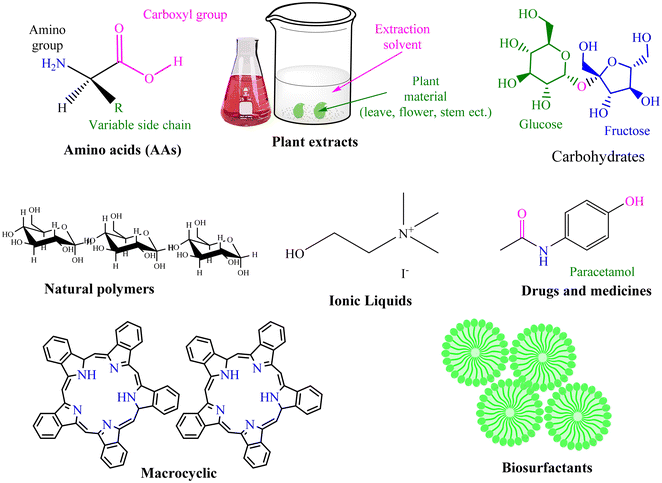 | ||
| Fig. 19 Some of the greener chemicals for the development of corrosion inhibitors [self-illustration, copyright permission is not required]. | ||
(i) Toxicity: The LD50 value should be >500 mg kg−1 weight of a rat.
(ii) Biodegradation: The biodegradability should be 60% in 28 days.
(iii) Bioaccumulation: The capability of a chemical to accumulate in living organisms can be computed employing the partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w), where log
Po/w), where log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w limit < 3.0.
Po/w limit < 3.0.
A wide range of heterocyclic compounds has been reported as corrosion inhibitors, including pyridines,416 pyrimidines,417 pyrazole,418 imidazoles,419 triazoles,420 tetrazoles,421 indoles,347 thiazoles,422,423 oxadiazoles,424 benzotriazoles,425,426 benzimidazoles,427,428 and macrocyclic compounds,429,430 and biomolecules. Previously, conventional research in corrosion inhibition was limited to small molecules, such as benzotriazole and other simple compounds, with little or no attention to biological and macromolecules. However, recently, scientists and researchers have focused on exploring novel inhibition chemistries that can be effective, easier to obtain/synthesize, and environmentally suitable. Their application in the synthesis agrees with several provisos of green chemistry principles of environmentally benign and low toxicity reagents, renewable raw materials, and minimising waste production.56,408 The major categories of green corrosion inhibitors include (i) the extracts from plants and tree parts409 such as roots, stems, leaves, bark, shells, seeds, flowers, and seeds. These parts contain several phytochemicals that are complex compositions of naturally occurring chemicals and are environmentally safe; (ii) carbohydrates286,410 such as glucose, chitin, chitosan, cellulose, starch, pectin, etc. These large molecular weight chemicals contain abundant surface functional groups that allow considerable prospects of reactivity; (iii) amino acids431 such as glycine, proline, histidine, and others are among the essential amino acid member categories. These provide reactivity, solubility, and prospects for chemical functionalization. In addition, polyamino acids such as polyaspartic acid and proteins such as soy, gluten, casein, gelatin, etc. have also found diverse applications. It is understood that the abovementioned categories are of natural origin and have considerable biocompatibility. Other classes include (iv) heterocyclic biomolecules411 such as vitamins, hormones etc.; (v) pharmaceutical products, viz. drugs, and (vi) ILs (Table 5).411 These types of chemicals are abundant, are environmentally safe, and afford cheap costs. Notably, some of the green chemicals presented in Table 5 can find applications as environmentally safer corrosion inhibitors. In addition, some methods are proposed to develop novel derivatives of such greener chemicals to obtain greener corrosion inhibitors. These biomolecules have been reported for their environmentally benign nature and considerable corrosion inhibition efficiency in aggressive aqueous media. Further, to achieve an improvement in corrosion inhibition performance, different chemical functionalization methods have been explored.
Polymers have found wide applications in corrosion inhibition due to their high molecular surface area, which can afford superior adsorption. Additionally, the presence of abundant available sites for adsorption allows excellent interaction with the metallic substrate. Naturally derived polymers such as chitosan, carboxymethyl cellulose, dextran, maltodextrin, and starch have been reported to exhibit significant inhibition behaviour.432–434 These polymers can be extracted from natural sources or synthesized from living organisms.432 The diverse applications of these biological polymers originate from their intrinsic biodegradability combined with excellent properties such as non-toxic nature, biocompatibility, renewability, barrier action, low cost, and demonstrated potential application in corrosion inhibition. Several natural polymers, such as chitosan,410,435–437 cellulose,438,439 dextrins,440,441 and starch,442,443 have been utilized as corrosion inhibitors. Exudate gums, such as xanthan gum and Gum Arabic, have also been reported. Further, considering their interesting inhibition and protection performance, researchers have also attempted the chemical modification of several natural polymers and reported their performance as corrosion inhibitors. Recently, several polymeric nanoparticles443 and nano-biocomposites444,445 derived from naturally occurring polymers have also been reported as environmentally friendly corrosion inhibitors. Schemes for the synthesis of organic molecule-based corrosion inhibitors using chitosan Schiff bases, amino acids, and ionic liquids are presented in Scheme 6. Other carbohydrate derivatives have also been reported as inhibitors.446,447
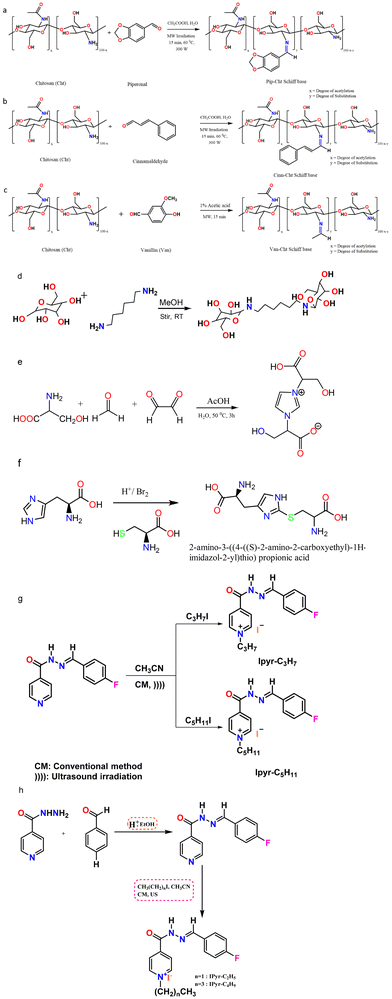 | ||
| Scheme 6 Schemes for the synthesis of corrosion inhibitors using environmentally safer reagents such as (a–c) chitosan,410,435–437 (d) glucose,446,447 (e and f) amino acids,448,449 and (g and h) ionic liquids450,451 [the synthetic schemes are reproduced from their respective publications with permission and copyright permissions have been obtained]. | ||
Amino acids are organic molecules composed of nitrogen, oxygen, and hydrogen, which are the building blocks of proteins.452 Amino acid molecules contain an –NH2 group, –COOH group, and a side chain (aliphatic/aromatic) specific to each amino acid molecule. These functional groups can adsorb on a desired metallic substrate, thereby promoting the corrosion protection by amino acids. Amino acids exist in aqueous solutions as zwitterions, i.e., containing separate positively (–NH3+) and negatively charged (–COOH−) groups, imparting high solubility. Moreover, amino acids are naturally occurring, biodegradable, cost-effective, and present as alternatives to commercially used toxic corrosion inhibitors.448,449 Several reports have emerged in the literature on the application of amino acids as corrosion inhibitors.18,431 The chemical modification of amino acids via functionalization strategies to develop amino acid derivative-based corrosion inhibitors has also been reported.
Natural extracts derived from plants present one of the leading categories of green corrosion inhibitors. Due to their natural origin, these extracts are considered green, and their application in corrosion inhibition agrees with environmental regulations. Extracts from almost all plant parts, e.g., leaves, fruits (including pulp, skin, shells, and seeds), stem, bark, flowers, and roots, are useful as corrosion inhibitors.128 The different sections of a plant are composed of various phytochemicals such as alkaloids, terpenoids, flavonoids, catechins, and co-enzymes, including carbohydrates, amino acids, vitamins, and proteins. Accordingly, plant extract-based corrosion inhibitors have been utilized in various corrosive media on different metal surfaces.128,409 Pharmaceutical products are organic compounds composed of heteroatoms (N, S, O, and P), functional groups, heterocyclic rings, phenyl rings, π-bonds, etc. in their molecular structure. Given that they are produced for human consumption, these products are green and environmentally friendly.
Besides, medicines can be easily solubilized by water and oils. Several research articles have been published on the use of drugs or chemical medicines as corrosion inhibitors.453,454 Almost all types of drugs, e.g., antimalarial, antibacterial, antifungal, antiviral, antihypertensive, and anticancer, have been utilized as corrosion inhibitors. Considering the cost of medicines, several expired pharmaceutical products have recently been reported as corrosion inhibitors.455–459 The use of oleochemicals in various applications aligns with the green chemistry principles and promotes the use of renewable, sustainable, and bio-based chemicals.460,461 Oleochemicals, which are fats and oils extracted from plant and animal sources, also represent this category. Due to their natural origin, oleochemicals are environmentally benign. Oleochemicals bear the structure of long-chain fatty acids, which aid in the effective adsorption on the target metal surfaces. Furthermore, the presence of other surface functionalities, in the form of polar functional groups, heteroatoms, heterocycles and/or phenyl rings π-bonds, facilitate their adsorption. Due to their ready availability, low toxicity, and biodegradable properties, oleochemicals present a major category of environmentally benign corrosion inhibitors.
Recently, the use of biosurfactants in corrosion protection has been gaining particular attention. It is important to note that generally, surfactants have found application together with conventional inhibitors, wherein these molecules aid in the dissolution of the inhibitors in corrosive media. However, a literature survey indicated that numerous cationic-, non-ionic-, Gemini- and ionic liquid-based surfactants have been widely reported as corrosion inhibitors.462,463 It also showed that ionic liquids are effective by adsorbing on metallic surfaces.464,465 Through their adsorption, ionic liquids block the active sites of metal surfaces responsible for corrosive damage. Similar to traditional organic corrosion inhibitors, the adsorption of ILs may follow the physisorption, chemisorption or physiochemisorption mechanism. Ionic liquids are also widely used as corrosion inhibitors for various metal/electrolyte systems.450,451 Macrocyclic compounds refer to large molecular structures similar to that commonly found in calixarenes, crown ethers, cyclodextrins, porphyrin rings, phthalocyanines, crown ethers, etc.466 Several antibiotics are based on macrocyclic compounds. These compounds can form highly stable chelates and complexes with transition metals and lanthanides. Their higher molecular weight affords them high metallic surface coverage. A greater number of heteroatom π-bonds, conjugation, additional phenyl rings, and heterocycles is favorable for surface adsorption on metallic substrates.353 Several studies by the authors are available on the effect of ring size and heteroatoms of macrocyclic compounds on their inhibition performance.429,466–470
2.6. Green corrosion inhibition using bio-based materials
The control of metallic corrosion using biobased compounds has gained significant momentum in response to the global campaign against the use and discharge of toxic chemicals into the environment. Various biobased compounds have captured keen interest from corrosion experts in recent years. This section provides a concise overview of the pertinent literature on biobased compounds, such as pharmaceutical drugs and essential oils, subjected to empirical testing for their anticorrosive activities. According to the literature, pharmaceutical drugs, which are known for their diverse clinical applications, including antihypertensive, anticonvulsant, antiviral, and antidepressant properties, represent biobased chemicals with substantial anticorrosion potential.471 These pharmaceutical drugs exhibit structural attributes similar to conventional organic inhibitors, thus prompting researchers to scrutinize their performance as corrosion inhibitors. Their key features such as conjugated multiple bonds, polar functional groups, planar structures, and heteroatoms contribute significantly to their inhibitory capabilities against corrosion. These attributes enable drug molecules to adsorb onto metal surfaces, forming a protective barrier against corrosive agents. Furthermore, pharmaceutical drugs possess the advantages of water-solubility, non-toxicity, ecofriendliness, cost-effectiveness and biodegradability. In this case, expired drugs are preferable as metallic corrosion inhibitors due to their environmental and economic benefits compared to fresh drugs.472,473The effectiveness of fresh and expired pharmaceutical drugs in inhibiting corrosion reactions has been extensively documented in the literature.471–475 In two distinct reports, investigations were carried out on the inhibition performance of azithromycin for stainless steel 316L in KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 and mild steel in 2 M HCl.477 These investigations employed electrochemical and surface analytical methods and revealed the ability of azithromycin to significantly reduce the corrosion rate even at minimal doses, functioning as a mixed inhibitor. The proposed inhibition mechanism for both cases involved physical adsorption, with the Freundlich isotherm governing the inhibition process in SS 316L/KOH and the Langmuir isotherm in MS/2 M HCl. Table 2 provides an overview of the significant research publications in 2023 pertaining to the utilization of pharmaceutical drugs as corrosion inhibitors.
476 and mild steel in 2 M HCl.477 These investigations employed electrochemical and surface analytical methods and revealed the ability of azithromycin to significantly reduce the corrosion rate even at minimal doses, functioning as a mixed inhibitor. The proposed inhibition mechanism for both cases involved physical adsorption, with the Freundlich isotherm governing the inhibition process in SS 316L/KOH and the Langmuir isotherm in MS/2 M HCl. Table 2 provides an overview of the significant research publications in 2023 pertaining to the utilization of pharmaceutical drugs as corrosion inhibitors.
| S. no. | Drug | Metal | Medium | Conc. | Max %IE (%) | Inhibitor category | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Mebendazole | 5Cr pipeline steel | 1 M HCl | 5 mM | 94.40 | Mixed/LAI | 478 |
| 2 | Ribavirin | Q235 steel | 1 M HCl | 10 mM | 97.70 | Mixed/LAI | 479 |
| 3 | Ibuprofen | Copper | 0.5 M H2SO4 | 5 × 10−3 M | 95.25 | LAI | 480 |
| 4 | Ampicillin | Mild steel | 5% HCl | 20 mM | 96.70 | Mixed/LAI | 481 |
| 5 | Aspirin | Aluminium | 0.5 M H2SO4 | 300 ppm | 96.98 | LAI | 482 |
| 6 | Aspirin | Carbon steel XC48 | 1 M HCl | 5 × 10−3 M | 96.50 | LAI | 483 |
| 7 | Bifonazole | Carbon steel | 1 M HCl | 375 mg L−1 | 92.08 | Mixed/LAI | 484 |
| 8 | Terconazole | Carbon steel | 1 M HCl | 375 mg L−1 | 94.19 | Mixed/LAI | 484 |
| 9 | Thiamazole | Copper | 3% NaCl | 10−4 M | 97.00 | Mixed/LAI | 485 |
| 10 | Gabapentin | Mild steel | 1 M HCl | 400 ppm | 90.30 | Mixed/LAI | 486 |
| 11 | Gabapentin | Zinc | 0.1 M HCl | 400 mg L−1 | 84.90 | Mixed/LAI | 487 |
| 12 | Vildagliptin | Mild steel | 1 M HCl | 1 mM | 97.50 | Mixed | 488 |
| 13 | Ethambutol hydrochloride | Mild steel | 0.5 M H2SO4 | 1000 ppm | 92.78 | Mixed/LAI | 489 |
| 14 | Vilazodone | Aluminium | 1 M HCl | 150 ppm | 95.00 | Mixed/LAI | 490 |
| 15 | Omeprazole | Al–Mg–Si alloy | 0.5 M H2SO4 | 1.5 g L−1 | 90.50 | Mixed/LAI | 491 |
| 16 | Clonazepam | Mild steel | 3.5% NaCl | 500 ppm | 91.40 | Mixed/LAI | 492 |
| 17 | Linagliptin | Mild steel | 1 M HCl | 7.5 × 10−3 M | 96.30 | Mixed/LAI | 493 |
| 18 | Chlorpheniramine | Mild steel | 2 M HCl | 800 mg L−1 | 95.10 | Mixed/LAI | 494 |
| 19 | Furosemide | Carbon steel | 1 M HCl | 300 ppm | 90.50 | Mixed/LAI | 495 |
| 20 | Tizanidine | E24 carbon steel | 10% HCl | 7 × 10−3 M | 97.10 | Mixed/LAI | 496 |
| 21 | Pipotiazine | Mild steel | 1 M HCl | 1000 ppm | 73.59 | Mixed/LAI | 497 |
| 22 | Famciclovir | Carbon steel | 1 M HCl | 1 mM | 96.87 | Mixed/LAI | 498 |
| 23 | Ebastine | Carbon steel | 1 M HCl | 17.03 × 10−5 M | 95.81 | Mixed/LAI | 499 |
| 24 | Fucoidan | 304 stainless steel | 3.5% NaCl | 200 ppm | 81.70 | Mixed/LAI | 500 |
| 25 | Favipiravir | Aluminium alloy | 1 M HCl | 100 ppm | 96.45 | Mixed/LAI | 501 |
Recent reports highlight the growing interest in hybrid systems, combining at least one type of drug with synergistic agents such as other drugs, halide ions, nanoparticles and natural gums to enhance the corrosion resistance of metallic materials. For instance, incorporating cyclodextrin into piroxicam resulted in outstanding resistance in a steel/1 M HCl system, achieving an impressive 98.65% inhibition at a concentration as low as 0.4 mM. This synergistic inhibitor exhibited a significantly superior performance to several individual drugs tested as corrosion inhibitors.502 Nevertheless, it remains crucial to determine the optimal dosage ratio of the inhibitor components to maximize the protection performance of these hybrid systems. Recent research efforts have focused on uncovering the remarkable inhibitory effects of drug-based hybrid inhibitors in investigating the corrosion behaviour of diverse metal/electrolyte systems.503–508
In the realm of employing computational tools to elucidate the inhibition mechanisms of drug molecules, Mrani et al.509 conducted comprehensive computational studies on seven sulfa drugs as inhibitors of steel corrosion. These investigations combined in silico toxicity assessments with DFT and MC simulation. The findings demonstrated that the tested drug compounds can be classified as non-toxic and highly effective steel corrosion inhibitors. A QSAR model was developed in a separate study to predict the inhibition performance of 250 commercial drugs employing an autoregressive with exogenous input (ARX) approach. This model utilized hard–soft acid–base (HSAB) descriptors derived from third-order DFTB. Its reliability was assessed through a five-fold cross validation process, and subsequently subjected to external validation, yielding exceptionally favorable results. To substantiate the credibility of the developed model, the authors further validated it by assessing newly synthesized lidocaine using electrochemical methods, achieving an impressive peak %IE of 92.5% (EIS) and 87.51% (PDP) at a concentration of 100 ppm. Remarkably, these experimental results are closely aligned with the predicted %IE of 87.51% obtained from the ARX model.510 Similarly, a recent report by Abeng and Anadebe harnessed the capability of artificial neural network (ANN) and adaptive neuro-fuzzy inference system (ANFIS) models to evaluate the protective performance of the doxorubicin drug. These modelling assessments were performed alongside well-established experimental and theoretical techniques. Both models showed remarkable agreement between the experimental and predicted inhibition performances of doxorubicin in a 0.5 M H2SO4 solution. Conclusively, the ANFIS model exhibited superior accuracy to the ANN model.511
Essential oils are natural, biobased and volatile liquids extracted from plants, which are well-known for their versatile applications across various domains, including cosmetics and medicine. These oils are distinguished by their purity and natural origin, natural abundance, high volatility with pronounced fragrances, liquid state at room temperature and lower density than water. In terms of their anticorrosive properties, essential oils stand out due to their natural abundance, non-toxicity, zero/minimal environmental impact, affordability, excellent biodegradability and outstanding adsorption capabilities.512 Most essential oils contain a rich assortment of chemical constituents, including monoterpene phenols, ketones, alcohols, hydrocarbons, and esters, many of which contribute to their anticorrosive abilities.513 These extracts are often derived from various parts of plants, with leaves being a common source.514,515 However, essential oils can also be obtained from seeds/nuts, flowers and fruits.516 The primary method employed in extracting essential oils, as frequently reported in the literature, is hydrodistillation, although other techniques, such as hydro-extractive steam distillation are also used.517 The choice of extraction method typically depends on the nature of the plant materials and the physicochemical characteristics of the essential oil being extracted.516 To identify the chemical constituents of essential oils, researchers often employ analytical tools such as FTIR, gas chromatography with flame ionization detection (GC-FID), GC and GC-MS.518
In assessing the protective performances of essential oils, gravimetric and electrochemical methods such as WL, OCP, LPR, PDP and EIS are commonly utilized.519 Using these techniques, the corrosion behaviour of a metallic specimen in the presence and absence of the essential oils is evaluated. For instance, using PDP and EIS methods, the inhibition performance of essential oils derived from the needles of black pine (Pinus nigra) was found to be 97% and 96%, respectively, at a concentration of 200 ppm.520 Furthermore, modern analytical instruments such as FTIR, UV-vis, SEM-EDX, AFM, XPS, XRD, contact angle tests, and Raman spectroscopy are increasingly employed to investigate the structural and morphological changes on metal surfaces in the presence and absence of essential oils.517,521 For instance, the morphological assessment of the anticorrosion performance of essential oils derived from Elettaria cardamomum (ECPE).522 The study revealed that the addition of 250 ppm ECPE reduced the average surface roughness and the contact angle of MS from 316.73 (blank) to 297.8 nm (ECPE) and 124.9° (blank) to 74.0° (ECPE), respectively, indicating their efficacy.
As a complement to experimental assessments, quantum chemistry approaches such as DFT and DFTB are now being explored to gain insights into the anticorrosive mechanism of essential oils by elucidating the electronic properties of their primary constituents. Beniaich et al.523 utilized the DFT method to study the adsorption of Artemisia herba-alba essential oils on metal surfaces, providing spatial distributions in the aqueous phase (Fig. 20). Molecular simulations are also employed to investigate the interactions between the essential oil components and metal surfaces, yielding critical parameters such as binding energy, stable adsorption configuration and diffusion behaviour.524–526 Moreover, recent studies have introduced response surface modelling (RSM) using a quadratic model to optimize the interactive effects of concentration, exposure time and temperature on the maximum inhibition performance of essential oils. For instance, Ansari et al.527 employed RSM and achieved a peak %IE of 88.10% for Ocimum basilicum essential oil on C38 steel in sulfuric acid under the optimal conditions. Similarly, Loto et al. employed statistical analysis, demonstrating the outstanding protection performances of essential oils from ginger, grapefruit and tea trees against steel corrosion in sulfuric acid. This study reported peak %IE of 99.56%, 98.32% and 98.17% for ginger, grapefruit and tea tree essential oils, respectively.528
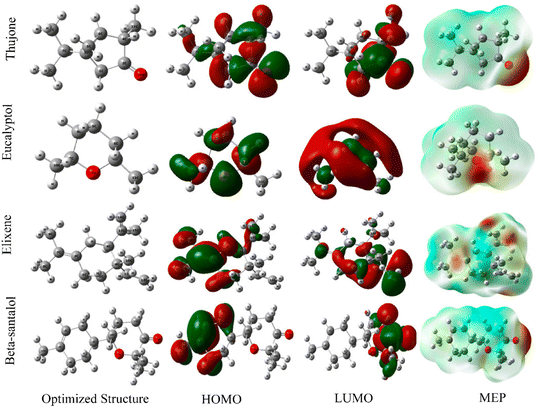 | ||
| Fig. 20 Spatial distributions of Artemisia herba-alba compounds in an aqueous medium523 [reproduced from ref. 523, open access publication, copyright permission not required]. | ||
Several studies have also explored the effect of combining various essential oils on the corrosion characteristics of metals.521,529,530 These synergistic actions have demonstrated enhanced resistance against the infiltration of corrosion ions into metal surfaces. The mechanism by which essential oils inhibit corrosion entails an electrocatalytic effect, active site blocking effect and geometric blocking effect. Most essential oils combine these actions, engaging with the metal surface through physical and chemical adsorption. The abundance of π-electrons in double and triple bonds and the presence of heteroatoms in essential oil molecules play a vital role in their anticorrosive properties. Furthermore, the efficacy of essential oils has been investigated across various metal types and corrosive environments. Studies have shown the effectiveness of essential oil to inhibit copper corrosion in 0.5 M HCl,518,531 aluminium alloy corrosion in 3.5% NaCl,532 and Cu–Zn alloy corrosion in 3% NaCl.533 In addition, other biobased compounds such as protein extracts, biodiesels and glycerol have been documented as effective, efficient and environmentally benign inhibitors of metallic corrosion.534–542
However, despite the recorded success in utilizing biobased chemicals as green corrosion inhibitors, there is still the need for the further exploration of the potential of synergistic/hybrid systems in controlling corrosion control for different types of metal/medium systems. In addition, integrating modern machine learning tools for modelling inhibition behaviour and predicting the performance of these biobased chemicals is crucial to reducing costs, saving time, and minimising extensive experimental trials. In industrial applications of pharmaceutical drugs such as green corrosion inhibitors, addressing the high procurement costs is essential, and comprehensive investigations into their toxicity assessment and environmental concerns are imperative. Emerging studies revealed that the chemical functionalization of existent drugs offers a promising prospect for achieving effective and sustainable control of metallic degradation on a large scale.543
2.7. Green corrosion inhibition using efficiency enhancement (synergism, self-healing, etc.)
Synergism has attracted significant interest in corrosion inhibitors due to its potential to enhance the effectiveness of many organic compounds that typically exhibit low to moderate inhibition capabilities. This has led to corrosion experts exploring synergistic opportunities across diverse corrosive environments. This section delves into the concept of “efficiency enhancement” as a fresh approach to elevating the performance of environmentally friendly corrosion inhibitors. The research aims to enhance the corrosion inhibition efficiency of various green inhibitors, including plant extracts and biodegradable polymers, by employing innovative techniques such as nanostructuring, surface modification, and synergistic combinations. The discoveries from these studies offer promising insights into sustainable corrosion prevention methods, significantly contributing to developing eco-conscious solutions for industries grappling with corrosion-related challenges.The concept of “green corrosion inhibition” addresses the dual objectives of safeguarding assets from corrosion, while minimizing the adverse environmental repercussions of corrosion prevention methods. This paradigm shift has led to innovative strategies that leverage the forces of nature, biodegradable materials, and sustainable chemistry to combat corrosion effectively. However, in this pursuit, a crucial question arises: How can the effectiveness of green corrosion inhibitors be heightened without compromising their environmentally friendly attributes? This section explores this pivotal question by delving into the novel “efficiency enhancement” concept within the context of green corrosion inhibition. It seeks to bridge the gap between sustainable corrosion prevention and optimal performance, offering a pathway towards corrosion mitigation solutions that are both efficacious and environmentally conscious. To accomplish this goal, studies should focus on various green corrosion inhibitors, such as plant extracts, biodegradable polymers, and amino acids, while investigating innovative techniques such as nanostructuring, surface modification, and synergistic combinations to elevate their corrosion inhibition efficiency.
Furthermore, the synergistic combinations of multiple inhibitors can enhance their effectiveness. These combinations of corrosion inhibitors collaborate to offer multiple layers of protection and target different aspects of the corrosion process. This can lead to improved inhibition effectiveness compared to a single inhibitor. Combinations such as organic corrosion inhibitors with their inorganic counterparts or mixtures of various organic inhibitors can be employed to enhance the synergistic effect of corrosion inhibitors. In the subsequent sections, we delve into the methodology, findings, and implications of the approaches used to improve the corrosion inhibition capabilities of these inhibitors. This will shed light on the promising advancements in green corrosion inhibition.
 | ||
| Fig. 21 Effect of nanostructure on the efficiency enhancement [self-illustration, copyright permission not required]. | ||
Basheer et al.54 developed a novel TiO2·B2O3-[TBID] nanocomposite as a corrosion inhibitor. They assessed its impact on carbon steel at concentrations in the range of 100 to 500 ppm in a 1 M HCl solution at 35 °C. The highest observed inhibition efficiency (IE%) was 99.91% at a 100 ppm concentration of the TiO2·B2O3-[TBID] inhibitor in an acidic medium. In a separate investigation,544 researchers examined the efficacy of an olive leaf extract-mediated chitosan (CHT)–CuO nanocomposite as a corrosion inhibitor for X60 carbon steel exposed to a 5% HCl solution. The corrosion inhibition performance displayed the following trend: CHT1.0–CuO nanocomposite (90.35%) exhibited a superior performance compared to CHT0.5–CuO (90.16%) and CHT2.0–CuO (89.52%). These nanocomposites substantially reduced the corrosion rate of X60 steel when immersed in a 5% HCl solution, which was particularly evident at a temperature of 25 °C, as illustrated in Fig. 22. However, as the temperature increased from 40 °C to 60 °C, the corrosion rates escalated in both the presence and absence of nanocomposites, peaking at 60 °C.
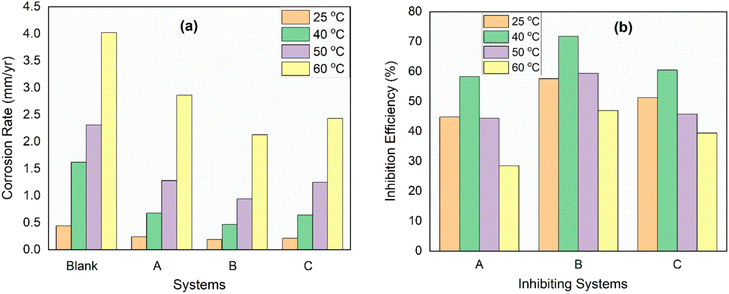 | ||
| Fig. 22 Plots of (a) corrosion rate and (b) inhibition efficiency for X60 steel in 5% HCl without and with (A) 0.5% of CHT0.5–CuO, (B) CHT1.0–CuO, and (C) CHT2.0–CuO nanocomposites at different temperatures [reproduced from ref. 544, open access publication, copyright permission not required]. | ||
In their study, Al-Mhyawi and colleagues545 synthesized silver nanoparticles using tobacco leaf extract at 3, 5, and 10 mM concentrations to investigate their corrosion inhibition properties on carbon steel exposed to 1.0 N HCl. They employed various chemical, electrochemical, and analytical techniques to assess the impact of concentration (ranging from 50 to 200 ppm) and temperature (ranging from 303 to 333 K) on the ability of the material to inhibit corrosion. Their findings indicated that as the temperature increased, the inhibitory effectiveness decreased, while higher concentrations resulted in increased inhibition. The most significant inhibition effectiveness, achieving a remarkable 98%, was obtained when utilizing 200 ppm of the nanomaterials. This outcome was attributed to the formation of a safeguarding barrier film, which acts as a shield, guarding the metal against corrosive elements. Also, the adsorption and bonding of the nanomaterial inhibitor to the metal surface contributed to this result.
In their study,546 Aslam and colleagues explored the inhibitory characteristics of a graphene/Fe3O4 nanocomposite functionalized with glycine called Gr/Fe@Gly NC. The objective was to assess its ability to reduce corrosion in mild steel when exposed to acidic environments. Gr/Fe@Gly NC exhibited remarkable effectiveness and stability, maintaining its inhibitory properties even at temperatures as high as 60 °C. The most effective concentration of 50 ppm resulted in an impressive inhibition efficiency of 98.28%. An analysis of the adsorption isotherm revealed that the inhibitor closely conformed to the Langmuir isotherm, indicating a strong preference for chemical adsorption. Furthermore, Aslam and colleagues547 introduced a novel nanocomposite called ZrO2–glycine, also called ZrO2–Gly NC. To evaluate its potential for inhibiting corrosion, they conducted WL and electrochemical tests using different concentrations of ZrO2–Gly NC on mild steel immersed in a 1 M HCl solution in the temperature range of 40–80 °C. With an increase in the concentration and temperature, the percentage inhibition efficiency of ZrO2–Gly NC demonstrated an upward trend, reaching its peak of approximately 81.01% at 500 ppm and 70 °C, with a slight decrease observed at 80 °C, where it exhibited a 73.5% inhibition efficiency. Based on polarization measurements, it was determined that ZrO2–Gly NC predominantly acted as a mixed-type inhibitor, with a primary focus on suppressing the cathodic process.
2.7.2.1. Combinations of organic and inorganic inhibitors. Incorporating organic and inorganic inhibitors is applicable across various industries such as oil and gas, automotive, aerospace, and infrastructure. This combination of organic and inorganic inhibitors presents a promising and well-established strategy. As industries continually adapt to increasingly challenging corrosion environments, the collaboration between these two types of inhibitors offers a versatile and adaptable solution to effectively combat the relentless forces of corrosion, ultimately contributing to economic sustainability and safety. The introduction of inorganic salts and rare earth metals in organic compounds has demonstrated a synergistic effect, leading to improved inhibition efficiency.81,548–550 When salts are added to organic inhibitors, the primary objective is to enhance their adsorption capacity, while minimizing the concentration of the inhibitor in the aqueous phase. Researchers have also explored the influence of various metal ions on the inhibitory effect of organic compounds.79
A study551 focused on exploring the synergistic inhibitory effect of Ce4+ and melamine on the corrosion of aluminum alloy 2024 (AA2024) in a 3.5% NaCl solution. The combination of melamine and Ce4+ exhibited a significantly higher inhibition efficiency than the sum of their individual inhibitory effects. The highest inhibition efficiency, measured at 90.4% ± 1.8%, was achieved when a mixture containing 5 ppm Ce4+ and 5 ppm melamine was employed. The cooperative inhibitory effect of Ce4+ combined with 3,4-dihydroxybenzaldehyde on cold-rolled steel exposed both in sulfuric acid and hydrochloric acid solutions was investigated by Li et al.552 It should be noted that although this idea relied on the capacity of Ce4+ to form complexes using its open orbitals (4f, 5d, and 6s), no chemical study was done to verify the chemical composition of the complex. El-Lateef553 investigated the effects of a combination of Ce4+ and polyethylene glycols (PEG) on reducing corrosion in carbon steel exposed to diluted sulfuric acid solution. The author hypothesized that a complex between Ce4+ and PEG may be responsible for the increased corrosion resistance. However, it is crucial to stress that no additional research was done to support this theory.
Mohammed et al.554 conducted a study to assess the inhibitory impact of a non-ionic surfactant, specifically nonylphenoxy poly(ethyleneoxy) ethanol (NPPE), on the corrosion of carbon steel in oilfield formation water. Interestingly, they found that adding halide ions (KCl, KBr, and KI) to the inhibitor enhanced the inhibition efficiency of NPPE. In a separate investigation, Hu et al.555 examined the corrosion inhibition performance of a magnesium alloy (GW103) containing Mg-10Gd-3Y in corrosive water, according to the ASTM D1384-87 standards. They studied the combined effects of organic sodium aminopropyltriethoxysilicate (APTS–Na) and inorganic zinc nitrate on corrosion using electrochemical techniques and immersion tests. It was observed that a combination of 0.5 mM APTS–Na+ and 0.1 mM Zn(NO3)2 exhibited a high inhibition efficiency of 92%, significantly surpassing its individual components, which showed a weaker inhibition performance (65% for 0.5 mM APTS–Na+ and 60% for 0.1 mM Zn(NO3)2. This enhanced inhibition efficiency was attributed to the deposition of protective films comprised of Mg(OH)2 and Zn(Mg) silicates, resulting in a compact and protective layer on GW103.
Li et al.556 disclosed the impact of chloride ions on the inhibitory effectiveness of cetyltrimethylammonium bromide (CTAB) in solutions of 1.0–4.0 M H3PO4 for carbon steel (cold-rolled steel). Their findings indicated that the chloride ions acted synergistically to enhance the %IE of CTAB in various acid concentrations. In a different investigation,557 researchers explored the impact of halide salts, specifically NaCl, NaBr, and NaI, on the corrosion inhibition properties of a cationic Gemini surfactant known as 1,3-butan-bis-(dimethyl dodecyl ammonium bromide), referred to as 12-4-12, when applied to low carbon steel immersed in a 1 M HCl solution at a temperature of 20 ± 1 °C. The introduction of halide salts in the surfactant solution led to synergistic effects, enhancing the inhibition efficiency of the surfactant. For instance, at a concentration of 10−6 M, the surfactant alone exhibited an %IE of 62%. However, in the presence of 10−6 M surfactant and 0.1 M of NaI, NaBr, and NaCl, the %IE increased to 90%, 77.3%, and 84.4%, respectively. Additionally, adjusting the solution pH from 0 to 3 reduced the corrosion rate.
Mobin et al.549 investigated the influence of 0.01 M sodium iodide (NaI) and sodium salicylate (NaSal) on the corrosion inhibition properties of m-E2-m-type surfactants in an acidic environment for mild steel corrosion using WL measurements. The addition of NaSal exhibited a synergistic effect on the inhibition efficiency of the m-E2-m surfactants, as evidenced by the synergism parameter of greater than 1. This higher effect of NaSal can be attributed to its more significant reduction in the critical micelle concentration (CMC) compared to I−. Additionally, the interaction between organic anions (Sal−) and the positively charged surfactant, Gemini surfactant (GS) through cation–π interactions, as depicted in Fig. 23, likely contributed to the further decrease in the CMC. Consequently, aromatic counter ions such as Sal− have a more remarkable ability to penetrate the head group region of the surfactants, promoting micellar growth at lower concentrations, unlike the less penetrating inorganic counter ions (I−).
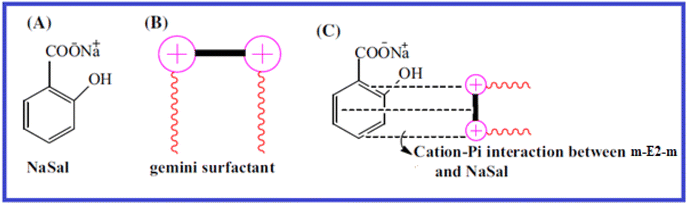 | ||
| Fig. 23 Structure of NaSal (a), gemini surfactant (b) and various interactions existing between the m-E2-m and the NaSal mixture (c) [reproduced from ref. 549, copyright permission not required]. | ||
In a separate investigation, Aslam and coworkers558 examined the impact of sodium tosylate (NaTos) on the corrosion inhibitory impact of 1,2-bis(N-hexadecyl-N,N-dimethylammonium) ethane dibromide, denoted as 16-2-16. The experimental results showcased an increase in the %IE of the 16-2-16 surfactant from 77% to 90.8% in the presence of 50 ppm of sodium tosylate (NaTos). The addition of the salt effectively elevated the ionic strength, which consequently reduced the electrostatic repulsion between the ionic heads of the surfactant and promoted its more significant adsorption on the steel surface. In a study by Khamis et al.,559 they investigated the corrosion inhibition of mild steel in a 0.5 M H2SO4 solution using cetyltrimethylammonium bromide (CTAB) in combination with various salts (NaCl, NaBr, and NaI). Their findings revealed a synergistic effect in the adsorption and inhibition action against acid corrosion of mild steel when CTAB was combined with different halides.
2.7.2.2. Mixtures of organic inhibitors. Studies have examined the impact of various organic additives, such as alcohols and surfactants, on the corrosion inhibition efficacy of environmentally friendly organic inhibitors such as polymers, amino acids, and plant extracts. The objective was to enhance the %IE of inhibitors, such as amino acids and polymers, through synergistic interactions, while preserving their environmentally friendly attributes. Asefi et al.560 combined a cationic Gemini surfactant, 1,3-butan-bis(dodecyl dimethyl ammonium bromide) (12-4-12), with non-ionic co-surfactants (C7H16O, C12H26O, and C5H12O) to inhibit corrosion on low carbon steel in an acidic environment. The results indicated that the corrosion rates decreased with an increase in surfactant concentration. Moreover, when the chain length difference between the surfactant and co-surfactant was lower, it resulted in better compatibility and improved inhibition efficiency behaviour of the surfactant and co-surfactant mixture. Among the mixtures studied, C12H26O + gemini surfactant exhibited the highest increase in %IE (84.5%) (which was 74.9% for C7H16O + gemini surfactant and 77.5% for C5H12O + gemini surfactant).
In a separate investigation conducted by Mobin et al.,561 they examined the anti-corrosive properties of Gemini surfactants, namely 1,2-ethane bis(dimethyl alkyl ammonium bromide) referred to as CmH2m+1, m-2-m, where m = 10 and 12, number of carbon in the hydrophobic chain. However, their focus in this study was on enhancing the %IE of these compounds using butanol. The %IE of the compounds varied with the inhibitor concentration and immersion time. The effectiveness of C4H9OH in increasing %IE values followed the order of 12-2-12 (95.4%) > 10-2-10 (90.8%). Mobin et al.562 investigated the corrosion inhibition performance of L-cysteine (CYS) in 1 M HCl solution for mild steel. They studied the impact of adding small concentrations of three surfactants, i.e., Triton X-100 (TX), SDS, and CPC (cetylpyridinium chloride). Among them, Cys + TX (97.76%) exhibited the highest inhibition performance compared to Cys + SDS (95.09%) > Cys + CPC (91.99%) > Cys (85.62%).
In the study by Zehra et al.,563 they examined the corrosion inhibition properties of the glycine derivative N-benzylidine-2((2-oxo-2-(10H-phenothiazine-10yl)ethyl)amino)acetohydrazide (BPAA) in 1 M HCl solution for mild steel. This study also considered the addition of surfactant additives such as SDS and CPC. It was found that the corrosion inhibition efficiency of the inhibitor was synergistically enhanced in the presence of the surfactant additives, SDS and CPC at lower concentrations. In another study conducted by Mobin et al.,564 they investigated the impact of SDS and CTAB on the corrosion inhibition behavior of L-methionine (LMT) for mild steel in a 0.1 M H2SO4 solution. When LMT was combined with surfactants, the corrosion rates of MS were further reduced compared to LMT alone. This observation suggested a synergistic effect between LMT and surfactants, with the mixture of LMT + CTAB exhibiting a higher inhibitory effect on steel corrosion than the combination of LMT + SDS.
In the study by Parveen and colleagues,565 they examined the impact of SDS and CPC on the corrosion inhibition capabilities of L-tyrosine (Tyr) for mild steel corrosion in a 1 M HCl environment at temperatures ranging from 30 °C to 60 °C. The findings indicated a substantial enhancement in the inhibition efficiency percentage of Tyr when SDS or CPC was introduced at different concentrations. Regardless of whether used independently or in conjunction with SDS or CPC, Tyr demonstrated mixed-type inhibition characteristics. Mobin and Parveen observed the effect of SDS and CTAB on L-cystine (LCY),566L-histidine (LHS),567 and L-tryptophan568 for mild steel in a 0.1 M H2SO4. The findings revealed that the corrosion performance of L-histidine was significantly improved in the presence of surfactants. Mobin and Khan569 researched the corrosion inhibition properties of polyvinyl alcohol (PVA) in a 0.1 M H2SO4 environment for mild steel. This study further explored the impact of SDS and CPC surfactants. It was observed that the inhibitory effect of PVA was significantly enhanced when a very small quantity of surfactants was added. The calculated synergism parameter exceeded one, indicating that the increased inhibition efficiency of PVA resulting from the surfactant addition was due to synergism.
In another report, Mobin et al.570 investigated the corrosion inhibition of mild steel in a sulfuric acid solution in the presence of starch. Starch exhibited moderate inhibition of mild steel corrosion in the tested medium, although this inhibition decreased as the temperature increased from 30 °C to 60 °C. Notably, the %IE of starch was significantly enhanced when both SDS and CTAB were added, and this enhancement was found to be synergistic. In a separate study, Mobin and Khan571 reported the adsorption and corrosion inhibition effects of gum acacia (GA), both alone and in the presence of SDBS and CTAB surfactants, for MS in a 0.1 M H2SO4 environment. The inhibitory action of GA was synergistically improved with the addition of a small amount of surfactants. Furthermore, the Freundlich adsorption model was applicable in describing the corrosion inhibition mechanism for GA alone and in combination with SDBS and CTAB on the surfaces of the mild steel at all the studied temperatures.
Mobin and Rizvi572 researched the inhibitory effect of xanthan gum (XG) in conjunction with synergistic surfactant additives, namely SDS, CPC, and TX, for mitigating mild steel corrosion in a 1 M HCl solution. The inhibitory action of XG was significantly enhanced with the addition of small amounts of surfactants, following the order of XG + SDS (83.17%) > XG + Triton X (82.31%) > XG + CPC (75.89%) > XG alone (74.24%). In a related study, Mobin and Rizvi573 further investigated the inhibitory effect of hydroxyethyl cellulose (HEC) in the presence of CPC and SDS for controlling A1020 carbon steel corrosion in a 1 M HCl solution employing various techniques. They observed that combining HEC and surfactants increased the inhibition efficiency more than either HEC or surfactants, indicating a synergistic effect between HEC and surfactants.
Aslam et al.574 conducted a study on the synergistic effect between Rhodamine blue (RhB) dye and a GS, namely 1,2-ethanediyl-bis(dimethyldecylammonium bromide), referred to as 10-2-10, for mitigating mild steel corrosion in a 1 M HCl medium. Although individual RhB and 10-2-10 GS exhibited a low inhibition efficiency, the inhibitory efficacy of RhB significantly increased when combined with a low concentration of 10-2-10 GS. In another investigation, Aslam et al.575 further explored the anti-corrosion performance of a polymer, the sodium salt of carboxymethyl cellulose (NaCMC), in combination with surfactants, specifically green cationic di-ester-bonded gemini surfactants known as ethane-1,2-diylbis(N,N-dimethyl-N-alkylammoniumacetoxy)dichloride, denoted as m-E2-m, where m = 12, 14, 16, for MS corrosion in a 1 M HCl solution. According to the experimental results, the inhibition efficiency and surface coverage values followed the order of NaCMC < NaCMC/12-E2-12 < NaCMC/14-E2-14 < NaCMC/16-E2-16. The maximum %IE (90.1%) was achieved with NaCMC/16-E2-16 at 30 °C, while the minimum (57.3%) was observed for NaCMC alone at the same temperature. The inhibitory characteristics of CPC in the presence of a copolymer of vinyl pyrrolidone and vinyl acetate were investigated for the corrosion of carbon steel in cyclohexane propionic acid (CHPA) using polarization, conductivity, and EIS measurements.576 This study also examined the influence of KCl on the %IE of the CPC/polymer system. The results revealed that CPC exhibited mixed-type inhibition behaviour, particularly when the copolymer was added (at pH > 4 or in the presence of Cl− ions).
Parveen et al.577 reported on the inhibitory effect of L-proline (LPr) and a combination of LPr with sodium benzoate (LPr + NaBenz) for mitigating mild steel corrosion in a 1 M HCl solution at temperatures of 30 °C, 40 °C, 50 °C, and 60 °C. The analysis of the polarization parameters indicated that both LPr and LPr + NaBenz acted as mixed-type inhibitors, with a more pronounced influence on the cathodic reaction. In another investigation, they565 presented findings regarding the inhibitory impact of L-tyrosine (Tyr) in combination with sodium dodecyl sulphate (Tyr + SDS) or cetylpyridinium chloride (Tyr + CPC) on the corrosion of mild steel in 1 M HCl in the temperature range of 30 °C to 60 °C. The results showed that Tyr, whether on its own or in combination with SDS or CPC, acted as a mixed-type inhibitor and adhered to the surface of mild steel following the Langmuir adsorption isotherm. Elsewhere, Mobin et al.578 explored the influence of adding minute amounts of SDS, CPC, and Triton X-100 on the corrosion inhibition performance of L-cysteine (CYS) for mild steel in aerated and unstirred 1 M HCl solution in the temperature range of 30–60 °C. Their findings indicated a substantial increase in the inhibition efficiency of CYS in the presence of all three surfactants.
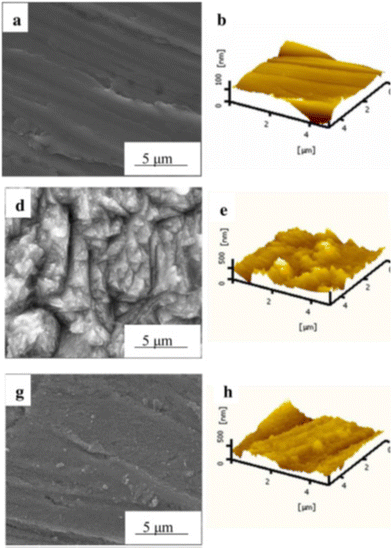 | ||
| Fig. 24 SEM (a, d, and g), AFM (b, e, and h), and contact angle images (c, f, and i) of CRS surfaces: (a–c) before immersion; (d–f) after 6 h of immersion at 20 °C in 1.0 M HCl solution; (g–i) after 6 h of immersion at 20 °C in 50 mg L−1 CS-SAS-AAGC + 1.0 M HCl solution [reproduced from ref. 583, open access publication, copyright permission is not required]. | ||
2.8. Green corrosion inhibition using computational modelings (reduction in trials)
The advancement in the implementation of computational chemistry as a burgeoning field has substantially emerged as a new era in the design and development of next-generation ‘green’ and sustainable corrosion inhibitors.584–586 Considering time, finances, and ecological constraints, as well as sometimes the inability to perform experimental approaches at every juncture to produce significant mechanistic insight into the interaction of metal surfaces and inhibitors, computational techniques are becoming more prevalent and straightforward approaches for researchers in the contemporary domain. Furthermore, computational modeling can be implemented as a substantial prediction tool for the ‘cherry picking’ of corrosion inhibitor scaffolds. For example, DFT can be used as an essential module to screen inhibitor molecules before their wet laboratory synthesis.584–586 Another positive aspect of computational modeling is its ability to anticipate the most suitable spatial orientation of organic compounds in the context of the inhibitory approach and in determining the tentative active sites responsible for interactions with metallic surfaces. Computational modeling techniques, specifically DFT-based quantum chemical calculations, molecular dynamics (MD), and Monte Carlo (MC) simulations, have recently been introduced as revolutionary and greener approaches to studying the adsorption behavior of aqueous phase corrosion inhibitors.585,587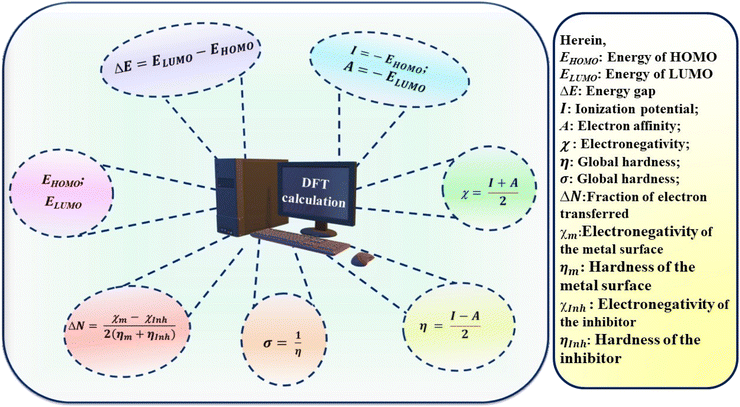 | ||
| Fig. 25 General equations used in DFT to calculate the electronic parameters [self-illustration, copyright permission is not required]. | ||
These parameters further help to comprehend the electron-donating and accepting (D–A) ability of green corrosion inhibitors to the metal surface. The adsorption property of molecules is highly reliant on their D–A capability. When inhibitors are added to the electrolytic solution, they are first adsorbed on the metal surface. Better surface coverage will result in greater inhibitory effectiveness. Accordingly, DFT analysis helps in screening the inhibitory function of molecules. EHOMO represents the ability of a molecule to donate electrons, whereas ELUMO signifies its ability to accept back-donated electrons. A higher EHOMO value often denotes the propensity of an inhibitor molecule to transfer electrons to an empty metal d-orbital. Additionally, a lower ELUMO value suggests a more effortless flow of electrons from the metallic matrix to the inhibitors. A lower value of ΔE reflects the higher tendency of electron donation and acceptance by inhibitors. Shao et al. reported the use of an amide derivative, namely N-[2-(3-indolyl)ethyl]-cinnamamide (IA) as a green corrosion inhibitor for Q235 steel in 0.5 M HCl medium.588 The DFT study showed that IA has greater EHOMO and lower ΔE value than tryptamine (TA), the precursor of IA. The geometry-optimized structures, HOMO, and LUMO, are presented in Fig. 26. To further comprehend the electrostatic potential (ESP) distribution of inhibitor molecules, the ESP distributions of TA and IA were further investigated (Fig. 26). The arrows in the picture shown in green and blue denote the positive and negative electrostatic potential, respectively. It was observed that the electron-rich sites are situated mainly on the heteroatoms such as N and O.
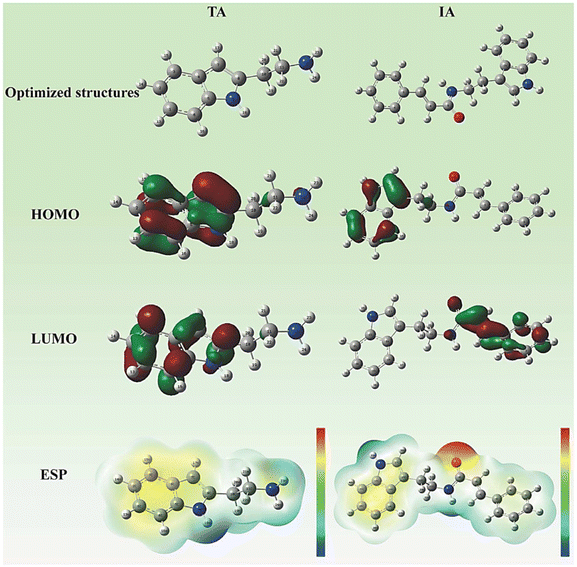 | ||
| Fig. 26 Geometry-optimized structures, density distributions in HOMO–LUMO, and electrostatic potential (ESP) distributions of TA and IA588 [reproduced from ref. 588, open access publication, copyright permission not required]. | ||
A recent study reported the use of garlic peel as a green corrosion inhibitor for protecting AISI 1020 mild steel.589 The electrochemical analysis was conducted in 0.5 M HCl, 0.5 M NaOH and 0.5 M NaCl to understand the corrosion suppression ability of the green inhibitor at different pH. WL tests, electrochemical investigations (EIS and PDP) and morphological analysis (SEM) were performed to investigate the corrosion inhibition ability of the two main components of garlic peel extract, alliin (ALL) and allicin (ALC) molecules, and computational analysis was performed to validate the experimental findings. Since there is only one amine group, it gets protonated and becomes positively charged (ALL+) at acidic pH levels. Alternatively at neutral pH, one protonated amine group and one deprotonated carboxyl group result in zero total charge (ALL+/−). A negatively charged deprotonated carboxyl group is present in ALL at basic pH, leading to the formation of ALL−. The electronic parameters derived from the theoretical study assisted in explaining the correlation between the molecules and their inhibitory effects with respect to the pH level. The inhibitor response at different pH levels was presented with quantum chemical calculations, which showed that the number of active and adsorbable species increases at basic pH.
Similarly, the corrosion inhibition effectiveness of bispyranopyrazoles, namely, 4,4′-(1,4-phenylene)bis(6-amino-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile) (BP-1) and 4,4′(1,4-phenylene)bis(6-amino-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile) (BP-2) was perceived via both experiments and DFT approach.590 All the quantum chemical parameters were calculated for the neutral and protonated BP-1 and BP-2. The higher energy value of HOMO, lower energy gap, higher fraction of electrons transferred, and higher global softness value for BP-2 than that of BP-1 strongly imply that BP-2 binds to the Fe metal via strong bonds given that BP-2 exhibits higher inhibition effectiveness (98.01%) than BP-1 (96.02%). Also, 93.13% corrosion inhibition efficiency was achieved from petals of B. glabra for mild steel in 0.5 M H2SO4.591 The potentiodynamic study, EIS, and gravimetric techniques were utilised to evaluate the surface protective nature of petals of B. glabra with varying concentrations (93.13% corrosion inhibition efficiency at 250 ppm) and varying temperatures (up to 40 °C). Metallurgical microscopy and SEM analysis were employed for the surface assessment. Furthermore, a DFT study was performed to support the experimental data. Gallic acid, quercetin, ascorbic acid, betalain, and peltogynoid molecules were found to be present in the B. glabra petal extract, as confirmed by the spectroscopic analyses such as UV-vis, FTIR, and nuclear magnetic resonance (NMR) studies. The DFT calculation was carried out for the three main components. To investigate the electron distribution in the HOMO and LUMO, chemical potential, electron affinity, electron density distribution per atom, van der Waals surfaces, DFT and adsorption locator investigations were carried out.
Several DFT-based local descriptors can be obtained through Fukui indices (fk). Frontier electron theory has a strong foundation on the fk. The following reactive sites for nucleophilic, electrophilic, and radical attacks can be obtained using Mulliken population analysis. The first derivative of electron density, 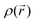 , with respect to the number of electrons, N, at a fixed external potential,
, with respect to the number of electrons, N, at a fixed external potential, 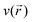 , is commonly used to formulate fk, according to eqn (2).
, is commonly used to formulate fk, according to eqn (2).
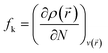 | (2) |
| fk+ = qk(N + 1) − qk(N) | (3) |
| fk− = qk(N) − qk(N − 1) | (4) |
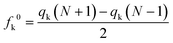 | (5) |
| Eint = ET − (Em+sol + EInh) | (6) |
| Ebind = −Eint | (7) |
The inhibitory effect of pectin-amino acid derivatives, namely pectin-glycine (P-Gly) and pectin-lysine (P-Lys), on N80 carbon steel in the presence of CO2-saturated groundwater was explored.598 P-AAs exhibited good corrosion inhibition efficiencies at a low concentration of 800 mg L−1, where P-Gly (99.01%) and P-Lys (99.36%) showed sustained outstanding adsorption stability for 120 h, as observed from electrochemical and gravimetric experiments. DFT and MD simulation studies were performed for theoretical corroboration. The Fe (110) surface was selected as a representative of N80 carbon steel on which the studied molecules could be adsorbed in a corrosive medium (herein, water). These molecules got adsorbed on the metal plane in a planar fashion, as visualized in Fig. 28. The Eads followed the order of P-Lys (−799.957 kJ mol−1) > P-Gly (−635.425 kJ mol−1) > pectin (−536.251 kJ mol−1), which indicates the better adsorption of P-Lys compared to P-Gly and pectin. P-Lys can get adsorbed more significantly due to its higher negative binding energy. Accordingly, this outcome is consistent with the aforementioned experimental findings.
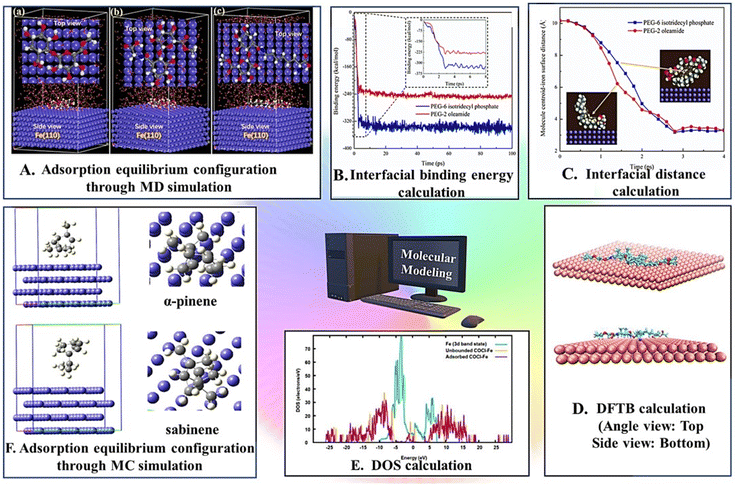 | ||
| Fig. 28 A. Equilibrium adsorption configurations of (a) pectin, (b) P-Gly, and (b) P-Lys on the Fe (110) surface598 [reprinted with permission, Copyright, Elsevier, 2022]; B. change in interfacial binding energies during a simulated physisorption process599 [reprinted with permission, Copyright, Elsevier, 2023]; C. relative distances of two corrosion inhibitors from the iron surface during simulated physisorption process599 [reprinted with permission, Copyright, Elsevier, 2023]; D. DFTB calculation for the interaction between COCI and Fe (110) surface600 [reprinted with permission, Copyright, Elsevier, 2020]; E. change in density of states (DOS)600 [reprinted with permission, Copyright, Elsevier, 2020]; and F. adsorption equilibrium configuration of on the Fe (110) surface obtained from MC simulation78 [reprinted with permission, Copyright, Elsevier, 2021]. | ||
In a recent work, two environmentally friendly corrosion inhibitors with heteroatoms of phosphorous (PEG-6 isotridecyl phosphate) and nitrogen (PEG-2 oleamide) were used to reduce the tribo-corrosion assault on 1018 carbon steel in water-based emulsion drilling fluids (WBEs).599 Using a pin-on-disk tribometer connected to an electrochemical workstation, the effects of the corrosion inhibitors on the tribo-corrosion behavior of steel samples submerged in the manufactured WBEs with and without inhibitors at varied sliding speeds were assessed. Employing a 3D optical profilometer, potentiostatic and potentiodynamic measurement, it was possible to analyze the effect of corrosion inhibitors on improving the anti-tribo-corrosion properties of steel and to calculate the volume losses brought on by wear, corrosion, and wear-corrosion synergy. SEM-EDS and XPS studies were performed to investigate the shape and chemical makeup of the wear tracks, respectively. First-principles calculations and MD simulations were conducted to better comprehend the underlying mechanism for the performance disparity between the PEG-6 isotridecyl phosphate and PEG-2 oleamide corrosion inhibitors. In the MD simulation study, the COMPASS-II forcefield was applied under the NVT ensemble, maintaining a temperature of 300 K for 100 ps. The driving force on the N-containing molecular fragment is primarily responsible for grabbing the PEG-2 oleamide molecule to the iron surface. In contrast, the PEG-6 isotridecyl phosphate molecule gets attracted to the iron surface. This is mainly attributed to the driving force on the C–H chain tail and is consistent with the electrostatic potential distribution of each molecule.
Fig. 28(B) depicts the changes in interfacial binding energies throughout the physisorption process for the two species. It is clear from this figure that the PEG-6 isotridecyl phosphate molecule binds to the iron surface much more strongly than the PEG-2 oleamide molecule through physisorption. The relative distances of the two corrosion inhibitors from the iron surface during the physisorption process are shown in Fig. 28(C). It can be observed from the comparison that the speeds of the two molecules approaching the iron surface are relatively similar. However, the interfacial distance of PEG-6 isotridecyl phosphate is much greater because of its larger size. The density-functional tight-binding (DFTB) method is another important theoretical approach for better understanding the anticorrosive process and to get a qualitative description of these adsorption systems. Self-consistent charge density-functional tight-binding (SCC-DFTB) based on the DFT-derived second-order expansion of the total energy estimated around a reference density was used to predict the interaction of castor oil-based corrosion inhibitor (COCI) and Fe (110) surface (Fig. 28(D)).600
Furthermore, the projected density of states (PDOS), another important tool in computational modeling, was utilized to vividly illustrate the COCI–Fe (110) interaction before and after their adsorption. According to Fig. 28(D), COCI has several distinct bands close to the Fermi energy state of the iron surface (between −7 and 2 eV) before it interacts with the Fe surface, and their peak notably decreases after the interaction. This data implies that an electron transition from the inhibitor to the Fe 3d band state occurs in the COCI–Fe (110) surface interaction. Adsorption typically refers to an exothermic process in which energy gets released in the form of heat. Employing the computational approach, the scale and corrosion inhibition aptitudes of green polyaspartic acid (PASP), polyepoxysuccinic acid (PESA), oxidized starch (OS) and carboxymethyl cellulose (CMC) were examined. With and without water, the relationship between the inhibitor and the surfaces of CaCO3 (110), CaCO3 (104), CaSO4 (020), and Fe (110) was explored using DFT, MD and radial distribution function (RDF) analysis.601 The RDF, also known as the pair correlation function, is the possibility that another particle will be scattered in space once the location of the center atom has been established. RDF analysis was adopted to ascertain the interaction between the oxygen atoms of the carboxyl group of the inhibitors with the metal and metal oxide crystal surfaces. In general, r = 3.5 Å indicates chemical bond formation between the adsorbate and the adsorbent, i.e., chemisorption occurs.
Similarly, a non-bonded interaction is facilitated throughout the 3.5 Å < r < 5.0 Å range. Accordingly, physisorption occurs.602,603 Herein, Fig. 29 depicts the RDF plot of the inhibitors and calcite. The bond length between the Ca and O atoms is 2.39, with a noticeable peak at about 2.36. This demonstrates that the inhibitor may be associated with the CaCO3 surface through chemisorption given that at this point, the oxygen atom of the inhibitor and Ca-atom form an ionic bond. The peak value in the presence of water is lower than the highest value in the absence of water. This observation illustrates that the interaction between the inhibitor and the calcite surface is weakened with an increase in the number of water molecules. The peak values of the four inhibitors follow the order of PESA > PASP > OS > CMC (Fig. 29), which corresponds to the earlier binding energy results obtained from MD.
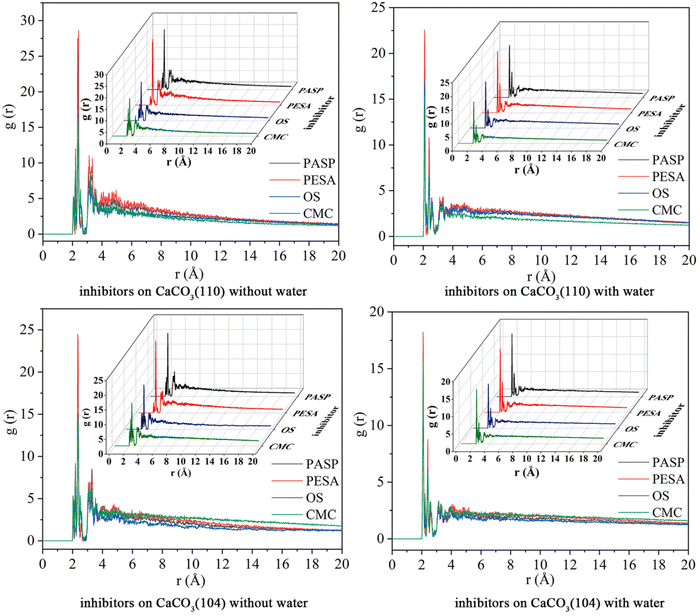 | ||
| Fig. 29 Radial distribution function plot for the distance between the oxygen atom of inhibitors and the Ca-atom of calcite crystal surface601 [reprinted with permission, Copyright, Elsevier, 2021]. | ||
The energy and other parameters may be estimated due to the interaction and adsorption of the concerned inhibitor species at the atomic and molecular levels using MC simulation, which is a vital corrosion inhibition monitoring tool. The crucial variables such as ET, Eint, deformation energy (Edef), adsorption energy (Eads), rigid adsorption energy (Erigid) and metal–inhibitor configuration energy (dEads/dNi) of the system can potentially be determined with the assistance of MC simulation. ET and Eads can be calculated as follows (eqn (8) and (9), respectively):584,602,604
| ET = EInh + Erigid + Edef | (8) |
| Eads = Erigid + Edef | (9) |
MC simulation was carried out for nutmeg oil on the Fe (110) surface.78 The obtained binding energies over the Fe surface follow the order of β-pinene < limonene < α-pinene < sabinene, which suggests that sabinene and α-pinene get adsorbed readily over the Fe surface. Using experimental and theoretical investigations, the ability of three naphthyridine derivatives (viz., 5-amino-9-hydroxy-2-phenylchromeno[4,3,2-de][1,6]-naphthyridine-4-carbonitrile abbreviated as N-1, 5-amino-9-hydroxy-2-(p-tolyl) chromeno-[4,3,2-de][1,6]naphthyridine-4-carbonitrile abbreviated as N-2, and 5-amino-9-hydroxy-2-(4-methoxyphenyl)chromeno[4,3,2-de][1,6]naphthyridine-4-carbonitrile abbreviated as N-3) to suppress mild steel corrosion in a 1 M HCl solution was thoroughly investigated. The MC simulation findings revealed that the naphthyridine derivatives adhere to Fe (110) surfaces in a nearly horizontal orientation. The binding energies for N-1, N-2, and N-3 were, 8.024 kcal mol−1, 8.376 kcal mol−1, 8.469 kcal mol−1, respectively, which are consistent with the experimentally determined trend of inhibitory efficiency, i.e., N-1 < N-2 < N-3. Not only for iron surfaces, but there are also several significant applications of MD and MC simulations to comprehend the interaction of inhibitor molecules on other non-ferrous metal surfaces. An MC simulation study was successfully applied for understanding the interaction phenomena between the Cu (111) surface and the inhibitors (jujube shell extract) in the presence of H2O (55 no), H3O+ (2 no) and Cl− (2 no) ions to mimic the 1 M HCl environment.43 The computation of the adsorption energy provided by the MC simulation explained the spontaneity of the adsorption of ascorbic acid (AA) linoleic acid (LA), oleanolic acid (OA), and triterpenoic acid (TA) present in jujube shell extract on the copper surface.
2.9. Green corrosion inhibition using waste materials (economy)
Green chemistry underscores the critical need to safeguard the environment and well-being of humans within a challenging economic landscape, which can be achieved by eliminating toxic elements and reducing waste via valorization. Green corrosion inhibition using waste materials is an innovative and environmentally conscious approach to tackle corrosion challenges in various industries. Traditional corrosion inhibitors often involve toxic or harmful chemicals, negatively impacting human health and the ecosystem. However, utilizing waste materials as corrosion inhibitors presents a sustainable alternative. Ongoing research delves into developing and applying these green corrosion inhibitors, simultaneously mitigating corrosion-related issues, while minimizing environmental harm, which appears increasingly promising, heralding a more sustainable future for corrosion protection. By repurposing waste substances such as agricultural byproducts, discarded plant matter, and industrial residues,544,545 researchers aim to create effective corrosion inhibitors that efficiently protect metals and alloys and contribute to waste reduction and resource optimization. Research indicates that household-generated food waste comprised of fresh fruits and vegetables accounts for nearly half of this type of waste.546According to the Food and Agriculture Organization (FAO), fruits and vegetables exhibit the highest rates of losses and waste compared to other food categories, with potential levels of wastage reaching up to 60%. The processes involved in fruit and vegetable production generate considerable by-products, constituting around 25% to 30% of the total volume within this commodity group.547 This residual material primarily includes seeds, peels, juice, leaves, and stems, which contain a variety of bioactive compounds with potential value. These compounds inherently possess antioxidant and anti-corrosion attributes, effectively inhibiting corrosion and extending the lifespan of metal structures.548 Furthermore, using waste as a corrosion inhibitor presents a series of merits, including formidable anti-corrosion efficacy, accessibility, renewability, cost-effectiveness, minimal environmental risks, and the ability to form protective layers on metal surfaces.79,551 This approach serves not only to address corrosion control but also contributes to waste reduction and aligns with the principles of a circular economy. Also, this approach is consistent with the principles of green chemistry, promoting the transformation of waste into valuable assets. By establishing a nexus between waste management and corrosion control, this section endeavours to showcase the feasibility of utilizing fruit, vegetable, industrial, and agricultural waste as efficient and environmentally friendly corrosion inhibitors.
In recent years, numerous endeavours have been undertaken to enhance the utilization of agro-waste biomass across diverse domains. However, estimating the precise quantity of agrowaste remains challenging, and due to its relatively low market value, this residue often yields economic returns that are outpaced by the costs associated with its collection, transportation, and processing for beneficial applications. Annually, a staggering amount of nearly one billion tons of this waste is generated,555–557 with this volume steadily rising. A substantial 80% of the total solid waste found on agricultural lands is constituted by organic waste. Although agrowaste was once indiscriminately incinerated on fields, technological advancements have transformed this material into valuable resources for numerous applications.548,549,558 Harnessing agricultural waste for corrosion inhibition boasts multiple merits, representing a sustainable and ecologically sound solution to address corrosion issues.
By repurposing agricultural waste, which is materials that would otherwise be discarded or left to decompose, we can alleviate the burden on landfills and reduce the environmental repercussions of waste disposal. Furthermore, the abundance and cost-effective nature of agricultural waste make it an appealing choice for corrosion inhibition, particularly in regions or countries with constrained resources. Various agricultural waste types have been explored for their potential as corrosion inhibitors. For instance, residues such as straw, husks, and stalks, which are rich in polyphenolic compounds, lignin, cellulose, and other bioactive constituents, exhibit excellent corrosion inhibition potential. These compounds intrinsically possess antioxidative and metal-chelating attributes, enabling them to effectively curtail corrosion rates and hinder the formation of rust on metal surfaces.606 Other forms of agricultural waste, including fruit peels, seed coats, and oilcakes, also display corrosion inhibition potential due to their distinct chemical compositions. To assess the efficacy of agricultural waste as corrosion inhibitors, researchers have employed diverse experimental techniques, including electrochemical tests, surface analysis, and corrosion rate assessments.560 The upcoming section will delve into the outcomes of a literature survey, aiming to enhance our comprehension of the corrosion performance provided by agricultural waste and its operational mechanisms.
Sanni and colleagues conducted a study607 investigating the adsorption and corrosion inhibitory performance of palm kernel shell extract (PKSE) as an eco-friendly corrosion inhibitor on stainless steel (SS) within a simulated seawater environment. The findings of this study indicated that the corrosion inhibition efficiency of PKSE was notably effective, surpassing 90% inhibition efficiency. PKSE acts as a dual-mode inhibitor, demonstrating chemical and physical adsorption on the stainless-steel surface by the Langmuir adsorption model. Furthermore, a closer examination of the surface morphology by SEM revealed that PKSE forms a protective film on the stainless-steel surface, exhibiting firm attachment. Similarly, Oyewole and co-authors explored the efficacy of palm kernel leaf extract (PKLE)608 using central composite design (CCD). This investigation involved conducting a phytochemical analysis of the extract. The process variables were optimized, including the extract concentration (ranging from 0.5 to 1.5 g L−1), duration (3 to 5 days), and temperature (30 °C to 50 °C). The optimal conditions were determined as inhibitor concentration (1.500 g l−1), temperature (30 °C), and time (3 days), leading to an inhibition efficiency of 96.74%. The PKLE extract derived from the agricultural waste of Elaeis guineensis was proven to be an effective inhibitor. Additionally, the lignin polymer obtained from this waste source was modified for use as a green corrosion inhibitor,300 involving the incorporation of aromatic scavengers (2-naphthol: AHN EOL and 1,8-dihydroxyanthraquinone: AHD EOL).
Bhardwaj and co-researchers conducted an assessment609 of the corrosion inhibition characteristics of Phyllanthus emblica seed extract (PESE), which was derived from discarded seeds, within a 15% HCl medium on stainless steel (SS-410). EIS unveiled a remarkable 92.43% corrosion inhibition efficiency when employing 4 g L−1 of PESE. Further analysis through SEM, AFM and XRD confirmed the adsorption of PESE on the SS-410 surface. The adherence of PESE to the Langmuir adsorption isotherm corroborated the formation of a PESE monolayer on the SS-410 surface. The theoretical investigation confirmed that 3,4,8,9,10-pentahydroxy-dibenzo[b,d] pyran-6-one played a pivotal role as the key phytochemical responsible for the anti-corrosive effect. Another instance involving seed extract pertains to the research conducted by Fernine and colleagues610 on Ocimum basilicum seed extract (OBSE) as a green corrosion inhibitor for mild steel in a 1 M HCl solution. This study entailed varying the concentration of OBSE. The polarization results indicated the mixed adsorption behavior of the inhibitor on the mild steel surface in the corrosive environment of 1 M HCl. At an inhibitor concentration of 1.0 g L−1 and a temperature of 298 K, OBSE demonstrated an inhibition efficiency of 95.12%. Surface morphology examination utilizing SEM facilitated the identification of a protective thin film on the external surface of mild steel, guarding it against HCl. Notably, the mild steel samples retained a smooth appearance after 6 h of immersion in a 1 M HCl solution with 1 g L−1 of the extract, reducing the dissolution of iron. Theoretical calculations based on the chemical quantum and MC methodologies unequivocally demonstrated the adsorption of most molecules on the metal surface.
Shahmoradi et al.611 explored the use of pistachio nut shell extracts containing eco-friendly compounds such as cellulose and lignin as organic inhibitors for steel corrosion in 1 M HCl solution. Using 800 ppm of the inhibitor, they achieved a 92% inhibition efficiency within 6 h through EIS testing. The inhibitor exhibited mixed-type action in polarization tests. Adsorption of the compounds followed the Langmuir isotherm, involving both chemical and physical processes.
Wu and colleagues612 investigated the efficacy of walnut green husk extract (WGHE) as a corrosion inhibitor for magnesium alloys in a NaCl solution. Electrochemical assessments unveiled an optimal inhibition efficiency of merely 44.8% with 1.0 g L−1 WGHE. Interestingly, elevating the WGHE concentration failed to enhance this efficiency further. However, immersing magnesium alloy samples in a 1.0 g L−1 WGHE solution for 48 h remarkably elevated the inhibition efficiency to 92.5% under the same corrosive conditions. Notably, menadione, a component in WGHE, emerged as the key contributor to the enhanced corrosion resistance. El-Deeb et al.613 extracted lignin from wheat straw agricultural waste through soda pulping, subsequently modifying and characterizing it using spectroscopic and thermal analysis. The investigation focused on two modified lignin compounds, LG–OH and LG–COOH, as corrosion inhibitors for aluminum in 1 M NaOH, comparing their performance to unmodified lignin (LG). PDP measurements and morphological analysis were employed. The findings revealed that the modified lignin compounds shifted the corrosion of aluminium and open circuit potentials toward more noble values. Moreover, they reduced the corrosion current density compared to the blank solution. Among the compounds, LG–COOH exhibited the highest inhibition efficiency, followed by LG–OH and LG. This difference can be attributed to the more significant number of active adsorption sites in LG–COOH. The Langmuir adsorption isotherm with a physical nature provided the best fit, and the thermodynamic parameters indicated the endothermic nature of the corrosion process with more organized activated complexes in the presence of lignin compounds. Kaban et al.28 investigated the anti-corrosion behaviour of liquid smoke derived from rice husk ash in a 1 M HCl solution in a separate study. The corrosion tests revealed that the inhibitor acted as a mixed-type inhibitor, achieving the optimal inhibition at 80 ppm and 323 K, displaying an impressive 99% inhibition efficiency. AFM analysis demonstrated a smoother surface with a lower skewness parameter of −0.5190 nm on the treated mild steel. Employing an ANN model showcased the reduced overfitting on inhibited steel, a higher prediction accuracy of 81.08%, and a lower loss rate of 0.6001. This neural network successfully modelled the correlation between EIS and PDP and the development of the passive layer on treated mild steel.
Asra et al.614 explored the potential of nanosilicate extracts from rice husk ash (RHA) as a corrosion inhibitor for carbon steel in distilled water. The nanosilicates were extracted through wet chemical methods, resulting in a powder form with particle sizes in the range of 5 to 10 nm. This size range was verified using TEM and zeta size analysis. Remarkably, corrosion measurements exhibited a high %IE of up to 98% with the nanosilicate extract. The adsorption behavior of the inhibitor on the carbon steel surface was found to align with the Temkin isotherm. The potentiodynamic polarization outcomes of this study indicated that the inhibitors functioned as mixed type. The EIS results showcased increased charge transfer resistance and inhibition efficiency percentage with an increase in the nanosilicate concentration. Moreover, the surface analysis revealed that the specimens treated with nanosilicate exhibited smoother surfaces with fewer corrosion products than the untreated specimens. In a study conducted by Matos et al.,615 the potential of barley agro-industrial waste (AW) extract as a corrosion inhibitor for AISI 304 stainless steel in H2SO4 was explored. The inhibitor demonstrated an impressive inhibition efficiency of 97%. However, the investigation needed to assess the stability and long-term durability of the inhibitor. Similarly, Raghavendra et al.616 delved into the effectiveness of a yellow color ripe (YCR) husk extract when applied to aluminum surfaces and exposed to a 0.5 M HCl solution. The researchers evaluated the performance of the extract using EIS and WL measurements. A direct relationship was observed between the inhibitor concentration and corrosion inhibition efficiency.
Shahmoradi and coauthors617 harnessed the extract from the green peel of walnut fruit as a corrosion inhibitor in a 1 M HCl solution. Their approach involved a straightforward, environmentally friendly water-based extraction method to isolate the chemical compounds from the walnut green husk. The presence of organic compounds and the inhibitory agent juglone in the green husk were validated through XPS, FT-IR, and DFT assessments. Their study strongly supported the formation of effective organic–inorganic complexes between the juglone molecules and metal cations, resulting in corrosion inhibition. This assertion was substantiated by the remarkable 95% inhibition efficiency observed in this study. Lin et al.618 studied the inhibitory capabilities of pomelo peel extract in a 1 M H3PO4 solution. The EIS test revealed a corrosion inhibition efficiency of 92.8% after 224 h. Additionally, surface analysis using field emission scanning electron microscopy (FE-SEM) indicated a smooth, corrosion-free metal surface in the presence of 5.0 g L−1 of pomelo peel extract. This observation suggests the successful adsorption of pomelo peel extract on the mild steel surface, contributing to effectively mitigating corrosion. In their research, Paul and co-researchers619 utilized papaya seed extract to evaluate its corrosion-inhibiting potential on carbon steel. The adsorption analysis indicated that the inhibition mechanism aligns with the Temkin isotherm adsorption model, which is likely attributed to the physical adsorption of the benzyl isothiocyanate molecule present in papaya seed. Additionally, both EIS and polarization tests yielded significant results, showcasing a remarkable 90% inhibition efficiency upon exposure to a papaya extract concentration of 1500 ppm.
Larissa Aparecida Corrêa Matos et al.626 employed barley agro-industrial waste (AW) as an environmentally friendly source to minimize corrosion phenomena. Their approach involved utilizing AW extract to inhibit corrosion in a sulfuric acid environment on AISI 304 stainless steel. The extract showcased an impressive inhibition efficiency of up to 97%, which was attributed to the physical bonds formed between the adsorbed extract components and the steel surface. Fig. 30 shows that the addition of the AW acid extract to the reaction medium promoted a decrease in the corrosion current density and increased the polarization resistance of the stainless-steel samples, suggesting that the extract inhibits the corrosion process.
 | ||
| Fig. 30 Tafel plots obtained for AISI 304 stainless steel in 1.5 mol L−1 H2SO4 with and without the AW acid extract at a 1 mV s−1 scanning rate626 [reproduced for ref. 626, open access publication, copyright permission is not required]. | ||
The SEM images also revealed that the presence of the extract curtailed metal oxidation reactions (Fig. 31). Furthermore, this study reported a shift in the cathodic mechanism reaction on the inhibitor-free surface, indicating the alteration in the adsorption mechanism of the AW extract.
 | ||
| Fig. 31 SEM images of AISI 304 stainless steel: (A) before immersion, (B) after immersion in H2SO4, and (C) after immersion in H2SO4 containing 5 g L−1 of AW acid extract (1500× increase). [reproduced for ref. 626, open access publication, copyright permission is not required]. | ||
Gusti and colleagues627 explored coffee bean husk waste as a corrosion inhibitor for mild steel in a 0.75 M H2SO4 environment. This waste contained chemical compounds such as tannins and flavonoids that augment adsorption, providing a broad surface area. Using the WL methodology, the addition of 10 g L−1 of coffee bean husk waste achieved an inhibition efficiency of approximately 80%. Over 72 h, this efficiency climbed to 97.5%, causing the authors to recommend coffee bean husk waste as an effective inhibitor under the tested conditions. Ayoola et al.628 investigated the inhibition potential of waste citrus limonum peel extract for A36 mild steel in a 0.5 M sulfuric acid environment. This study employed various techniques, demonstrating that a concentration of 0.4 w/v% of citrus limonum led to a remarkable 94% inhibition at temperatures ranging from 18 °C to 28 °C. The efficiency only decreased by 2% at higher temperatures up to 45 °C, illustrating the robust reactivity of the extract as an eco-friendly corrosion inhibitor across a wide temperature range. The SEM/EDX analysis further supported the efficient adsorption of the inhibitor, requiring only a small quantity.
Salinas-Solano et al.629 explored the eco-friendly corrosion inhibition potential of rice bran oil-based agro-industrial waste. The process involved extracting and filtering rice bran oil using the Soxhlet method with hexane as the solvent. Their investigation applied various electrochemical techniques to examine the corrosion inhibition on 1018 steel. The addition of 25 ppm of the inhibitor remarkably elevated the inhibition process, achieving over 99% inhibition. This enhancement was attributed to the formation of a protective barrier film on the metal surface, as confirmed by the EIS spectra and adsorption isotherm modeling. This thin film increased the charge-transfer resistance of the metallic material. Vera et al.630 evaluated the corrosion-inhibiting potential of an extract from Fuji apple (Malus Domestica) skin collected in Chile's Valparaiso region. Their study involved carbon steel immersed in a saline medium. WL measurements, impedance measurements, and polarization curves were utilized to estimate the inhibitory performance, which reached 90% efficiency at a concentration of 1000 ppm of the extract. In 2019, Cruz-Zabalegui et al. employed a Gemini-surfactant synthesized from waste avocado oil as a CO2 corrosion inhibitor for X-52 steel.631 This inhibitor exhibited effective mixed-type corrosion inhibition, achieving an impressive 94% efficiency at a concentration of just 10 ppm. It reduced the corrosion rate by forming a thin film due to the adsorption of inhibitor components on the steel interface, forming a physical bond, according to the Langmuir adsorption isotherm.
Halambek et al.632 investigated tomato peel waste-derived pectin (TPP) as a tin corrosion inhibitor. They aimed to promote natural inhibitors over synthetic ones and enhance the biopolymer production. Electrochemical evaluations revealed an inhibition efficiency of 62.19% for CAP and 65.48% for TPP at a pectin concentration of 4 g L−1. Microscopic analysis indicated that pectins prevented the degradation of tin surfaces in aggressive media. Kusumaningrum et al.633 assessed Artocarpus heterophyllus peel extract as a non-toxic fruit waste inhibitor. The extract effectively reduced the corrosion rates and protected pure copper in contact with 1 M HNO3. The highest efficiency reached 98% at an optimal concentration of 800 ppm, confirming the potential of this extract for tin corrosion protection.
Bhardwaj et al.634 undertook a study to explore the viability of Beta vulgaris peel (BVP) waste material as a green corrosion inhibitor for SS 410 in a 15% HCl solution. The PDP analysis revealed that a concentration of 4 g L−1 of BVP extract exhibited the highest %IE of 90.17% in the HCl solution. The experimental findings were consistent with the Langmuir adsorption isotherm, confirming the creation of a monolayer on the SS 410 surface due to the peel extract. The anti-corrosive effect was primarily attributed to vitexin, as supported by the theoretical and computational analyses of the phytochemical composition. Molecular simulation techniques were used to explore the interactions of vitexin with SS 410. Additionally, the study revealed that the B. vulgaris peels acted as anodic inhibitors. The peel extract components adsorbed on the metal surfaces, forming a protective layer, which effectively reduced the corrosion rates due to its protective properties.
Fekri et al.635 introduced turnip peel extract (TPE) as an innovative, eco-friendly, and cost-effective solution for inhibiting copper corrosion in a 3.5 wt% NaCl solution. This study demonstrated that increasing TPE concentration enhanced the inhibition efficiency, reaching an impressive maximum %IE of 91.2%. Furthermore, it assessed the impact of temperature variations (298–338 K) on the performance of TPE as a corrosion inhibitor, indicating a gradual decline in IE with an increase in temperature. Nonetheless, even at 338 K, the %IE remained acceptably high at approximately 64%. A comprehensive analysis revealed that the TPE molecules primarily underwent physical adsorption on the copper surface. The positive ΔH value and the agreement between the experimental data and the Langmuir isotherm model supported this conclusion. Notably, the investigation of the TPE components revealed the presence of heteroatoms (S, O, and N) in their molecular structures, playing a crucial role in forming a protective film on the copper surface and effectively inhibiting the corrosion processes. Radi et al.636 investigated the corrosion inhibitory potential of pumpkin seeds for the 7075-T6 aluminum alloy in a 3.5% NaCl solution using electrochemical, surface, and theoretical analyses. This study revealed an impressive inhibition efficiency of 95% at 298 K and 1 g L−1 concentration. The Tafel polarization analysis suggested that the pumpkin seeds function as cathodic inhibitors. The adsorption of the pumpkin seeds on the aluminum alloy surface followed the Langmuir adsorption isotherm. However, longer immersion times led to a decrease in the protection efficiency.
2.10. Green corrosion inhibition using material design
Material design offers a promising pathway to green corrosion inhibition by combining sustainable principles with innovative techniques. The cutting-edge field of green corrosion prevention through material design is examined in this section. Developing effective and environmentally friendly inhibitors is essential to address corrosion challenges, while minimizing ecological impacts. We explore the production of intelligent coatings, use of nanotechnology, and development of sustainable materials. The discussion includes predictive modelling methods, AI-driven optimization, and green manufacturing procedures. The emphasis is on collaborative research initiatives and regulatory assistance as change agents. The research alludes to a future where environmentally friendly corrosion inhibition protects crucial infrastructure and fosters environmental sustainability by adhering to these standards. As research advances, material design will play a pivotal role in shaping the future of green corrosion protection.As already mentioned, corrosion tremendously impacts metal structures in various sectors, resulting in substantial financial losses, environmental issues, and safety risks.637 Traditional corrosion inhibitors frequently use hazardous chemicals, which raises concerns for the environment and human health. In response, designing materials according to green principles has become a potential strategy for creating efficient and environmentally friendly corrosion inhibitors. The intriguing “green corrosion inhibition through material design” method focuses on successfully developing materials with specific features to fight corrosion, while reducing their environmental impact.13 One of these tactics is designing materials to build a protective film on the metal surface. This layer acts as a barrier to prevent corrosive substances from directly touching the metal, delaying the corrosion process.638,639 These substances are frequently called corrosion inhibitors. Several elements are considered when creating these materials to provide green corrosion inhibition.
Toxic or harmful components should not be used, and the materials should be eco-friendly. This ensures that no harmful compounds are released into the environment during the corrosion inhibition process. Additionally, the materials must successfully halt corrosion, while remaining stable over time. They should have qualities such as strong adherence to the metal surface, decent resistance to deterioration, and the capacity to repair themselves after being harmed. This guarantees that the metal structure will be protected longer.529,640
Academics and industry professionals use different material design strategies to achieve green corrosion inhibition. These strategies involve using organic substances, polymers, nanomaterials, and bioinspired materials. These materials may be tuned for certain corrosion avoidance applications by adjusting their composition, structure, and surface qualities. Overall, green corrosion inhibition via material design is a novel and sustainable method for preventing corrosion, while reducing its adverse environmental effects. It has enormous potential to increase the toughness and longevity of metal structures across a range of industries, from manufacturing and infrastructure to transportation and infrastructure. This section emphasizes the significant ideas, approaches, and most recent developments in this area, emphasizing using materials design tactics to develop green corrosion inhibitors.
 | ||
| Fig. 32 General depiction of the importance of material selection in inhibitor design [self-illustration, copyright permission is not required]. | ||
(i) Environmental sustainability: The minimal negative effects on the environment are the core of green corrosion inhibition.644–646 In line with the sustainability tenets, materials made from renewable resources or biodegradable should be used. These materials support a healthy ecosystem by lowering the production of toxic waste.
(ii) Non-toxic nature: Corrosion inhibitors should be safe for humans and the environment. Through careful material selection, the inhibitors are made safe for users, consumers, and ecosystems by preventing the release of toxic compounds into the environment.640
(iii) Reduced carbon footprint: Climate change may be mitigated by choosing materials with a smaller carbon footprint during manufacturing and use. Choosing materials involving less energy-intensive manufacturing processes or emissions can reduce the environmental effect.
(iv) Biocompatibility: The chosen inhibitors must be biocompatible when the protected materials interact with live species, such as in agricultural or marine applications.647 This guarantees that the inhibitors will not hurt aquatic, terrestrial, or plant life.
(v) Renewable resources: Utilizing renewable resources lessens the reliance on finite resources and supports sustainable practices. Examples of these materials are plant extracts,13,648,649 natural polymers,15,650,651 and waste byproducts.178,652,653
(vi) Integration into existing processes: Green corrosion inhibitors must be easily incorporated into current industrial processes without requiring substantial changes. Implementing green corrosion inhibition techniques across sectors is facilitated by selecting materials compatible with existing production processes.
(vii) Performance and durability: The chosen materials must have corrosion inhibition capabilities that are comparable with or better than conventional inhibitors. They should also continue to work well over time, providing materials with long-lasting protection.
(viii) Adaptability to varied conditions: Corrosion protection that can adjust to changing circumstances is needed for various locations and applications. The materials should be adaptable in multiple environments without sacrificing their inhibitory qualities.306
(ix) Innovation and research: Innovation in creating green corrosion inhibitors is made possible by investigating innovative materials and combinations. Researchers can create composite materials with improved performance and little environmental effect or find novel chemicals.
(x) Regulatory compliance: Standards and regulations that control the use of chemicals and materials apply to many different sectors. Implementing green corrosion inhibitors will go more smoothly using products that adhere to these requirements.
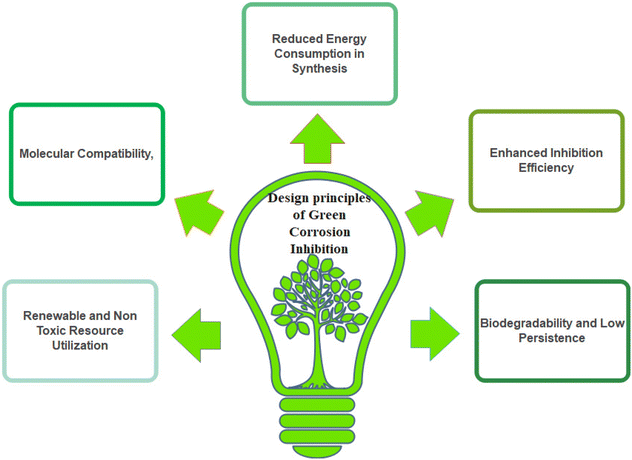 | ||
| Fig. 33 Outline of the major design principles for green corrosion inhibition [self-illustration, copyright permission is not required]. | ||
(i) Renewable resource utilization: Environmentally friendly corrosion inhibitors must utilise organic materials, biopolymers, and plant extracts as renewable resources. The inherent biodegradability of these materials prevents the accumulation of potentially harmful residues. For instance, employing plant compounds conserves non-renewable resources and provides a sustainable alternative to conventional inhibitors.
(ii) Non-toxic nature: The selection of materials that are safe for the environment and human health is strongly encouraged by design guidelines. This idea agrees with the principles of green chemistry and ensures that inhibitors do not introduce dangerous substances into ecosystems. Using non-toxic inhibitors makes it possible to handle, store, and dispose of dangers more safely.
(iii) Molecular compatibility: Design standards strongly recommend using materials safe for the environment and human health. This notion guarantees that inhibitors do not introduce harmful compounds into ecosystems and is consistent with the principles of green chemistry. Handling, storing, and disposing of risks more securely when non-toxic inhibitors are used are feasible.
(iv) Biodegradability and low persistence: Natural biodegradable eco-friendly inhibitors should eventually break down into harmless components. Biodegradability reduces the persistence of inhibitor residues in the environment, minimising their long-term effects. Furthermore, it promotes the broad aims of environmental stewardship.
(v) Reduced energy consumption in synthesis: Green inhibitor design encourages using less energy-intensive and less-byproduct-producing processes. Employing energy-efficient synthesis techniques may significantly reduce the environmental effect of manufacturing inhibitors. This idea promotes the overall sustainability of the manufacture of inhibitors.
(vi) Enhanced inhibition efficiency: The effectiveness of the corrosion prevention by an inhibitor should not be hampered by its environmental friendliness. To maximize the inhibitory performance, design concepts emphasize striking a balance among molecular interactions, film-forming ability, and long-term stability. This ensures that environmentally friendly inhibitors are both effective material protectors and safe for the environment.
2.11. Green corrosion inhibition using safer chemicals (ILs, biodegradable polymers, surfactants and dyes)
The nexus between chemical and environmental sciences has necessitated the development of chemical substances that are viable for numerous industrial applications and eco-friendly. A solution-focused approach to the challenges of chemical production has prompted the design and development of safer chemicals to replace toxic traditional chemicals.654 Safer chemicals, now widely known as green chemicals, reduce or eliminate adverse impacts on human health and the environment. These chemical substances are tailored to minimize or have zero environmental impact in their production, use and disposal, while still serving their intended purpose. Some main characteristics of safer chemicals include potency, efficiency, low cost, facile synthesis, non-toxicity, biodegradability, tolerability and non-bioaccumulative properties.655 In recent years, the stringent campaigns and regulations for using safer chemicals in various industries have led to the advancement of green chemistry initiatives. The field of corrosion science has also embraced the progressive view of utilizing safer chemicals to develop highly effective metallic inhibitors. This section overviews the use of prominent, safer chemicals such as ILs, biodegradable polymers, biosurfactants and dyes as effective, efficient and environmental benign inhibitors.Due to their exceptional biocompatibility, solubility, and adsorption capacity, ILs have emerged as promising eco-friendly alternatives to traditional corrosion inhibitors. Recent advancements in corrosion science, engineering, and technology have showcased the viability of employing ILs for inhibiting metallic corrosion. Over the past decade, the adoption of ILs as environmentally friendly corrosion inhibitors has witnessed a significant upsurge in research activity.659,660 ILs possess the necessary attributes of high-performance inhibitors, featuring multiple heteroatoms, π-electrons, varying alkyl chain lengths, and numerous reactive centers, which facilitate their robust adsorption onto metal surfaces.659,661 Their inhibition capabilities are remarkable, with numerous studies reporting impressive %IE ranging from 90% to 99% at low concentrations. In most instances, ILs often display a mixed-type effect and become effective by adhering to the metal substrate, aligning with the Langmuir adsorption isotherm. The mechanism of action has been widely observed to be both physical and chemical adherence to the metal surface.
Among the diverse classes of ILs explored, imidazolium-based ILs (Im-ILs) have garnered the most attention in the literature. These ILs, similar to others, predominantly inhibit metallic degradation through a mechanism in which their polar hydrophilic groups attach to the metal substrate. Simultaneously, their nonpolar hydrocarbon segments interact with the solution end. For instance, in the study by Haldhar et al.,662 they illustrated the hydrophilic and hydrophobic interactions of three Im-ILs in an HCl environment, specifically for applications in oil and gas pipelines (Fig. 34). In a general context, several critical parameters have been identified as influential factors in determining the inhibitive properties of ILs. These factors include inhibitor concentration, immersion time, temperature, electrolyte composition, alkyl chain length, nature of the alkyl group, effect of synergism, and the type of anion/cation. Extensive research has been devoted to investigating the impact of these variables on the effectiveness of ILs as corrosion inhibitors.392,663–667 Extending present knowledge on the relationship between the structural attributes of ILs and their inhibition behaviour, several authors have constructed quantitative structure–activity relationship (QSAR) models.668–670 The outstanding inhibition behaviour of ILs derived from lignin,671 pyridinium,672,673 pyrrolidinium,674,675 polymers,676,677 and amino acids77,678 has also been recently reported. These reports have demonstrated the inhibiting capability of different ILs to suppress corrosion in different metal/electrolyte system. Table 3 presents a summary of recent reports on the inhibition of different metals using ILs.
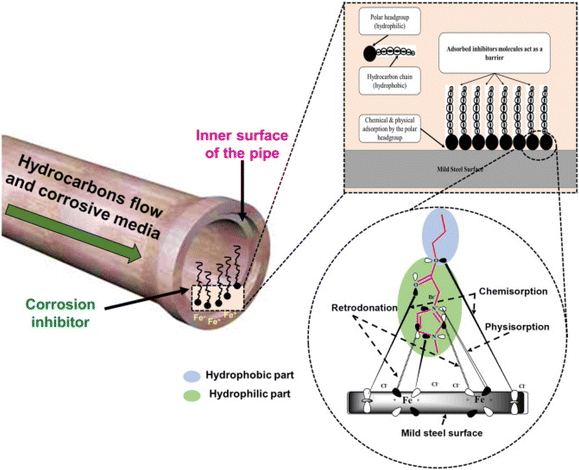 | ||
| Fig. 34 Mechanism of action of Im-ILs in 1 M HCl662 [reproduced from ref. 662 with permission, Copyright, Elsevier, 2023]. | ||
| Ionic liquid | Substrate | Medium | Max %IE | Ref. |
|---|---|---|---|---|
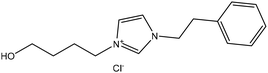
|
Mild steel | 1 M HCl | 96.20% | 679 |
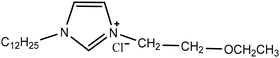
|
Stainless steel | 2 M HCl | 95.40% | 680 |
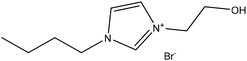
|
Carbon steel | 0.3 M NaCl | 88.90% | 681 |
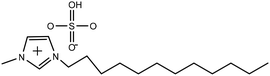
|
Carbon steel | CO2-saturated NaCl brine | 94.46% | 682 |
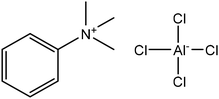
|
Carbon steel | 1 N HCl | 96.00% | 683 |
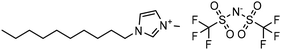
|
AZ31B magnesium | 0.5 wt% NaCl | 93.91% | 684 |
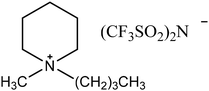
|
Q235 steel | 1 M HCl | 95.80% | 685 |
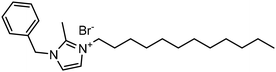
|
Aluminium | 1 M HCl | 80.90% | 686 |
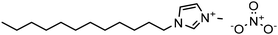
|
Copper | 0.5 M H2SO4 | 96.60% | 687 |
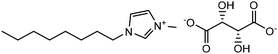
|
Copper | 3.5% NaCl | 95.00% | 688 |
A notable achievement in corrosion control in the chemical and oil industry has recently emerged with the development and evaluation of innovative and environmentally friendly supramolecular ILs.689,690 In these pioneering studies, Berdimurodov et al. introduced two distinct types of supramolecular ILs, namely bromide–cucurbit[7]uril supramolecular ILs (BrCU)689 and cucurbit[6]uril-based[3]rotaxane supramolecular ILs (CB6),690 both designed to mitigate the corrosion of CS. These ILs were investigated in two distinct corrosive environments, namely NaCl saturated with H2S and CO2 in a NaCl medium and the other in a 1 M OH− + 1 M Cl− solution. To assess the inhibitive performance of these supramolecular ILs, a comprehensive array of test methods was employed, including cyclic voltammetry (CV), electrochemical noise (EN), EIS, PDP, SEM, EDX, DFT, and MD simulation. Remarkably, the results demonstrated that the inclusion of merely 100 mg L−1 of BrCU and CB6 yielded an outstanding %IE of 97.54% and 97.97%, respectively, at a temperature of 303 K. The analysis of the Tafel plots indicated that both supramolecular ILs acted as mixed-type inhibitors, predominantly influencing the anodic corrosion process. Subsequent investigations unveiled that these ILs shielded the metal substrate from corrosive ions through physical and chemical interactions. The exceptional corrosion inhibition exhibited by these novel ILs can be attributed to several key factors, including their heightened solubility, substantial planar structure featuring numerous heteroatoms, and the presence of delocalized pi-electrons. This innovative approach marks a significant leap forward in the quest for green and effective corrosion control strategies, promising sustainable solutions for the chemical and oil industry.
Cellulose and starch are the most encountered plant polysaccharides, which are celebrated for their widespread applications across various industries. Cellulose is regarded as the most abundant biopolymer on Earth.302 The appeal of cellulose and starch appeal lies in their affordability, biocompatibility, and favorable physicochemical characteristics. However, in the case of employing them as corrosion inhibitors, especially in their unaltered native forms, their utility is somewhat constrained due to various associated challenges.570,694,695 These challenges are intimately linked to their source, concentration, composition, and solubility, collectively contributing to their suboptimal inhibitive effects. For instance, irrespective of its source, starch is inherently insoluble and necessitates a substantial dosage exceeding 700 mg L−1 to achieve an inhibition performance comparable to traditional inhibitors administered at a mere 50 mg L−1.302 Nevertheless, modifying starch and cellulose has emerged as a transformative approach, effectively mitigating these limitations. When exposed to corrosive environments, modified starch and cellulose derivatives exhibit improved solubility and enhanced adsorption capacities.
In a series of extensive studies on grafted cassava starch conducted by the Chinese research group led by Prof. Li, it was conclusively demonstrated that physical and chemical modification enhanced its water solubility in the test medium and significantly reinforced its corrosion resistance. One of these studies showcased the protective capabilities of cassava starch graft copolymers synthesized from the grafting of acryl amide using gravimetric, electrochemical techniques and surface imaging tools. This study offered a promising avenue for corrosion protection of 90.6% with 1 g L−1 at 293 K for Al in H3PO4.579 Water-soluble moieties such as –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the inhibitor were found to bond and interact with the metal surface coordinatively. The modification of cellulose with the introduction of hydroxyl propyl696 and carboxymethyl697 showed that functionalized cellulose can lower the metal corrosion rate in given media.
O groups in the inhibitor were found to bond and interact with the metal surface coordinatively. The modification of cellulose with the introduction of hydroxyl propyl696 and carboxymethyl697 showed that functionalized cellulose can lower the metal corrosion rate in given media.
Lignin is the second most prevailing biopolymer on Earth after cellulose. It is a phenolic macromolecule with many functional groups, including hydroxyl, carboxyl, methoxy, aldehyde, and phenolic moieties. These diverse functional groups serve as potential centers for adhesion to metallic surfaces. Notably, these functional groups contain double bonds and electron pairs on oxygen atoms, endowing lignin with the remarkable capability to adhere to metal surfaces effectively. This adhesion mechanism facilitates the formation of a protective barrier in the metal/electrolyte system. The recent study conducted by Rahayu et al. carried out lignin extraction from sugarcane bagasse, and subsequently utilized it as a corrosion inhibitor with noteworthy results. The authors reported a peak performance of 80.79% when employing a concentration of 10 g L−1 after a 6 h exposure period.698
Natural gums derived from plants have emerged as exceptional adsorption agents, primarily owing to their remarkable complexation capabilities facilitated by functional groups, extensive surface coverage on metal substrates, and the presence of heteroatoms that serve as centers for adsorption. These natural gums are often composed of polysaccharides, contributing to their robust inhibitory potential. Numerous studies have been conducted to elucidate the inhibitory properties of natural gums and their modified forms.699,700 A case study used Gum Arabic variants from Mauritania, Senegal, and Morocco as effective anticorrosive agents. These variants were rigorously tested for their anticorrosive efficacy against mild steel in a highly corrosive environment of 1 M HCl. The investigation included various electrochemical, surface analytical, and computational analyses. Remarkably, the study yielded promising results, with all three Gum Arabic variants demonstrating inhibition efficiencies ranging from 94% to 96%. The inhibitory mechanism for these variants was elucidated by applying the Langmuir single-layer model. Furthermore, advanced techniques such as AFM and XPS conclusively established a protective layer on the steel sample. To further enhance the understanding of the inhibitory process, a QSAR model was developed. The final model revealed a consistent influence of lipophilicity on the inhibition process, with a commendable R2 value exceeding 0.7.701 These findings underscore the significant potential of natural gums as effective and environmentally friendly corrosion inhibitors, paving the way for sustainable corrosion control solutions. Table 4 portrays other popular natural gums reported as corrosion inhibitors of metals in different electrolytic media.
| Natural gums | Substrate | Medium | Max %IE | Ref. |
|---|---|---|---|---|
| Gum Arabic | Mild steel | 1 M HCl | 94.00% | 702 |
| Locust bean | Stainless steel | 0.15 M NaCl | 99.99% | 703 |
| Guar gum | Aluminium | 1 M HCl | 82.85% | 704 |
| Exudate gum | N80 carbon steel | 1 M HCl | 95.50% | 705 |
| Alginate | Copper | 1 M HCl | 83.00% | 706 |
| Pectin | Carbon steel | 0.5 M HCl | 89.50% | 707 |
| Dextrin | Reinforced steel | 1 M HCl | 85.00% | 708 |
| Inulin | Reinforced steel | 1 M HCl | 93.00% | 708 |
| Xanthan gum | X80 steel | 1 M H2SO4 | 94.85% | 709 |
| Almond gum | Mild steel | 1 M HCl | 96.37% | 710 |
| Category | LD50 value (mg kg−1) | Identification | Symbol |
|---|---|---|---|
| Key: green inhibitors belong to category 4 and 5.a International standard for chemical safety, globally harmonized system of classification and labelling of chemicals (GHS), United Nations Institute for Training and Research Program Advisory Group. | |||
| 1 | 5 | Fatal if swallowed |

|
| 2 | 50 | Fatal if swallowed |

|
| 3 | 300 | Toxic if swallowed |

|
| 4 | >300 ≤ 2000 | Environmentally benign green inhibitors |

|
| 5 | >2000 ≤ 5000 | ||
Chitosan is widely recognized as a linear polysaccharide characterized by numerous functional moieties along its polymer chain. These functional groups play a pivotal role in enhancing the solubility and facilitating robust adhesion to metallic surfaces. Chitosan-based biopolymers have attracted significant attention due to their diverse industrial, environmental, and biological applications.711 Their appeal stems from their exceptional biological tolerance, non-toxicity, minimal environmental footprint, and inherent biodegradability.712 As integral components of the green corrosion inhibitors family, chitosan and its derivatives excel in their capacity to effectively bind to metallic substrates, thereby preventing the infiltration of corrosive ions. Multiple research studies have provided compelling evidence of the anti-corrosive capabilities of chitosan, particularly in safeguarding mild steel and various other metal types.713,714 Research into the functionalization of chitosan has unveiled a remarkable enhancement in its anti-corrosive properties. Chitosan derivatives have undergone comprehensive examination as aqueous corrosion inhibitors across various solutions and for diverse metal types, consistently demonstrating exceptional %IE values that outweigh its pure forms.715 Several studies have demonstrated the ability of chitosan derivatives to adhere to metal surfaces to deter the invasion of corrosive ions under different experimental conditions.715–717
Green synthetic biopolymers have also been reported as effective and eco-friendly metallic inhibitors under different environments. Among these synthetic biopolymers, PEG is prominent, which is characterized by its high molecular weight, ranging from 200 to several tens of thousands.691 PEG has earned recognition for its exceptional properties, including high solubility, strong adhesive characteristics, effective dispersion, cost-effectiveness, and environmentally friendly attributes. Its applications span a broad spectrum, encompassing food science, biology, pharmaceuticals, and chemistry. According to a comprehensive review of the existing literature, it becomes evident that the inhibitory capabilities of PEG are dependent on its molecular weight. Lower molecular weight PEG variants are associated with diminished %IE, implying that the effectiveness of PEG as a corrosion inhibitor is intricately linked to its specific molecular characteristics. A literature review also showed that a host of biodegradable polymers including poly(vinyl alcohol),718 poly(lactic acid), poly(acrylic-maleic acid),719 and polyvinyl pyrrolidone720 are capable of acting as sustainable anticorrosive agents.
2.12. Green corrosion inhibition in industry (oil-well, petrochemicals, acidization, etc.)
Several factors require careful consideration when addressing corrosion issues within the oil and gas sector. These factors encompass the composition of reservoir rocks, the use of acids for stimulation, the selection of tubing and casings in oil well operations, and the prevailing operational conditions. Among these variables, oxygen stands out as a critical element. It is typically absent in productive formations but introduced during the drilling phase when oxygen-contaminated fluids appear, significantly influencing the corrosive potential. Thus, mismanagement of drilling mud can damage well casings, drilling equipment, pipelines, and mud-handling apparatus. Furthermore, whether naturally occurring or introduced for secondary recovery, water and carbon dioxide can induce substantial corrosion in oil well steels. Moreover, it is worth noting that the acids employed for scale removal can readily corrode metal and dealing with hydrogen sulfide (H2S) introduces challenges. Thus, successfully navigating these diverse corrosion scenarios necessitates the expertise of a corrosion engineer, particularly given the demanding conditions of high temperatures, pressures, and stresses prevalent in drilling and production. The role of corrosion engineers is progressively gaining significance.732 The primary culprits behind oil and gas production corrosion are the combination of carbon dioxide (CO2) and H2S gases with water. Additionally, this industry commonly employs water flooding techniques to enhance oil recovery, while simultaneously reinjecting production water into the reservoir to maintain the pressure and stability. As oilfields age, the proportion of water in the produced fluids increases significantly, often surpassing 95%, exacerbating corrosion challenges.733 Consequently, extensive efforts have been dedicated to enhancing their corrosion resistance.734 In scenarios where environmental conditions prove to be excessively harsh for uncoated carbon steels, using inhibitors or corrosion-resistant alloys presents two viable alternatives for mitigating corrosion issues.735 Heating HCl solutions to stimulate oil wells can lead to considerable corrosion issues affecting production tubing, downhole equipment, and casing.736,737 Oil well stimulation is an overarching term encompassing various procedures conducted on a well to enhance its productivity.Matrix acidization is a carefully controlled technique employed to address the production challenges within the wellbore without inducing fractures or harming the reservoir. It introduces acid into the wellbore at specific rates and pressures.738 Typically, hydrofluoric acid (HF) acid is chosen for addressing issues related to sandstone or silica, while HCl or CH3COOH (acetic acid) is preferred for problems associated with limestone or carbonate formations. Matrix acidization primarily mitigates damage caused by factors such as drilling, completion, work-over fluids, and the accumulation of deposits from produced water. Indeed, in carbonate reservoirs, fracture acidization is an alternative strategy to the conventional hydraulic fracturing and propping methods. This technique begins with hydraulic fracturing, followed by the application of acid to etch the fracture surfaces, generating linear flow channels that facilitate the flow of fluids to the wellbore.739 Fractures are initiated by injecting fluid into the well, thereby increasing the pressure and causing pre-existing fractures within the formation to expand and open. Propping agents such as glass beads, sand, epoxy, and silica sand are commonly employed to maintain the newly created fractures and keep them open. The acid reaction can be expressed using the following equations.740
| 2HCl + CaCO3 → CaCl2 + H2O + CO2 |
| CaMg(CO3)2 + 4HCl → CaCl2 + MgCl2 + 2H2O + 2CO2 |
Inhibitor additives are introduced into completed wells to prevent acid-induced corrosion of the steel casing and maintain the structural integrity of the well.741 Various well-stimulation techniques combine inorganic, organic, and surfactant acids. In conventional acidizing treatments, a range of acids is commonly used, with the following acids being among the most prevalent: HCl, HF, CH3COOH, HCOOH (formic acid), H2NSO3H (sulfamic acid), and ClCH2COOH (chloroacetic acid).740,742 These acids are often applied individually or in specific formulations tailored to the particular application. Typically, the weight concentration of HCl utilized in the field is 15%. However, its concentration can vary from 5% to approximately 35%.743 HCl has been proven to be highly effective in dissolving carbonates such as limestone and dolomite. Conversely, acetic acid (HAc) is a slowly reacting, weakly ionized acid. Although the cost of using acetic acid to dissolve a given weight of limestone is higher compared to HCl, there are notable advantages associated with its use.744 These include the ease of inhibiting corrosion, maintaining prolonged contact with the tubing or casing without significant corrosion risk, and natural sequestration properties that prevent iron precipitation. The use of surfactants is imperative in acidization techniques to reduce the surface and interfacial tension, prevent the formation of emulsions, and promote the wetting of the formation. These surfactants play a pivotal role in enhancing the efficiency of the acid treatment.745 Suspending agents, typically composed of polymers or surfactants, prevent insoluble particles from settling and forming bridges, which can lead to blockages in the formed pores or fractures. In addition to their emulsion-preventing properties, certain suspending particles aid in improving the formation wetting by reducing the surface tension of the fresh and spent acid.744,746
Sequestering agents play a vital role in acidization by complexing ions present in iron and other metallic salts, thereby preventing their precipitation. Allowing the precipitation of insoluble iron complexes, such as ferric hydroxide, during acidizing can result in the redeposition of these complexes near the wellbore, leading to persistent clogging issues. During the use of HCl, HAc is employed to maintain a low pH, while citric acid is a valuable chelating agent, which is particularly effective when dealing with higher iron concentrations. Sequestered HCl acid is commonly applied to treat injection and disposal wells.747 Anti-sludge chemicals are used when specific crude oils, particularly heavy asphaltic crudes, encounter acid to prevent the formation of insoluble sludge. Sludge is typically comprised of components such as asphaltenes, resins, paraffin waxes, high molecular weight hydrocarbons, and fine materials or clays from the formation.748 Certain surfactants can prevent sludge formation by maintaining the dispersion of colloidal materials. Moreover, these surfactants that prevent sludge formation often act as emulsion inhibitors. Managing sludge can be challenging, especially when using potent acids.749
Inhibitors in the oil and gas industry are chemical substances employed to safeguard the surfaces of metals from corrosion.750 They achieve this protection by either bonding with the metals or reacting with environmental impurities that may lead to pollution. These inhibitors are applied to metals as a solution, subsequently creating a thin layer or film over the metal surface, effectively shielding it. By altering the anodic and cathodic polarization behaviors, inhibitors deter and reduce the diffusion of ions onto the metallic surface. They also contribute to developing electrical resistance across the metal surface. Several factors must be considered before utilizing a corrosion inhibitor in the oil and gas sector, including its toxicity, environmental compatibility, availability, and cost.751 Corrosion inhibitors employed in this context can be categorized into various types based on their modes of operation, including vapor phase, cathodic, anodic, passivating, film forming, neutralizing, and reactive inhibitors.751
Employing acid treatment on wells is a prevalent technique for boosting oil and gas extraction. Acidization improves the permeability of geological layers and facilitates the movement of oil and gas towards the wellbore.752 Its application extends to well testing and preparation, as well as the cleansing of oil and gas conduits and water transmission lines. This process eliminates salt deposits from the inner metal surfaces.753 Utilizing a concentrated HCl solution to stimulate oil and gas wells is crucial in enhancing the production and mitigating formation damage.754 The acids employed during this procedure engage with acid-sensitive substances within the well due to their heightened chemical reactivity. Consequently, unless controlled, the acid may be expended before achieving a satisfactory penetration depth within the formation. Thus, several approaches have been suggested to curtail the rapid reaction of these acids, including the utilization of aqueous acid emulsions within oil-rich settings or dissolving the acids within non-aqueous solutions.755 The tubing near the surface encounters acids at lower temperatures throughout the acid treatment procedure, while the bottom of the well comes into contact with hotter acids. Additionally, the microstructure of the inner tubing is uneven, even when employing alloys of the same API grade throughout the well.754 Consequently, corrosion-related challenges arise with the acid treatment process. Thus, to mitigate the effect of these highly corrosive acids, inhibitors are added.756,757 Choosing appropriate inhibitors for these conditions involves evaluating factors such as the acid type, fluid temperature, and flow velocity.758 The inhibitors frequently employed include alkenyl phenones, aromatic aldehydes, acetylenic alcohols, quaternary salts, nitrogen-containing heterocycles, and condensation products of carbonyls and amines. Nevertheless, these inhibitors have drawbacks, primarily their reliance on high concentrations and their adverse environmental impact due to their toxicity. Hence, there is a pressing need to explore innovative environmentally-friendly corrosion inhibitors that are both efficient and safe for application in oil well steel, particularly N80 steel, in 15% HCl systems.
Corrosion prevention in the oil and gas industry is complex, requiring specialized inhibitors designed for specific applications such as refineries, wells, recovery units, and pipelines. Corrosive gases such as H2S, CO2, and organic acids further compound the challenge of inhibiting corrosion in wells. Passivating, neutralizing, or adsorption-type inhibitors are commonly employed to mitigate wet corrosion in refineries. Slag inhibitors are employed in conjunction with corrosion inhibitors to reduce deposit formation. Both oil- and water-soluble inhibitors are used in pipelines, with adsorption-type inhibitors being a popular choice to prevent internal corrosion in pipelines transporting refined petroleum products. In environments with elevated operating temperatures and/or higher acid concentrations, using a relatively higher quantity of corrosion inhibitor is typically necessary. To ensure that the inhibitor establishes a protective coating on metal surfaces, introducing the inhibitor at a higher dosage rate is often recommended, normally ranging from 2000 to 5000 ppm. Once this protective layer has been formed, the dosage can be reduced to a maintenance level, typically ranging from 10 to 100 ppm. It is worth noting that nitrogen-based corrosion inhibitors tend to be less effective in high-temperature settings. Additionally, phosphorus substances may hinder the performance of various catalysts used to treat crude oil.752
Recently, a blend containing extracts of rosemary and Cinnamomum cassia essential oils demonstrated remarkable effectiveness in mitigating the deterioration of mild steel when subjected to diluted solutions of H2SO4 and HCl. This approach yielded exceptional inhibition outcomes, consistently exceeding 90% inhibition efficiency across all concentrations tested.765 A similar inhibition efficiency was observed for pipeline steel in the presence of Gum Arabic.766 Aribo et al.757 employed extracts from Tridax procumbens and Chromolaena odorata to protect super austenitic stainless steel in a corrosive environment consisting of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4 M HCl and a CO2-saturated 3.5% NaCl solution. The researchers achieved inhibition efficiencies exceeding 90%. Furtado et al.767 presented a novel green inhibitor using cashew nutshell liquid. They found that combining cardanol residue with butyl glycol and acetylenic alcohol produced a synergistic outcome, resulting in an impressive inhibition efficiency of 99.47% for API P110 carbon steel when exposed to 15% HCl.
1 mixture of 4 M HCl and a CO2-saturated 3.5% NaCl solution. The researchers achieved inhibition efficiencies exceeding 90%. Furtado et al.767 presented a novel green inhibitor using cashew nutshell liquid. They found that combining cardanol residue with butyl glycol and acetylenic alcohol produced a synergistic outcome, resulting in an impressive inhibition efficiency of 99.47% for API P110 carbon steel when exposed to 15% HCl.
Quraishi et al.768 conducted a study on environmentally friendly vanillin-modified chitosan, which displayed noteworthy corrosion inhibition characteristics when employed on carbon steel specimens exposed to diluted solutions of 15% HCl. The findings demonstrated a remarkable corrosion inhibition efficiency of 92%. Palimi et al.769 conducted an extensive investigation aimed at assessing the effects of three environmentally conscious inhibitors, which were derived from fatty acids, i.e., polyethylene glycol-2 oleamide, glycerol myristate, and glycerol linoleate, within drilling fluids based on emulsions. The central objective of this research was to address the specific issue of corrosion associated with 1018 carbon steel in environments saturated with CO2 and KCl. The findings of this study highlighted the substantial efficacy of these environmentally friendly inhibitors. Notably, polyethylene glycol-2 oleamide emerged as an exceptional performer, achieving an impressive inhibition efficiency of 99.7%.
The inhibition of API grade N80 steel in a 15% HCl solution was studied using two environmentally friendly compounds, namely 2-amino-N-octadecylacetamide (AOA) and 2-amino-N-octadecyl-3-(4-hydroxyphenyl) propionamide (AOHP). Various concentrations of these synthesized inhibitors were added to the test solution, and their inhibitory effectiveness was evaluated through mass loss, PDP and EIS. At a concentration of 150 ppm, the optimal inhibition performance was observed for both AOA and AOHP. In the WL tests, AOA achieved an efficiency of 90.04%, while AOHP demonstrated an even higher efficiency of 94.97%. This superiority of AOHP over AOA across all test parameters can be attributed to the more significant number of active centers and larger molecular size of AOHP compared to AOA. Electrochemical assessments, FTIR and SEM analyses determined that these inhibitors exhibit a hybrid nature. The inhibition mechanism of these compounds predominantly involves their absorption on the surface of the N80 steel alloy.770
An extensive research study assessed the effectiveness of 2-(2-pyridyl) benzimidazole, an environmentally friendly corrosion inhibitor, in mitigating CO2-induced corrosion. This meticulous investigation was conducted under particular conditions of turbulent fluid dynamics within a non-corrosive environment. Notably, the inhibitor demonstrated exceptional efficacy, achieving a remarkable 90% effectiveness rate during carefully executed experimental trials, all conducted at a consistent rotational speed of 2000 rpm.771 Moreover, their findings revealed an efficiency of at least 86% at 4000 rpm after 12 h. The subsequent introduction of thiobarbituric acid further heightened the effectiveness, resulting in an enhanced efficiency of 95.6% following a 12 h immersion period.771
In the context of corrosion inhibition, one proposed compound is the amalgamation of zinc sulfate and calcium gluconate. Moreover, investigations delving into the collaborative influence of aspartates and alkyl polyglucosides as both environmentally friendly and efficacious inhibitors have underscored the essential requirement for an optimal alkyl chain length to facilitate the creation of a corrosion protection film. These inhibitors demonstrated robust compatibility with fluids and equipment employed in oil fields. Nonetheless, achieving heightened effectiveness demands introducing more significant quantities of these inhibitors than their traditional counterparts.772 However, although the literature highlights the notable inhibition efficiency of various components, uncertainties continue to linger. For instance, the precise mechanism through which molecules present in plant extracts impede corrosion and the specific constituents responsible for this phenomenon remain elusive. Furthermore, crucial factors such as toxicity, biodegradation, and bioaccumulation necessitate consideration, although these parameters are frequently omitted from studies. Elaborate documentation regarding the extraction procedures of inhibitors from their sources needs to be presented, given that it is a pivotal facet for gauging the feasibility and economic viability of mass-producing these inhibitors. Ultimately, an imperative need persists for further research, encompassing authentic downhole scenarios and real-world environments, to effectively establish a hierarchy among environmentally friendly inhibitors, positioning them as potential substitutes for their current counterparts.
3. Conclusion, challenges and opportunities
The ongoing debate demonstrates that corrosion inhibition has extensively used green chemistry concepts and principles, especially in the last several decades. Considering the toxicity of inorganic inhibitors, organic compounds, primarily heterocycles, have emerged as one of the most effective, economical, and valuable substitutes. The development of environmentally benign and sustainable alternatives has advanced significantly due to the drawbacks of utilizing organic corrosion inhibitors, particularly their extreme environmental toxicity. This entails using various eco-friendly corrosion protection methods, materials, and procedures. Toxicology, bioaccumulation, and biodegradability are three indicators of the sustainability of a chemical. Thus, to evaluate the sustainability indices, OSPAR and REACH have established some standards. The lethal concentration (LD50) and effective concentration (EC50), which represent the concentration of a species that adversely affects the growth of 50% of the living population and kills 50% of the living population, respectively, are two ways to quantify the toxicity of a species. A chemical species may be considered green or harmless if its LC50/EC50 value is 10 mg kg−1 (oral, rat) or higher. The natural degradability of the impacted species is a further sustainability factor. If a chemical species dissolves 60% or more in 28 days, it can be considered eco-friendly according to the OSPAR and REACH regulations. The partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW or DOW) is the primary metric to measure bioaccumulation. Log
KOW or DOW) is the primary metric to measure bioaccumulation. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW or DOW is typically calculated using a water and octanol mixture, which should be less than 3 for a non-bioaccumulative species.
KOW or DOW is typically calculated using a water and octanol mixture, which should be less than 3 for a non-bioaccumulative species.
Among the natural green corrosion inhibitors, various bio-based resources are used as sustainable building materials, including plant extracts, natural polymers, gums, waste, amino acids, and carbohydrates. They are the most excellent eco-friendly alternatives given their natural nature, biodegradability, and lack of accumulation. However, their industrial usage is restricted because of their sensitivity to deterioration at high temperatures. Furthermore, obtaining, processing, and preparing extracts often requires significant work, time, and money. Consequently, the preparation and application of bio-based materials cannot be considered a cost-effective method, especially for extracts from species and decorative plants with a specific distribution. However, semi-synthetic substances such as carbohydrates (chitosan, cellulose, etc.) and amino acids derivatives that have undergone chemical modification have significantly improved long-term and effective corrosion inhibition. The development and application of organic compounds resulting from MW and US irradiation with and without the addition of MCRs have been widely studied. Future research on the usage of semi-synthetic chemicals, particularly those produced by MW and US irradiation in conjunction with MCRs, is warranted, given their many positive green attributes. Sustainable corrosion protection has also made tremendous progress using relatively safer chemicals such as ionic liquids and biodegradable synthetic polymers. Poly(vinyl alcohol) (PVA), poly(amino acids) (PAA), poly(lactic acid) (PLA), poly(caprolactone) (PCL), poly(malic acid) (PMA) and poly(ethylene glycol) (PEG) are among the biodegradable synthetic polymers (BDSPs) that are widely employed in corrosion prevention. However, there are drawbacks to using them as well. These materials are expensive and not advised for further research because of the increased cost of using costly catalysts, solvents, and chemicals.
In long-term corrosion prevention, the synergism (efficiency improvement) and self-healing (efficiency and durability improvement) have also significantly increased. Urea and thiourea are two hazardous chemical compounds employed to increase the corrosion inhibition potential of other metal cations and toxic organic compounds. However, only a small number of studies examined the impact of environmentally friendly synergists such as amino acids on the ability of organic inhibitors to stop corrosion. This calls for further research. Computational modeling and simulations have recently been recognized as potent, successful, economical, and environmentally friendly methods for evaluating the potential inhibition of organic inhibitors. Before being synthesized, the potential of a compound as an inhibitor can be assessed based on its effectiveness, solubility, toxicity, adsorption potential, additive effects, orientation on the metallic surface, charge-sharing molecular sites, etc. Although computational modeling and simulations are frequently used in assessing inhibition potential and supporting the experimental %IE, they occasionally need to improve their ability to forecast the features of the actual environment.
Author contributions
Conception: Mumtaz A. Quraishi, Priyabrata Banerjee and Akram AlFantazi; Writing first draft: Chandrabhan Verma, Ruby Aslam, Dheeraj Singh Chauhan, Jeenat Aslam, Taiwo W. Quadri, Saman Zehra and Dakeshwar Kumar Verma. Editing: Shikha Dubey, Tahir Rasheed and Chandrabhan Verma. Submission and correspondence: Chandrabhan Verma.Conflicts of interest
There are no conflicts to declare.References
- M. Uerdingen, C. Treber, M. Balser, G. Schmitt and C. Werner, Green Chem., 2005, 7, 321–325 RSC
.
-
G. Koch, Trends in oil and gas corrosion research and technologies, 2017, pp. 3–30 Search PubMed
.
-
C. Verma, Handbook of science & engineering of green corrosion inhibitors: modern theory, fundamentals & practical applications, Elsevier, 2021 Search PubMed
.
- R. Ganjoo, S. Sharma, C. Verma, M. Quraishi and A. Kumar, Int. J. Biol. Macromol., 2023, 123571 CrossRef CAS PubMed
.
- R. Ganjoo, C. Verma, A. Kumar and M. Quraishi, Adv. Colloid Interface Sci., 2022, 102832 Search PubMed
.
- M. Stranick, Corrosion, 1984, 40, 296–302 CrossRef CAS
.
- J. Godinez-Alvarez, J. L. Mora-Mendoza, E. Rodriguez-Betancourt, G. Zavala-Olivares and M. A. Gonzalez-Nunez, NACE CORROSION, NACE-04412, NACE, 2004.
-
M. A. A. Ali, Inhibition of mild steel corrosion in cooling systems by low and non toxic corrosion inhibitors (Doctoral Dissertation), The University ofManchester, United Kingdom, 2017 Search PubMed
.
- C. Verma, A. Thakur, R. Ganjoo, S. Sharma, H. Assad, A. Kumar, M. Quraishi and A. Alfantazi, Coord. Chem. Rev., 2023, 488, 215177 CrossRef CAS
.
- E. D. Akpan, O. Dagdag and E. E. Ebenso, Coord. Chem. Rev., 2023, 489, 215207 CrossRef CAS
.
- C. Verma, A. Alfantazi, M. Quraishi and K. Y. Rhee, Coord. Chem. Rev., 2023, 495, 215385 CrossRef CAS
.
- A. Miralrio and A. Espinoza Vázquez, Processes, 2020, 8, 942 CrossRef CAS
.
- A. Zakeri, E. Bahmani and A. S. R. Aghdam, Corros. Commun., 2022, 5, 25–38 CrossRef
.
- S. H. Alrefaee, K. Y. Rhee, C. Verma, M. Quraishi and E. E. Ebenso, J. Mol. Liq., 2021, 321, 114666 CrossRef CAS
.
- S. A. Umoren and U. M. Eduok, Carbohydr. Polym., 2016, 140, 314–341 CrossRef CAS PubMed
.
- M. Shahini, B. Ramezanzadeh and H. E. Mohammadloo, J. Mol. Liq., 2021, 325, 115110 CrossRef CAS
.
- L. Hamadi, S. Mansouri, K. Oulmi and A. Kareche, Egypt. J. Pet., 2018, 27, 1157–1165 CrossRef
.
- D. Zhang, B. Xie, L. X. Gao, Q. Cai, H. G. Joo and K. Y. Lee, Thin Solid Films, 2011, 520, 356–361 CrossRef CAS
.
- C. Verma, C. M. Hussain, M. Quraishi and A. Alfantazi, Adv. Colloid Interface Sci., 2022, 102822 Search PubMed
.
- C. Verma, E. E. Ebenso, M. Quraishi and C. M. Hussain, Mater. Adv., 2021, 2, 3806–3850 RSC
.
- C. Verma, J. Haque, M. Quraishi and E. E. Ebenso, J. Mol. Liq., 2019, 275, 18–40 CrossRef CAS
.
- C. Verma, M. Quraishi and E. E. Ebenso, Sustainable Chem. Pharm., 2018, 10, 134–147 CrossRef
.
- S. M. Powell, H. McMurray and D. Worsley, Corrosion, 1999, 55, 1040–1051 CrossRef CAS
.
- H. Wang and J. Zheng, Corros. Sci. Prot. Technol., 2002, 14, 275–279 CAS
.
- A. N. Kabra, M.-K. Ji, J. Choi, J. R. Kim, S. P. Govindwar and B.-H. Jeon, Environ. Sci. Pollut. Res., 2014, 21, 12270–12278 CrossRef CAS PubMed
.
- M. Sun, H. Lin, W. Guo, F. Zhao and J. Li, J. Ocean Univ. China, 2017, 16, 1167–1174 CrossRef CAS
.
- J. Greenwood, Br. J. Political Sci., 2007, 37, 333–357 CrossRef
.
- A. Kaban, W. Mayangsari, M. Anwar, A. Maksum, T. Aditiyawarman, J. W. Soedarsono and A. Ridhova, East.-Eur. J. Enterp. Technol., 2022, 5, 119 Search PubMed
.
-
W. P. Dinter, Federal Agency for Nature Conservation, Bonn, Germany, 2001, vol. 167 Search PubMed
.
- P. Heslenfeld and E. L. Enserink, ICES J. Mar. Sci., 2008, 65, 1392–1397 CrossRef
.
- A. Castano, M. Cantarino, P. Castillo and J. Tarazona, Chemosphere, 1996, 32, 2141–2157 CrossRef CAS
.
- J. Hermens, H. Canton, N. Steyger and R. Wegman, Aquat. Toxicol., 1984, 5, 315–322 CrossRef CAS
.
- A. L. Parra, R. S. Yhebra, I. G. Sardiñas and L. I. Buela, Phytomedicine, 2001, 8, 395–400 CrossRef PubMed
.
- A. Zakari and D. Kubmarawa, Nat. Prod. Chem. Res., 2016, 4, 2 Search PubMed
.
-
M. D. Summers, S. L. Blunk and B. M. Jenkins, Ecological Building Network, 2003 Search PubMed
.
- R. Wedmann, I. Ivanovic-Burmazovic and M. R. Filipovic, Interface Focus, 2017, 7, 20160139 CrossRef PubMed
.
- J. M. Conder, R. A. Hoke, W. d. Wolf, M. H. Russell and R. C. Buck, Environ. Sci. Technol., 2008, 42, 995–1003 CrossRef CAS PubMed
.
- S. A. van der Heijden and M. T. Jonker, Environ. Sci. Technol., 2009, 43, 8854–8859 CrossRef CAS PubMed
.
- W. M. Meylan, P. H. Howard, R. S. Boethling, D. Aronson, H. Printup and S. Gouchie, Environ. Toxicol. Chem., 1999, 18, 664–672 CrossRef CAS
.
- A. Thakur and A. Kumar, J. Bio- Tribo-Corros., 2021, 7, 1–48 CrossRef
.
- M. Lavanya and A. A. Machado, Sci. Total Environ., 2023, 168407 Search PubMed
.
- J. Panchal, D. Shah, R. Patel, S. Shah, M. Prajapati and M. Shah, J. Bio- Tribo-Corros., 2021, 7, 107 CrossRef
.
- A. Jmiai, A. Tara, S. El Issami, M. Hilali, O. Jbara and L. Bazzi, J. Mol. Liq., 2021, 322, 114509 CrossRef CAS
.
- P. van Gelder, P. Klaassen, B. Taebi, B. Walhout, R. van Ommen, I. van de Poel, Z. Robaey, L. Asveld, R. Balkenende and F. Hollmann, Int. J. Environ. Res. Public Health, 2021, 18, 6329 CrossRef PubMed
.
- M. Damej, S. Skal, J. Aslam, M. Zouarhi, H. Erramli, A. A. Alrashdi, H.-S. Lee and H. Lgaz, Colloids Surf., A, 2022, 643, 128745 CrossRef CAS
.
- C. Verma, A. Alfantazi, M. A. Quraishi and K. Y. Rhee, Sustainable Chem. Pharm., 2023, 31, 100943 CrossRef CAS
.
- J. Aslam, M. Mobin, Huda, A. Aslam and R. Aslam, Int. J. Environ. Sci. Technol., 2023, 20, 2441–2454 CrossRef CAS
.
- D. Darling and R. Rakshpal, CORROSION NACE, NACE, 1998.
- N. Chaubey, A. Qurashi, D. S. Chauhan and M. Quraishi, J. Mol. Liq., 2021, 321, 114385 CrossRef CAS
.
-
G. Koch, in Trends in Oil and Gas Corrosion Research and Technologies, ed. A. M. El-Sherik, Woodhead Publishing, Boston, 2017, pp. 3–30. DOI:10.1016/B978-0-08-101105-8.00001-2
.
- G. R. Meira, C. Andrade, C. Alonso, I. Padaratz and z. J. Borba, Corros. Sci., 2008, 50, 2724–2731 CrossRef CAS
.
- B. A. Al Jahdaly, Y. R. Maghraby, A. H. Ibrahim, K. R. Shouier, A. M. Alturki and R. M. El-Shabasy, Mater. Today Sustainability, 2022, 20, 100242 CrossRef
.
- M. M. Khalaf, A. H. Tantawy, K. A. Soliman and H. M. Abd El-Lateef, J. Mol. Struct., 2020, 1203, 127442 CrossRef CAS
.
- N. A. Basheer, A. A. Ali, R. H. Allawi and A. A. Mashaf, Chem. Pap., 2023, 77, 1655–1667 CrossRef CAS
.
-
V. S. Sastri, Green corrosion inhibitors: theory and practice, John Wiley & Sons, 2012 Search PubMed
.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC
.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC
.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef
.
- S. Marzorati, L. Verotta and S. P. Trasatti, Molecules, 2018, 24, 48 CrossRef PubMed
.
- A. Thakur, S. Sharma, R. Ganjoo, H. Assad and A. Kumar, Mater. Today: Proc., 2022, 66, 609–621 CAS
.
- N. Winterton, Clean Technol. Environ. Policy, 2021, 23, 2499–2522 CrossRef PubMed
.
-
J. H. Clark, A. Hunt, C. Topi, G. Paggiola and J. Sherwood, Sustainable Solvents: Perspectives from Research, Business and International Policy, Royal Society of Chemistry, 2017 Search PubMed
.
- V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. V. D. Long, M. Escribà-Gelonch, J. O. Tejada, S. Linke and K. Sundmacher, Green Chem., 2022, 24, 410–437 RSC
.
-
F. A. Etzkorn, Green chemistry: Principles and case studies, Royal Society of Chemistry, 2019 Search PubMed
.
-
W. Carroll, S. A. Green, H. Plaumann, M. Straka, L. Putman, L. Kolopajlo, M. Mio and M. Kerr, Sustainable Green Chemistry, Walter de Gruyter GmbH & Co KG, 2017 Search PubMed
.
- B. Borah, K. D. Dwivedi, B. Kumar and L. R. Chowhan, Arabian J. Chem., 2022, 15, 103654 CrossRef CAS
.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC
.
- J. Haque, V. Srivastava, D. S. Chauhan, M. Quraishi, A. M. Kumar and H. Lgaz, Sustainable Chem. Pharm., 2020, 16, 100260 CrossRef
.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC
.
-
R. D. Rogers and K. R. Seddon, Ionic liquids: industrial applications for green chemistry, ACS Publications, 2002 Search PubMed
.
- J. Haque, V. Srivastava, M. A. Quraishi, D. S. Chauhan, H. Lgaz and I.-M. Chung, Corros. Sci., 2020, 172, 108665 CrossRef CAS
.
- A. Badawi and I. Fahim, Int. J. Corros. Scale Inhib., 2021, 10, 1385–1406 CAS
.
- D. Wang, Y. Li, B. Chen and L. Zhang, Chem. Eng. J., 2020, 402, 126219 CrossRef CAS
.
- R. Farahati, S. M. Mousavi-Khoshdel, A. Ghaffarinejad and H. Behzadi, Prog. Org. Coat., 2020, 142, 105567 CrossRef CAS
.
- Q. Zhang, B. Hou, Y. Li, G. Zhu, Y. Lei, X. Wang, H. Liu and G. Zhang, Chem. Eng. J., 2021, 424, 130519 CrossRef CAS
.
- O. Ogunleye, A. Arinkoola, O. Eletta, O. Agbede, Y. Osho, A. Morakinyo and J. Hamed, Heliyon, 2020, 6, e03205 CrossRef CAS PubMed
.
- R. Aslam, M. Mobin, I. B. Obot and A. H. Alamri, J. Mol. Liq., 2020, 318, 113982 CrossRef CAS
.
- M. Abdallah, H. Altass, A. S. Al-Gorair, J. H. Al-Fahemi, B. Jahdaly and K. Soliman, J. Mol. Liq., 2021, 323, 115036 CrossRef CAS
.
- S. A. Umoren and M. M. Solomon, J. Environ. Chem. Eng., 2017, 5, 246–273 CrossRef CAS
.
- Y. Zhu, Q. Sun, Y. Wang, J. Tang, Y. Wang and H. Wang, Corros. Sci., 2021, 185, 109414 CrossRef CAS
.
- S. Umoren and M. Solomon, J. Ind. Eng. Chem., 2015, 21, 81–100 CrossRef CAS
.
- M. Djellab, H. Bentrah, A. Chala and H. Taoui, Mater. Corros., 2019, 70, 149–160 CrossRef CAS
.
- G. K. Shamnamol, J. M. Jacob, P. Rugma and J. R. Anoop Raj, J. Adhes. Sci. Technol., 2021, 35, 133–163 CrossRef
.
- C. Verma, E. E. Ebenso and M. Quraishi, J. Mol. Liq., 2017, 248, 927–942 CrossRef CAS
.
- F. Zhang, P. Ju, M. Pan, D. Zhang, Y. Huang, G. Li and X. Li, Corros. Sci., 2018, 144, 74–88 CrossRef CAS
.
-
L. Veleva, Paint and Coating Testing Manual, 2012, pp. 282–299 Search PubMed
.
- N. A. Awang, W. N. Wan Salleh, F. Aziz, N. Yusof and A. F. Ismail, J. Chem. Technol. Biotechnol., 2023, 98, 22–44 CrossRef CAS
.
- Y. L. Kobzar and K. Fatyeyeva, Chem. Eng. J., 2021, 425, 131480 CrossRef CAS
.
- S. Z. Salleh, A. H. Yusoff, S. K. Zakaria, M. A. A. Taib, A. A. Seman, M. N. Masri, M. Mohamad, S. Mamat, S. A. Sobri and A. Ali, J. Cleaner Prod., 2021, 304, 127030 CrossRef CAS
.
- M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh and Z. Sanaei, J. Ind. Eng. Chem., 2019, 77, 323–343 CrossRef CAS
.
- A. M. Vaysburd and P. H. Emmons, Cem. Concr. Compos., 2004, 26, 255–263 CrossRef CAS
.
-
G. Palanisamy, Corrosion inhibitors, 2019, pp. 1–24 Search PubMed
.
- R. B. Rebak and T. E. Perez, NACE CORROSION, 2017, NACE-2017-8933.
- M. Asadian, M. Sabzi and S. M. Anijdan, Int. J. Press. Vessels Pip., 2019, 171, 184–193 CrossRef CAS
.
- I. B. Obot, A. A. Sorour, C. Verma, T. A. Al-Khaldi and A. S. Rushaid, Eng. Fail. Anal., 2022, 107008 Search PubMed
.
- C. Verma and M. Quraishi, Coord. Chem. Rev., 2021, 446, 214105 CrossRef CAS
.
- C. Verma, L. O. Olasunkanmi, E. E. Ebenso, M. A. Quraishi and I. B. Obot, J. Phys. Chem. C, 2016, 120, 11598–11611 CrossRef CAS
.
- C. Verma, E. E. Ebenso and M. Quraishi, J. Mol. Liq., 2020, 316, 113874 CrossRef CAS
.
- D. S. Chauhan, C. Verma and M. Quraishi, J. Mol. Struct., 2021, 1227, 129374 CrossRef CAS
.
- A. A. Al-Amiery, W. N. R. W. Isahak and W. K. Al-Azzawi, Lubricants, 2023, 11, 174 CrossRef CAS
.
- W. R. de Souza Morais, J. S. da Silva, N. M. P. Queiroz, C. L. de Paiva e Silva Zanta, A. S. Ribeiro and J. Tonholo, Appl. Sci., 2023, 13, 7482 CrossRef CAS
.
-
K. M. O. G. Lipiar and A. J. M. Mohammad, in Corrosion Inhibitors, ed. S. Ambrish, IntechOpen, Rijeka, 2019, p. 5. DOI:10.5772/intechopen.81376
.
- K. M. S. Newaz, W. J. Basirun, H. B. M. Ali, F. L. Faraj and G. M. Khan, Int. J. Electrochem. Sci., 2015, 10, 6120–6134 CrossRef
.
- A. Peter, I. Obot and S. K. Sharma, Int. J. Ind. Chem., 2015, 6, 153–164 CrossRef CAS
.
- S. K. Sharma, A. Mudhoo, E. K. E. Khamis and G. Jain, J. Corros. Sci. Eng., 2008, 11, 1–14 Search PubMed
.
- A. Rajasekar, S. Maruthamuthu, N. Palaniswamy and A. Rajendran, Microbiol. Res., 2007, 162, 355–368 CrossRef CAS PubMed
.
- M. Mobin, M. Rizvi, L. O. Olasunkanmi and E. E. Ebenso, ACS Omega, 2017, 2, 3997–4008 CrossRef CAS PubMed
.
-
D. S. Chauhan, M. A. Quraishi, H. Al-Qahtani and M. A. Jafar Mazumder, Polymeric Corrosion Inhibitors for Greening the Chemical and Petrochemical Industry, 2022, pp. 1–22 Search PubMed
.
- M. A. A. Khan, O. M. Irfan, F. Djavanroodi and M. Asad, Sustainability, 2022, 14, 9502 CrossRef CAS
.
- N. Al Otaibi and H. H. Hammud, Molecules, 2021, 26, 7024 CrossRef CAS PubMed
.
-
F. Luborsky, Amorphous metallic alloys, 1983, vol. 1 Search PubMed
.
- L. E. Shoemaker, Superalloys, 2005, 718, 409–418 Search PubMed
.
- Y. Sun, S. Tian, P. Ciais, Z. Zeng, J. Meng and Z. Zhang, Nat. Commun., 2022, 13, 297 CrossRef CAS PubMed
.
- W. Chen, X. Yin and D. Ma, Appl. Energy, 2014, 136, 1174–1183 CrossRef
.
- Z. Liu, Y. Geng, S. Lindner and D. Guan, Energy, 2012, 45, 1059–1068 CrossRef
.
- G. Q. Chen and B. Zhang, Energy Policy, 2010, 38, 6180–6193 CrossRef CAS
.
- R. Clémençon, J. Environ. Dev., 2016, 25, 3–24 CrossRef
.
- R. Kumar and A. K. Sharma, Uttar Pradesh J. Zool., 2020, 41, 1–8 Search PubMed
.
- P. W. Griffin and G. P. Hammond, Glob Transit., 2021, 3, 72–86 CrossRef
.
- E. Mousa, C. Wang, J. Riesbeck and M. Larsson, Renewable Sustainable Energy Rev., 2016, 65, 1247–1266 CrossRef
.
- H. Muslemani, X. Liang, K. Kaesehage, F. Ascui and J. Wilson, J. Cleaner Prod., 2021, 315, 128127 CrossRef
.
- A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed
.
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547–4592 RSC
.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC
.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC
.
- D. K. Verma, S. Kaya, E. Ech-chihbi, F. El-Hajjaji, M. M. Phukan and H. M. Alnashiri, J. Mol. Liq., 2021, 329, 115531 CrossRef CAS
.
-
O. S. Shehata, L. A. Korshed and A. Attia, Corrosion inhibitors, principles and recent applications, 2018, vol. 121 Search PubMed
.
- C. Verma, E. E. Ebenso, I. Bahadur and M. A. Quraishi, J. Mol. Liq., 2018, 266, 577–590 CrossRef CAS
.
- K. Xhanari, M. Finšgar, M. K. Hrnčič, U. Maver, Ž Knez and B. Seiti, RSC Adv., 2017, 7, 27299–27330 RSC
.
- J. Buchweishaija, Tanz. J. Sci., 2009, 35, 77–92 Search PubMed
.
- S. Ghareba and S. Omanovic, Corros. Sci., 2010, 52, 2104–2113 CrossRef CAS
.
- Z. Shang and J. Zhu, J. Mater. Res. Technol., 2021, 15, 5078–5094 CrossRef CAS
.
- B. El Ibrahimi, Colloid Interface Sci. Commun., 2020, 37, 100279 CrossRef CAS
.
- K. Alaoui, M. Ouakki, A. S. Abousalem, H. Serrar, M. Galai, S. Derbali, K. Nouneh, S. Boukhris, M. E. Touhami and Y. El Kacimi, J. Bio- Tribo-Corros., 2018, 5, 1 Search PubMed
.
- S. Mo, L. J. Li, H. Q. Luo and N. B. Li, J. Mol. Liq., 2017, 242, 822–830 CrossRef CAS
.
- Y. M. Abdallah, J. Mol. Liq., 2016, 219, 709–719 CrossRef CAS
.
- M. Yadav, T. K. Sarkar and I. B. Obot, RSC Adv., 2016, 6, 110053–110069 RSC
.
- Y. Li, S. Zhang, Q. Ding, D. Feng, B. Qin and L. Hu, Tribol. Int., 2017, 114, 121–131 CrossRef CAS
.
- Y. Guo, B. Xu, Y. Liu, W. Yang, X. Yin, Y. Chen, J. Le and Z. Chen, J. Ind. Eng. Chem., 2017, 56, 234–247 CrossRef CAS
.
- P. Arellanes-Lozada, O. Olivares-Xometl, N. V. Likhanova, I. V. Lijanova, J. R. Vargas-García and R. E. Hernández-Ramírez, J. Mol. Liq., 2018, 265, 151–163 CrossRef CAS
.
- F. A. Azeez, O. A. Al-Rashed and A. A. Nazeer, J. Mol. Liq., 2018, 265, 654–663 CrossRef CAS
.
- S. Cao, D. Liu, T. Wang, A. Ma, C. Liu, X. Zhuang, H. Ding, B. B. Mamba and J. Gui, Colloids Surf., A, 2021, 616, 126280 CrossRef CAS
.
- V. Saraswat and M. Yadav, ChemistrySelect, 2020, 5, 7347–7357 CrossRef CAS
.
- Y. Ye, D. Yang, H. Chen, S. Guo, Q. Yang, L. Chen, H. Zhao and L. Wang, J. Hazard. Mater., 2020, 381, 121019 CrossRef CAS PubMed
.
- J.-j. Fu, S.-n. Li, Y. Wang, X.-d. Liu and L.-D. Lu, J. Mater. Sci., 2011, 46, 3550–3559 CrossRef CAS
.
- P. Muthukrishnan, B. Jeyaprabha, P. Tharmaraj and P. Prakash, Res. Chem. Intermed., 2015, 41, 5961–5984 CrossRef CAS
.
- A. Ousslim, K. Bekkouch, B. Hammouti, A. Elidrissi and A. Aouniti, J. Appl. Electrochem., 2009, 39, 1075–1079 CrossRef CAS
.
- M. I. Awad, J. Appl. Electrochem., 2006, 36, 1163–1168 CrossRef CAS
.
- V. Rajeswari, D. Kesavan, M. Gopiraman and P. Viswanathamurthi, J. Surfactants Deterg., 2013, 16, 571–580 CrossRef CAS
.
- M. Yadav, D. Behera, S. Kumar and R. R. Sinha, Ind. Eng. Chem. Res., 2013, 52, 6318–6328 CrossRef CAS
.
- R. Aslam, M. Mobin, S. Zehra, I. B. Obot and E. E. Ebenso, ACS Omega, 2017, 2, 5691–5707 CrossRef CAS PubMed
.
- P. Ekins and D. Zenghelis, Sustainability Sci., 2021, 16, 949–965 CrossRef PubMed
.
- P. Bolouri, R. Salami, S. Kouhi, M. Kordi, B. Asgari Lajayer, J. Hadian and T. Astatkie, Molecules, 2022, 27(24), 8999 CrossRef CAS PubMed
.
- X. Li, S. Deng and H. Fu, Prog. Org. Coat., 2010, 67, 420–426 CrossRef CAS
.
- N. Hossain, M. Aminul Islam and M. Asaduzzaman Chowdhury, Results Chem., 2023, 5, 100883 CrossRef
.
- R. M. Saleh, A. A. Ismall and A. A. El Hosary, Br. Corros. J., 1982, 17, 131–135 CrossRef CAS
.
- K. Srivastav and P. Srivastava, Br. Corros. J., 1981, 16, 221–223 CrossRef
.
- F. S. de Souza, C. Giacomelli, R. S. Gonçalves and A. Spinelli, Mater. Sci. Eng., C, 2012, 32, 2436–2444 CrossRef
.
- H. Wang, M. Gao, Y. Guo, Y. Yang and R. Hu, Desalination, 2016, 398, 198–207 CrossRef CAS
.
- E. Khamis and N. AlAndis, Mater. Werkst., 2002, 33, 550–554 CrossRef CAS
.
- A. Y. El-etre, Corros. Sci., 1998, 40, 1845–1850 CrossRef CAS
.
- A. Y. El-Etre and M. Abdallah, Corros. Sci., 2000, 42, 731–738 CrossRef CAS
.
- R. A. L. Sathiyanathan, S. Maruthamuthu, M. Selvanayagam, S. Mohanan and N. Palaniswamy, Indian J. Chem. Technol., 2005, 12, 356–360 CAS
.
- A. Hamdy and N. S. El-Gendy, Egypt. J. Pet., 2013, 22, 17–25 CrossRef
.
- T. Ibrahim, H. Alayan and Y. A. Mowaqet, Prog. Org. Coat., 2012, 75, 456–462 CrossRef CAS
.
- A. Ait Aghzzaf, D. Veys-Renaux and E. Rocca, Mater. Corros., 2020, 71, 148–154 CrossRef CAS
.
- H. Ashassi-Sorkhabi, S. Mirzaee, T. Rostamikia and R. Bagheri, Int. J. Corros., 2015, 2015, 197587 Search PubMed
.
- A. Harouat, A. Bezzar and L. Sail, Eur. J. Environ. Civ. Eng., 2023, 1–34, DOI:10.1080/19648189.2023.2220788
.
- A. Buyuksagis, M. Dilek and M. Kargioglu, Prot. Met. Phys. Chem. Surf., 2015, 51, 861–872 CrossRef CAS
.
- H. Nazari Mehdi, J. Yu and X. Shi, J. Mater. Civ. Eng., 2020, 32, 04020281 CrossRef
.
- R. Saratha and V. G. Vasudha, E-J. Chem., 2010, 7, 162375 Search PubMed
.
- A. Saxena, K. K. Thakur, K. K. Saxena, S. Chambyal and A. Sharma, Mater. Today: Proc., 2020, 26, 1360–1367 CAS
.
- D. Prabhu, P. R. Prabhu and P. Rao, Chem. Pap., 2021, 75, 653–667 CrossRef CAS
.
- E. E. Oguzie, M. A. Chidiebere, K. L. Oguzie, C. B. Adindu and H. Momoh-Yahaya, Chem. Eng. Commun., 2014, 201, 790–803 CrossRef CAS
.
- S. Sutthiruangwong, C. Wongpaiboon, N. Sritha and N. Anukulkich, Metals, 2023, 13, 262 CrossRef CAS
.
- R. Haldhar, D. Prasad and N. Bhardwaj, Arab. J. Sci. Eng., 2020, 45, 131–141 CrossRef CAS
.
- P. B. Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 113–116 CrossRef CAS
.
- A. Pal and C. Das, Ind. Crops Prod., 2020, 151, 112468 CrossRef CAS
.
- A. Singh, V. K. Singh and M. A. Quraishi, Int. J. Corros., 2010, 2010, 275983 Search PubMed
.
- M. Kliškić, J. Radošević, S. Gudić and V. Katalinić, J. Appl. Electrochem., 2000, 30, 823–830 CrossRef
.
- M. Abdallah, Port. Electrochim. Acta, 2004, 22, 161–175 CrossRef CAS
.
- S. Martinez and I. Štern, J. Appl. Electrochem., 2001, 31, 973–978 CrossRef CAS
.
- E. E. Oguzie, Pigm. Resin Technol., 2005, 34, 321–326 CrossRef CAS
.
- G. O. Avwiri and F. O. Igho, Mater Lett., 2003, 57, 3705–3711 CrossRef CAS
.
- L. R. Chauhan and G. Gunasekaran, Corros. Sci., 2007, 49, 1143–1161 CrossRef CAS
.
- K. O. Orubite and N. C. Oforka, Mater. Lett., 2004, 58, 1768–1772 CrossRef CAS
.
- B. Müller, Corros. Sci., 2002, 44, 1583–1591 CrossRef
.
- A. Bouyanzer, B. Hammouti and L. Majidi, Mater. Lett., 2006, 60, 2840–2843 CrossRef CAS
.
- A. Chetouani, B. Hammouti and M. Benkaddour, Pigm. Resin Technol., 2004, 33, 26–31 CrossRef CAS
.
- R. T. Loto and O. Olowoyo, Procedia Manuf., 2019, 35, 310–314 CrossRef
.
- E. Chaieb, A. Bouyanzer, B. Hammouti and M. Benkaddour, Appl. Surf. Sci., 2005, 246, 199–206 CrossRef CAS
.
- E. E. Oguzie, Corros. Sci., 2007, 49, 1527–1539 CrossRef CAS
.
- E. E. Oguzie, Mater. Chem. Phys., 2006, 99, 441–446 CrossRef CAS
.
- A. Y. El-Etre, M. Abdallah and Z. E. El-Tantawy, Corros. Sci., 2005, 47, 385–395 CrossRef CAS
.
- A. Y. El-Etre, Appl. Surf. Sci., 2006, 252, 8521–8525 CrossRef CAS
.
- A. Y. El-Etre, Corros. Sci., 2003, 45, 2485–2495 CrossRef CAS
.
- A. Y. El-Etre, Corros. Sci., 2001, 43, 1031–1039 CrossRef CAS
.
- Y. Li, P. Zhao, Q. Liang and B. Hou, Appl. Surf. Sci., 2005, 252, 1245–1253 CrossRef CAS
.
- F. Zucchi and I. H. Omar, Surf. Technol., 1985, 24, 391–399 CrossRef CAS
.
- M. I. Awad, J. Appl. Electrochem., 2006, 36, 1163–1168 CrossRef CAS
.
- B. Monica and S. Ioan, J. Biotechnol. Biomed.
Sci., 2018, 1, 35–43 CrossRef
.
- R. T. Loto and A. Busari, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 770, 012046 CAS
.
- M. Bendahou, M. Benabdellah and B. Hammouti, Pigm. Resin Technol., 2006, 35, 95–100 CrossRef CAS
.
- I. Dhouibi, F. Masmoudi, M. Bouaziz and M. Masmoudi, Arabian J. Chem., 2021, 14, 102961 CrossRef CAS
.
- K. Boumhara, H. Harhar, M. Tabyaoui, A. Bellaouchou, A. Guenbour and A. Zarrouk, J. Bio- Tribo-Corros., 2018, 5, 8 CrossRef
.
- N. Al-Akhras and Y. Mashaqbeh, J. Build. Eng., 2021, 35, 101848 CrossRef
.
- M. Znini, L. Majidi, A. Bouyanzer, J. Paolini, J. M. Desjobert, J. Costa and B. Hammouti, Arabian J. Chem., 2012, 5, 467–474 CrossRef CAS
.
- M. Znini, G. Cristofari, L. Majidi, A. Ansari, A. Bouyanzer, J. Paolini, J. Costa and B. Hammouti, Int. J. Electrochem. Sci., 2012, 7, 3959–3981 CrossRef CAS
.
- M. Mansouri, Y. El Ouadi, M. Znini, J. Costa, A. Bouyanzer, J. M. Desjobert and L. Majidi, J. Mater. Environ. Sci., 2015, 6, 631–646 Search PubMed
.
- S. Andreani, M.-C. De Cian, J. Paolini, J.-M. Desjobert, J. Costa and A. Muselli, Chem. Biodiversity, 2013, 10, 2061–2077 CrossRef CAS PubMed
.
- D. B. Hmamou, R. Salghi, A. Zarrouk, H. Zarrouk, M. Errami, B. Hammouti, L. Afia, L. Bazzi and L. Bazzi, Res. Chem. Intermed., 2013, 39, 973–989 CrossRef
.
- T. Benabbouha, M. Siniti, H. El Attari, K. Chefira, F. Chibi, R. Nmila and H. Rchid, J. Bio- Tribo-Corros., 2018, 4, 39 CrossRef
.
- J. K. Patra and K.-H. Baek, Molecules, 2016, 21(3), 388 CrossRef PubMed
.
- P. López, C. Sánchez, R. Batlle and C. Nerín, J. Agric. Food Chem., 2007, 55, 4348–4356 CrossRef PubMed
.
- S. Ağaoğlu, N. Dostbil and S. Alemdar, Bull. Vet. Inst. Pulawy, 2007, 51, 53–57 Search PubMed
.
- M. Boudalia, R. M. Fernández-Domene, M. Tabyaoui, A. Bellaouchou, A. Guenbour and J. García-Antón, J. Mater. Res. Technol., 2019, 8, 5763–5773 CrossRef CAS
.
- O. Ouachikh, A. Bouyanzer, M. Bouklah, J. M. Desjobert, J. Costa, B. Hammouti and L. Majidi, Surf. Rev. Lett., 2009, 16, 49–54 CrossRef CAS
.
- N. Hechiche, D. Boughrara, A. Kadri, N. Dahmani and N. Benbrahim, Anal. Bioanal. Electrochem., 2019, 11, 1129–1147 CAS
.
- W. Daoudi, A. El Aatiaoui, O. Dagdag, K. Zaidi, R. Haldhar, S.-C. Kim, A. Oussaid, A. Aouinti, A. Berisha, F. Benhiba, E. E. Ebenso and A. Oussaid, Coatings, 2023, 13, 611 CrossRef CAS
.
- M. Benabdellah, M. Benkaddour, B. Hammouti, M. Bendahhou and A. Aouniti, Appl. Surf.
Sci., 2006, 252, 6212–6217 CrossRef CAS
.
- K. Boumhara, F. Bentiss, M. Tabyaoui, J. Costa, J. M. Desjobert, A. Bellaouchou, A. Guenbour, B. Hammouti and S. S. Al-Deyab, Int. J. Electrochem. Sci., 2014, 9, 1187–1206 CrossRef
.
- K. Boumhara, M. Tabyaoui, C. Jama and F. Bentiss, J. Ind. Eng. Chem., 2015, 29, 146–155 CrossRef CAS
.
- A. Hbika, A. Bouyanzer, M. Jalal, N. Setti, E. Loukili, A. Aouniti, Y. Kerroum, I. Warad, B. Hammouti and A. M. Zarrouk, Anal. Bioanal. Electrochem., 2023, 15, 17–35 CAS
.
- M. Ali, B. Kim, K. D. Belfield, D. Norman, M. Brennan and G. S. Ali, Mater. Sci. Eng., C, 2016, 58, 359–365 CrossRef CAS PubMed
.
- A. Belakhdar, H. Ferkous, S. Djellali, R. Sahraoui, H. Lahbib, Y. B. Amor, A. Erto, M. Balsamo and Y. Benguerba, Colloids Surf., A, 2020, 606, 125458 CrossRef CAS
.
- B. A. Al Jahdaly, Arabian J. Chem., 2023, 16, 104411 CrossRef CAS
.
- R. T. Loto, Results Phys., 2018, 8, 172–179 CrossRef
.
- M. Tezeghdenti, L. Dhouibi and N. Etteyeb, J. Bio- Tribo-Corros., 2015, 1, 16 CrossRef
.
- R. Haldhar and D. Prasad, J. Bio- Tribo-Corros., 2020, 6, 48 CrossRef
.
- A. M. Abdel-Gaber, H. T. Rahal and F. T. Beqai, Int. J. Ind. Chem., 2020, 11, 123–132 CrossRef CAS
.
- S. M. Z. Hossain, S. A. Razzak and M. M. Hossain, Arab. J. Sci. Eng., 2020, 45, 7137–7159 CrossRef CAS
.
- A. Bouoidina, M. Chaouch, A. Abdellaoui, A. Lahkimi, B. Hammouti, F. El-Hajjaji, M. Taleb and A. Nahle, Anti-Corros. Methods Mater., 2017, 64, 563–572 CrossRef CAS
.
- P. Parthipan, M. S. AlSalhi, S. Devanesan and A. Rajasekar, Bioprocess Biosyst. Eng., 2021, 44, 1441–1452 CrossRef CAS PubMed
.
- L. El Hattabi, A. A. Guenbour, A. Ballaouchou and M. Tabyaoui, Mor. J. Chem., 2016, 2016, 4 Search PubMed
.
-
S. Maloy, in Brenner's Encyclopedia of Genetics, ed. S. Maloy and K. Hughes, Academic Press, San Diego, 2nd edn, 2013, pp. 108–110. DOI:10.1016/B978-0-12-374984-0.00051-6
.
-
R. Malak, in Introduction to Corrosion, ed. S. Ambrish, IntechOpen, Rijeka, 2023, p. 3. DOI:10.5772/intechopen.109816
.
- D.-Q. Zhang, Q.-R. Cai, L.-X. Gao and K. Y. Lee, Corros. Sci., 2008, 50, 3615–3621 CrossRef CAS
.
- D. G. de Matos and C. C. Furnus, Theriogenology, 2000, 53, 761–771 CrossRef CAS PubMed
.
- M. B. Petrović, M. B. Radovanović, A. T. Simonović, S. M. Milić and M. M. Antonijević, Int. J. Electrochem. Sci., 2012, 7, 9043–9057 CrossRef
.
- A. T. Simonović, M. B. Petrović, M. B. Radovanović, S. M. Milić and M. M. Antonijević, Chem. Pap., 2014, 68, 362–371 Search PubMed
.
- A. Aouniti, K. F. Khaled and B. Hammouti, Int. J. Electrochem. Sci., 2013, 8, 5925–5943 CrossRef CAS
.
- Z. Zhang, G. Yan and L. Ruan, Adv. Mater. Res., 2012, 415–417, 964–967 CAS
.
- D. Bouzidi, A. Chetouani, B. Hammouti, S. Kertit, M. Taleb and S. S. Al-Deyab, Int. J. Electrochem. Sci., 2012, 7, 2334–2348 CrossRef CAS
.
- R. Cui, N. Gu and C. Li, Mater. Corros., 2011, 62, 362–369 CrossRef CAS
.
- A. D. Zapata-Loría and M. A. Pech-Canul, Chem. Eng. Commun., 2014, 201, 855–869 CrossRef
.
- A. A. Muhammad, A. Uzairu, J. F. Iyun and H. Abba, IOSR J. Appl. Chem., 2014, 7, 50–62 CrossRef CAS
.
- H. Ashassi-Sorkhabi, Z. Ghasemi and D. Seifzadeh, Appl. Surf. Sci., 2005, 249, 408–418 CrossRef CAS
.
- A. Yurt, G. Bereket and C. Ogretir, J. Mol. Struct.: THEOCHEM, 2005, 725, 215–221 CrossRef CAS
.
- A. Abdel Nazeer, N. K. Allam, G. I. Youssef and E. A. Ashour, Ind. Eng. Chem. Res., 2011, 50, 8796–8802 CrossRef CAS
.
- G. M. A. El-Hafez and W. A. Badawy, Electrochim. Acta, 2013, 108, 860–866 CrossRef
.
- G. Kilinççeker and H. Demir, Prot. Met. Phys. Chem. Surf., 2013, 49, 788–797 CrossRef
.
- D.-Q. Zhang, Q.-R. Cai, X.-M. He, L.-X. Gao and G.-D. Zhou, Mater. Chem. Phys., 2008, 112, 353–358 CrossRef CAS
.
- G. Kılınççeker and H. Demir, Anti-Corros. Methods Mater., 2013, 60, 134–142 CrossRef
.
- D.-Q. Zhang, H. Wu and L.-X. Gao, Mater. Chem. Phys., 2012, 133, 981–986 CrossRef CAS
.
- H. A. Rahman, A. Moustafa and M. Awad, Int. J. Electrochem. Sci., 2012, 7, 1266–1287 CrossRef
.
- M. Kuruvilla, S. John and A. Joseph, Res. Chem. Intermed., 2013, 39, 3531–3543 CrossRef CAS
.
- D.-Q. Zhang, B. Xie, L.-X. Gao, Q.-R. Cai, H. G. Joo and K. Y. Lee, Thin Solid Films, 2011, 520, 356–361 CrossRef CAS
.
- L. Wang, X. Yin, W. Wang, L. Jin and Z. Li, Int. J. Electrochem. Sci., 2014, 9, 6088–6102 CrossRef
.
- N. V. Makarenko, U. V. Kharchenko and L. A. Zemnukhova, Russ. J. Appl. Chem., 2011, 84, 1362–1365 CrossRef CAS
.
- M. A. Amin and K. Khaled, Corros. Sci., 2010, 52, 1194–1204 CrossRef CAS
.
- D.-Q. Zhang, Q.-R. Cai, X.-M. He, L.-X. Gao and G. S. Kim, Mater. Chem. Phys., 2009, 114, 612–617 CrossRef CAS
.
- K. M. Ismail, Electrochim. Acta, 2007, 52, 7811–7819 CrossRef CAS
.
-
E. A. MacGregor, in Encyclopedia of Physical Science and Technology, ed. R. A. Meyers, Academic Press, New York, 3rd edn, 2003, pp. 207–245. DOI:10.1016/B0-12-227410-5/00064-8
.
-
D. S. Chauhan, M. A. Quraishi, H. Al-Qahtani and M. A. Jafar Mazumder, in Polymeric Corrosion Inhibitors for Greening the Chemical and Petrochemical Industry, 2022, pp. 1–22. DOI:10.1002/9783527835621.ch1
.
- M. H. Shahini, B. Ramezanzadeh and H. E. Mohammadloo, J. Mol. Liq., 2021, 325, 115110 CrossRef CAS
.
- A. Toghan and A. Fawzy, Polymers, 2023, 15(14), 3144 CrossRef CAS PubMed
.
- C. P. Jiménez-Gómez and J. A. Cecilia, Molecules, 2020, 25, 3981 CrossRef PubMed
.
- J. Scheerder, R. Breur, T. Slaghek, W. Holtman, M. Vennik and G. Ferrari, Prog. Org. Coat., 2012, 75, 224–230 CrossRef CAS
.
- M. Moradi, Z. Song and T. Xiao, J. Mater. Sci. Technol., 2018, 34, 2447–2457 CrossRef CAS
.
- M. Mobin and M. Rizvi, Carbohydr. Polym., 2016, 136, 384–393 CrossRef CAS PubMed
.
- P. I. Murungi, A. A. Sulaimon, O. Ssembatya and P. Nwankwo, SPE Nigeria Annual Interntional Conference and Exhibition, SPE, 2022D021S007R003 Search PubMed.
- H. Ashassi-Sorkhabi and A. Kazempour, Carbohydr. Polym., 2020, 237, 116110 CrossRef CAS PubMed
.
- P. A. Rasheed, A. Alfantazi, K. A. Jabbar and K. A. Mahmoud, J. Bio- Tribo-Corros., 2021, 7, 103 CrossRef
.
- Q. H. Zhang, N. Xu, Z. N. Jiang, H. F. Liu and G. A. Zhang, J. Colloid Interface Sci., 2023, 640, 1052–1067 CrossRef CAS PubMed
.
- D. S. Chauhan, M. A. Quraishi, A. A. Sorour, S. K. Saha and P. Banerjee, RSC Adv., 2019, 9, 14990–15003 RSC
.
- N. K. Gupta, P. G. Joshi, V. Srivastava and M. A. Quraishi, Int. J. Biol. Macromol., 2018, 106, 704–711 CrossRef CAS PubMed
.
- M. Srivastava, S. K. Srivastava, G. J. Nikhil and R. Prakash, Int. J. Biol. Macromol., 2019, 140, 177–187 CrossRef CAS PubMed
.
- P. Kong, H. Feng, N. Chen, Y. Lu, S. Li and P. Wang, RSC Adv., 2019, 9, 9211–9217 RSC
.
- R. Menaka and S. Subhashini, Polym. Int., 2017, 66, 349–358 CrossRef CAS
.
- S. A. Umoren, M. J. Banera, T. Alonso-Garcia, C. A. Gervasi and M. V. Mirífico, Cellulose, 2013, 20, 2529–2545 CrossRef CAS
.
- S. N. Dalhatu, K. A. Modu, A. A. Mahmoud, Z. U. Zango, A. B. Umar, F. Usman, J. O. Dennis, A. Alsadig, K. H. Ibnaouf and O. A. Aldaghri, Polymers, 2023, 15, 398 CrossRef CAS PubMed
.
- M. A. Quraishi, K. R. Ansari, D. S. Chauhan, S. A. Umoren and M. A. J. Mazumder, Cellulose, 2020, 27, 6425–6443 CrossRef CAS
.
- W. Zhang, Y. Zhang, B. Li, H. Guo, X. Dou, K. Lu and Y. Feng, Bioelectrochemistry, 2023, 150, 108330 CrossRef CAS PubMed
.
- O. S. I. Fayomi, I. G. Akande and A. P. I. Popoola, J. Bio- Tribo-Corros., 2018, 4, 73 CrossRef
.
- I. G. Arwati, E. H. Majlan, S. Alva and W. Muhammad, Energies, 2022, 15, 8511 CrossRef CAS
.
- K. El Mouaden, B. El Ibrahimi, R. Oukhrib, L. Bazzi, B. Hammouti, O. Jbara, A. Tara, D. S. Chauhan and M. A. Quraishi, Int. J. Biol. Macromol., 2018, 119, 1311–1323 CrossRef CAS PubMed
.
- A. Jmiai, B. El Ibrahimi, A. Tara, R. Oukhrib, S. El Issami, O. Jbara, L. Bazzi and M. Hilali, Cellulose, 2017, 24, 3843–3867 CrossRef CAS
.
- M. N. El-Haddad, Int. J. Biol. Macromol., 2013, 55, 142–149 CrossRef CAS PubMed
.
- A. Jmiai, B. El Ibrahimi, A. Tara, S. El Issami, O. Jbara and L. Bazzi, J. Mol. Struct., 2018, 1157, 408–417 CrossRef CAS
.
-
S. A. Umoren, M. M. Solomon and V. S. Saji, in Polymeric Materials in Corrosion Inhibition, ed. S. A. Umoren, M. M. Solomon and V. S. Saji, Elsevier, 2022, pp. 271–286. DOI:10.1016/B978-0-12-823854-7.00014-X
.
- P. Kesari, G. Udayabhanu, A. Roy and S. pal, J. Ind. Eng. Chem., 2023, 122, 303–325 CrossRef CAS
.
- S. M. Tawfik, RSC Adv., 2015, 5, 104535–104550 RSC
.
- W. Zhang, B. Nie, H.-J. Li, Q. Li, C. Li and Y.-C. Wu, Carbohydr. Polym., 2021, 260, 117842 CrossRef CAS PubMed
.
- I. B. Obot, I. B. Onyeachu and A. M. Kumar, Carbohydr. Polym., 2017, 178, 200–208 CrossRef CAS PubMed
.
- M. Fardioui, M. Rbaa, F. Benhiba, M. Galai, T. Guedira, B. Lakhrissi, I. Warad and A. Zarrouk, J. Mol. Liq., 2021, 323, 114615 CrossRef CAS
.
- M. Mariana, T. Alfatah, H. P. S. Abdul Khalil, E. B. Yahya, N. G. Olaiya, A. Nuryawan, E. M. Mistar, C. K. Abdullah, S. N. Abdulmadjid and H. Ismail, J. Mater. Res. Technol., 2021, 15, 2287–2316 CrossRef CAS
.
- C. Gao, X. Zhao, P. Fatehi, X. Dong, K. Liu, S. Chen, S. Wang and F. Kong, Ind. Crops Prod., 2021, 168, 113585 CrossRef CAS
.
- M. H. Hussin, A. A. Rahim, M. N. Mohamad Ibrahim and N. Brosse, Measurement, 2016, 78, 90–103 CrossRef
.
- Y. Ren, Y. Luo, K. Zhang, G. Zhu and X. Tan, Corros. Sci., 2008, 50, 3147–3153 CrossRef CAS
.
- M. H. Hussin, A. A. Rahim, M. N. Mohamad Ibrahim and N. Brosse, Mater. Chem. Phys., 2015, 163, 201–212 CrossRef CAS
.
- J. J. Liao, N. H. A. Latif, D. Trache, N. Brosse and M. H. Hussin, Int. J. Biol. Macromol., 2020, 162, 985–1024 CrossRef CAS PubMed
.
- L. Huang, W.-Q. Chen, S.-S. Wang, Q. Zhao, H.-J. Li and Y.-C. Wu, Environ. Chem. Lett., 2022, 20, 3235–3264 CrossRef CAS
.
- R. C. Cioc, E. Ruijter and R. V. Orru, Green Chem., 2014, 16, 2958–2975 RSC
.
- M. Palomar-Pardavé, M. Romero-Romo, H. Herrera-Hernández, M. Abreu-Quijano, N. V. Likhanova, J. Uruchurtu and J. Juárez-García, Corros. Sci., 2012, 54, 231–243 CrossRef
.
- A. Singh, S. Thakur, B. Pani and G. Singh, New J. Chem., 2018, 42, 2113–2124 RSC
.
- J. Haque, V. Srivastava, D. S. Chauhan, H. Lgaz and M. A. Quraishi, ACS Omega, 2018, 3, 5654–5668 CrossRef CAS PubMed
.
- K. Ansari, M. Quraishi and A. Singh, Corros. Sci., 2015, 95, 62–70 CrossRef CAS
.
- A. Singh, K. Ansari, P. Bedi, T. Pramanik, I. H. Ali, Y. Lin, P. Banerjee and S. Zamindar, J. Phys. Chem. Solids, 2023, 172, 111064 CrossRef CAS
.
- C. B. Verma, M. Quraishi and A. Singh, J. Taiwan Inst. Chem. Eng., 2015, 49, 229–239 CrossRef CAS
.
- A. Singh, S. Thakur, B. Pani, B. Chugh, H. Lgaz, I.-M. Chung, P. Chaubey, A. Pandey and J. Singh, J. Mol. Liq., 2019, 283, 788–803 CrossRef CAS
.
- S. B. Aoun and M. Messali, Int. J. Electrochem. Sci., 2018, 13, 3757–3776 CrossRef CAS
.
- D. S. Chauhan, M. J. Mazumder, M. Quraishi, K. Ansari and R. Suleiman, Int. J. Biol. Macromol., 2020, 158, 231–243 CrossRef CAS PubMed
.
- J. Haque, V. Srivastava, D. Chauhan, H. Lgaz and M. Quraishi, ACS Omega, 2018, 3(5), 5654–5668 CrossRef CAS PubMed
.
- C. Verma, P. Singh, I. Bahadur, E. Ebenso and M. Quraishi, J. Mol. Liq., 2015, 209, 767–778 CrossRef
.
- P. Singh, M. Quraishi, E. Ebenso and C. B. Verma, Int. J. Electrochem. Sci., 2014, 9, 7446–7459 CrossRef CAS
.
- A. K. Singh, S. Thakur, B. Pani, E. E. Ebenso, M. A. Quraishi and A. K. Pandey, ACS Omega, 2018, 3, 4695–4705 CrossRef CAS PubMed
.
- A. Khanra, M. Srivastava, M. P. Rai and R. Prakash, ACS Omega, 2018, 3, 12369–12382 CrossRef CAS PubMed
.
- N. Palaniappan, I. Cole, F. Caballero-Briones, S. Manickam, K. J. Thomas and D. Santos, RSC Adv., 2020, 10, 5399–5411 RSC
.
- X. Luo, R. Bai, D. Zhen, Z. Yang, D. Huang, H. Mao, X. Li, H. Zou, Y. Xiang and K. Liu, Ind. Crops Prod., 2019, 129, 405–413 CrossRef CAS
.
- C. He, X.-Q. Li, G.-L. Feng and W.-J. Long, Green Chem., 2022, 24, 5842–5855 RSC
.
- B. A. Al Jahdaly, M. F. Elsadek, B. M. Ahmed, M. F. Farahat, M. M. Taher and A. M. Khalil, Sustainability, 2021, 13, 2127 CrossRef
.
- Y. Ye, D. Zhang, Y. Zou, H. Zhao and H. Chen, J. Cleaner Prod., 2020, 264, 121682 CrossRef CAS
.
- Z. Xu, Y. Gan, J. Zeng, J. Chen, A. Fu, X. Zheng and W. Li, Chem. Eng. J., 2023, 144425 CrossRef CAS
.
- A. Mishra, C. Verma, S. Chauhan, M. Quraishi, E. E. Ebenso and V. Srivastava, J. Bio- Tribo-Corros., 2018, 4, 1–15 CrossRef CAS
.
-
K. Tanaka, Solvent-free organic synthesis, John Wiley & Sons, 2009 Search PubMed
.
- K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074 CrossRef CAS PubMed
.
- R. Dubadi and M. Jaroniec, Nanomaterials, 2023, 13, 2262 CrossRef CAS PubMed
.
- J. F. Fernandez-Bertran, Pure Appl. Chem., 1999, 71, 581–586 CrossRef CAS
.
-
K. Tkáčová, Mechanical activation of minerals, Elsevier, Amsterdam, Tokyo New York, NY, 1989 Search PubMed
.
-
A. D. McNaught and A. Wilkinson, Compendium of Chemical Terminology: IUPAC Recommendations, IUPAC Chemical Data Series, 1997 Search PubMed
.
- S. L. James and T. Friščić, Chem. Soc. Rev., 2013, 42, 7494–7496 RSC
.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., 2020, 132, 1030–1041 CrossRef
.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed
.
- A. Stolle, T. Szuppa, S. E. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC
.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Nat. Rev. Chem., 2023, 7, 51–65 CrossRef CAS PubMed
.
- R. R. Bolt, J. A. Leitch, A. C. Jones, W. I. Nicholson and D. L. Browne, Chem. Soc. Rev., 2022, 51, 4243–4260 RSC
.
- R. Laskar, T. Pal, T. Bhattacharya, S. Maiti, M. Akita and D. Maiti, Green Chem., 2022, 24, 2296–2320 RSC
.
- F. Penteado, B. Monti, L. Sancineto, G. Perin, R. G. Jacob, C. Santi and E. J. Lenardão, Asian J. Org. Chem., 2018, 7, 2368–2385 CrossRef CAS
.
-
T. Kimura, in Sonochemistry and the Acoustic Bubble, Elsevier, 2015, ch. 171, pp. 171–186 Search PubMed
.
- D. C. Martin, J. Chen, J. Yang, L. F. Drummy and C. Kübel, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 1749–1778 CrossRef CAS
.
- A. Tiusanen, J. Ruiz-Jimenez, K. Hartonen and S. K. Wiedmer, Environ. Sci.: Process. Impacts, 2023, 25, 1263–1287 CAS
.
- S. Hwang, S. Grätz and L. Borchardt, Chem. Commun., 2022, 58, 1661–1671 RSC
.
- K. Vanchinathan, G. Bhagavannarayana, K. Muthu and S. Meenakshisundaram, Phys. B, 2011, 406, 4195–4199 CrossRef CAS
.
-
A. I. Vogel, Practical Organic Chemistry, 5th edn, 1956 Search PubMed
.
- B. Ahmed, R. A. Khan and M. Keshari, Tetrahedron Lett., 2009, 50, 2889–2892 CrossRef CAS
.
- N. K. Gupta, C. Verma, R. Salghi, H. Lgaz, A. Mukherjee and M. Quraishi, New J. Chem., 2017, 41, 13114–13129 RSC
.
- C. Verma, M. Quraishi, E. Ebenso, I. Obot and A. El Assyry, J. Mol. Liq., 2016, 219, 647–660 CrossRef CAS
.
- C. Verma, P. Singh and M. Quraishi, J. Assoc. Arab Univ. Basic Appl. Sci., 2016, 21, 24–30 Search PubMed
.
- H. G. Alvim, E. N. da Silva Junior and B. A. Neto, RSC Adv., 2014, 4, 54282–54299 RSC
.
- M. Puripat, R. Ramozzi, M. Hatanaka, W. Parasuk, V. Parasuk and K. Morokuma, J. Org. Chem., 2015, 80, 6959–6967 CrossRef CAS PubMed
.
- A. Stadler and C. O. Kappe, J. Comb. Chem., 2001, 3, 624–630 CrossRef CAS PubMed
.
- C. Verma, J. Haque, M. A. Quraishi and E. E. Ebenso, J. Mol. Liq., 2018, 275, 18–40 CrossRef
.
-
M. A. Quraishi, D. S. Chauhan and V. S. Saji, Heterocyclic Organic Corrosion Inhibitors: Principles and Applications, Elsevier Inc., Amsterdam, 2020 Search PubMed
.
-
C. Verma, M. A. Quraishi and D. S. Chauhan, Green Corrosion Inhibition: Fundamentals, Design, Synthesis and Applications, Royal Society of Chemistry, 2022 Search PubMed
.
- P. Singh, E. E. Ebenso, L. O. Olasunkanmi, I. Obot and M. A. Quraishi, J. Phys. Chem. C, 2016, 120, 3408–3419 CrossRef CAS
.
- C. G. Neochoritis, T. Zarganes-Tzitzikas, K. Katsampoxaki-Hodgetts and A. Dömling, J. Chem. Educ., 2020, 97, 3739–3745 CrossRef CAS PubMed
.
- N. Isambert, M. d. M. S. Duque, J.-C. Plaquevent, Y. Genisson, J. Rodriguez and T. Constantieux, Chem. Soc. Rev., 2011, 40, 1347–1357 RSC
.
- S. Vidyacharan, A. H. Shinde, B. Satpathi and D. S. Sharada, Green Chem., 2014, 16, 1168–1175 RSC
.
- C. S. Graebin, F. V. Ribeiro, K. R. Rogério and A. E. Kümmerle, Curr. Org. Synth., 2019, 16, 855–899 CrossRef CAS PubMed
.
- C. Verma, M. Quraishi, K. Kluza, M. Makowska-Janusik, L. O. Olasunkanmi and E. E. Ebenso, Sci. Rep., 2017, 7, 44432 CrossRef CAS PubMed
.
- C. Verma, L. O. Olasunkanmi, E. E. Ebenso, M. A. Quraishi and I. Obot, J. Phys. Chem. C, 2016, 120, 11598–11611 CrossRef CAS
.
- D. K. Yadav, B. Maiti and M. A. Quraishi, Corros. Sci., 2010, 52, 3586–3598 CrossRef CAS
.
- J. Haque, K. Ansari, V. Srivastava, M. A. Quraishi and I. Obot, J. Ind. Eng. Chem., 2017, 49, 176–188 CrossRef CAS
.
- R. González-Olvera, V. Román-Rodríguez, G. E. Negrón-Silva, A. Espinoza-Vázquez, F. J. Rodríguez-Gómez and R. Santillan, Molecules, 2016, 21, 250 CrossRef PubMed
.
- K. R. Ansari, M. A. Quraishi and A. Singh, Measurement, 2015, 76, 136–147 CrossRef
.
- T. Kitanosono, K. Masuda, P. Xu and S. Kobayashi, Chem. Rev., 2018, 118, 679–746 CrossRef CAS PubMed
.
- R. A. Sheldon and D. Brady, ChemSusChem, 2019, 12, 2859–2881 CrossRef CAS PubMed
.
- B. H. Lipshutz, S. Ghorai and M. Cortes-Clerget, Chem. – Eur. J., 2018, 24, 6672–6695 CrossRef CAS PubMed
.
- T. Kitanosono and S. Kobayashi, Chem. – Eur. J., 2020, 26, 9408–9429 CrossRef CAS PubMed
.
- F. Zhou, Z. Hearne and C.-J. Li, Curr. Opin. Green Sustain. Chem., 2019, 18, 118–123 CrossRef
.
- T. Sahoo, J. Panda, J. Sahu, D. Sarangi, S. K. Sahoo, B. Nanda and R. Sahu, Curr. Org. Synth., 2020, 17, 426–439 CrossRef CAS PubMed
.
- E. Breynaert, M. Houlleberghs, S. Radhakrishnan, G. Grübel, F. Taulelle and J. A. Martens, Chem. Soc. Rev., 2020, 49, 2557–2569 RSC
.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed
.
- C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC
.
- C. Verma, M. A. Quraishi and E. E. Ebenso, Sustainable Chem. Pharm., 2018, 10, 134–147 CrossRef
.
- P. Dohare, K. Ansari, M. Quraishi and I. Obot, J. Ind. Eng. Chem., 2017, 52, 197–210 CrossRef CAS
.
- C. Verma, L. Olasunkanmi, I. Obot, E. E. Ebenso and M. A. Quraishi, RSC Adv., 2016, 6, 15639–15654 RSC
.
- K. R. Ansari, Sudheer, A. Singh and M. A. Quraishi, J. Dispersion Sci. Technol., 2015, 36, 908–917 CrossRef CAS
.
- C. Verma, M. A. Quraishi and A. Singh, J. Taibah Univ. Sci., 2016, 10, 718–733 CrossRef
.
- P. Singh, V. Srivastava and M. A. Quraishi, J. Mol. Liq., 2016, 216, 164–173 CrossRef CAS
.
- M. Tobiszewski, Anal. Methods, 2016, 8, 2993–2999 RSC
.
- F. Pena-Pereira, A. Kloskowski and J. Namieśnik, Green Chem., 2015, 17, 3687–3705 RSC
.
- T. Khezeli, A. Daneshfar and R. Sahraei, Talanta, 2016, 150, 577–585 CrossRef CAS PubMed
.
- Ł. Marcinkowski, F. Pena-Pereira, A. Kloskowski and J. Namieśnik, TrAC, Trends Anal. Chem., 2015, 72, 153–168 CrossRef
.
- D. L. Rocha, A. D. Batista, F. R. Rocha, G. L. Donati and J. A. Nobrega, TrAC, Trends Anal. Chem., 2013, 45, 79–92 CrossRef CAS
.
- R. K. Henderson, A. P. Hill, A. M. Redman and H. F. Sneddon, Green Chem., 2015, 17, 945–949 RSC
.
- T. R. Sekharan, R. M. Chandira, S. Tamilvanan, S. Rajesh and B. Venkateswarlu, Biointerface Res. Appl. Chem., 2022, 12, 847–860 CAS
.
- S. V. Dzyuba and R. A. Bartsch, Angew. Chem., Int. Ed., 2003, 42, 148–150 CrossRef CAS PubMed
.
- H. Zhao, S. Xia and P. Ma, J. Chem. Technol. Biotechnol., 2005, 80, 1089–1096 CrossRef CAS
.
- C. G. Yoo, Y. Pu and A. J. Ragauskas, Curr. Opin. Green Sustain. Chem., 2017, 5, 5–11 CrossRef
.
-
D. S. Chauhan, F. El-Hajjaji and M. Quraishi, in Ionic Liquid-Based Technologies for Environmental Sustainability, 2022, ch. 18, pp. 279–294. DOI:10.1016/B978-0-12-824545-3.00018-0
.
- X. Zeng, X. Zheng, L. Guo, Q. Xu, H. Huang and B. Tan, J. Mol. Liq., 2021, 324, 115063 CrossRef CAS
.
- D. Lozowski, Chem. Eng., 2010, 117, 15 Search PubMed
.
- X. Meng, Y. Wang, A. J. Conte, S. Zhang, J. Ryu, J. J. Wie, Y. Pu, B. H. Davison, C. G. Yoo and A. J. Ragauskas, Bioresour. Technol., 2022, 368, 128280 CrossRef PubMed
.
- S. Dahiya, A. N. Kumar, J. S. Sravan, S. Chatterjee, O. Sarkar and S. V. Mohan, Bioresour. Technol., 2018, 248, 2–12 CrossRef CAS PubMed
.
- F. Nemati, M. M. Hosseini and H. Kiani, J. Saudi Chem. Soc., 2016, 20, S503–S508 CrossRef CAS
.
- M. A. Quraishi, K. R. Ansari, D. K. Yadav and E. E. Ebenso, Int. J. Electrochem. Sci., 2012, 7, 12301–12315 CrossRef CAS
.
- P. Singh, A. Singh and M. A. Quraishi, J. Taiwan Inst. Chem. Eng., 2016, 60, 588–601 CrossRef CAS
.
- I. B. Onyeachu, D. S. Chauhan, M. Quraishi and I. Obot, Corros. Eng., Sci. Technol., 2020, 56, 154–161 CrossRef
.
- P. Singh, D. S. Chauhan, S. S. Chauhan, G. Singh and M. A. Quraishi, J. Mol. Liq., 2019, 298, 112051 CrossRef
.
- M. Vafaeezadeh and H. Alinezhad, J. Mol. Liq., 2016, 218, 95–105 CrossRef CAS
.
- R. A. Sheldon, J. Mol. Catal. A: Chem., 2016, 422, 3–12 CrossRef CAS
.
- C. Verma, L. Olasunkanmi, I. B. Obot, E. E. Ebenso and M. A. Quraishi, RSC Adv., 2016, 6, 15639–15654 RSC
.
- M. Quraishi and R. Sardar, Corrosion, 2002, 58, 748–755 CrossRef CAS
.
- C. Verma, M. A. Quraishi, L. Olasunkanmi and E. E. Ebenso, RSC Adv., 2015, 5, 85417–85430 RSC
.
- D. K. Yadav and M. A. Quraishi, Ind. Eng. Chem. Res., 2012, 51, 14966–14979 CrossRef CAS
.
- M. Bahrami, S. Hosseini and P. Pilvar, Corros. Sci., 2010, 52, 2793–2803 CrossRef CAS
.
- M. Kurian and A. Paul, Carbon Trends, 2021, 3, 100032 CrossRef CAS
.
- N. Chaubey, A. Qurashi, D. S. Chauhan and M. Quraishi, J. Mol. Liq., 2020, 114385, DOI:10.1016/j.molliq.2020.114385
.
- K. E. Mouaden, D. S. Chauhan, M. Quraishi, L. Bazzi and M. Hilali, Int. J. Biol. Macromol., 2020, 164, 3709–3717 CrossRef PubMed
.
- M. A. Quraishi, D. S. Chauhan and V. S. Saji, J. Mol. Liq., 2021, 341, 117265 CrossRef CAS
.
- OECD, Test No. 301: Ready Biodegradability, OECD Guidelines for the Testing of Chemicals, Section 3, 1992, DOI:10.1787/9789264070349-en.
- OECD, Guidance Document on Acute Oral Toxicity Testing, Organization for Economic Cooperation and Development, Paris, France, 1987, DOI:10.1787/20777876.
- OECD, Test No. 117: Partition Coefficient (n-octanol/water), HPLC Method, 2004, DOI:10.1787/9789264069824-en.
- D. S. Chauhan, M. Quraishi and A. Qurashi, J. Mol. Liq., 2021, 326, 115117 CrossRef CAS
.
- C. T. Ser, P. Žuvela and M. W. Wong, Appl. Surf. Sci., 2020, 512, 145612 CrossRef
.
- K. Rasheeda, D. Vijaya, P. Krishnaprasad and S. Samshuddin, Int. J. Corros. Scale Inhib., 2018, 7, 48–61 CAS
.
- M. Yadav, R. R. Sinha, T. K. Sarkar and N. Tiwari, J. Adhes. Sci. Technol., 2015, 29, 1690–1713 CrossRef CAS
.
- M. B. P. Mihajlović, M. B. Radovanović, ŽZ. Tasić and M. M. Antonijević, J. Mol. Liq., 2017, 225, 127–136 CrossRef
.
- M. ElBelghiti, Y. Karzazi, A. Dafali, B. Hammouti, F. Bentiss, I. Obot, I. Bahadur and E.-E. Ebenso, J. Mol. Liq., 2016, 218, 281–293 CrossRef CAS
.
- J. Seetharaman, E. A. Reny, D. A. Johnson, K. B. Sawant and V. Sivaswamy, Google Patents, 2021 Search PubMed
.
- M. A. Quraishi, W. Khan and M. Ajmal, J. Electrochem. Soc. India, 1997, 46, 133–138 CAS
.
- M. A. Quraishi, W. Khan, M. Ajmal, S. Muralidharan and S. V. Iyer, J. Appl. Electrochem., 1996, 26, 1253–1258 CrossRef CAS
.
- M. Quraishi and F. A. Ansari, J. Appl. Electrochem., 2006, 36, 309–314 CrossRef CAS
.
- M. Albini, P. Letardi, L. Mathys, L. Brambilla, J. Schröter, P. Junier and E. Joseph, Corros. Sci., 2018, 143, 84–92 CrossRef CAS
.
- C. Gattinoni and A. Michaelides, Faraday Discuss., 2015, 180, 439–458 RSC
.
- I. Obot and U. M. Edouk, J. Mol. Liq., 2017, 246, 66–90 CrossRef CAS
.
- I. Obot, A. Madhankumar, S. Umoren and Z. Gasem, J. Adhes. Sci. Technol., 2015, 29, 2130–2152 CrossRef CAS
.
- K. R. Ansari, S. Ramkumar, D. S. Chauhan, M. Salman, D. Nalini, V. Srivastava and M. A. Quraishi, Int. J. Corros. Scale Inhib., 2018, 7, 443–459 CAS
.
- S. Hadisaputra, S. Hamdiani, M. A. Kurniawan and N. Nuryono, Indones. J. Chem., 2017, 17, 431–438 CrossRef CAS
.
- D. S. Chauhan, M. Quraishi, V. Srivastava, J. Haque and B. El ibrahimi, J. Mol. Struct., 2021, 1226, 129259 CrossRef CAS
.
- A. Jha and A. Kumar, Bioprocess Biosyst. Eng., 2019, 42, 1893–1901 CrossRef CAS PubMed
.
- S. A. Umoren, M. M. Solomon, A. Madhankumar and I. B. Obot, Carbohydr. Polym., 2020, 230, 115466 CrossRef CAS PubMed
.
- S. A. Umoren and M. M. Solomon, Prog. Mater. Sci., 2019, 104, 380–450 CrossRef CAS
.
- D. S. Chauhan, M. J. Mazumder, M. A. Quraishi, K. Ansari and R. Suleiman, Int. J. Biol. Macromol., 2020, 158, 231–243 CrossRef CAS PubMed
.
- D. S. Chauhan, M. J. Mazumder, M. A. Quraishi and K. Ansari, Int. J. Biol. Macromol., 2020, 158, 127–138 CrossRef CAS PubMed
.
- M. A. Quraishi, K. R. Ansari, D. S. Chauhan, S. A. Umoren and M. A. J. Mazumder, Cellulose, 2020, 27, 6425–6443 CrossRef CAS
.
- S. A. Al Kiey, M. S. Hasanin and S. Dacrory, J. Mol. Liq., 2021, 338, 116604 CrossRef CAS
.
- H. M. Abd El-Lateef, W. Albokheet and M. Gouda, Cellulose, 2020, 27, 8039–8057 CrossRef
.
- C. Wang, C. Zou and Y. Cao, J. Mol. Struct., 2021, 1228, 129737 CrossRef CAS
.
- A. Altin, M. Krzywiecki, A. Sarfraz, C. Toparli, C. Laska, P. Kerger, A. Zeradjanin, K. J. Mayrhofer, M. Rohwerder and A. Erbe, Beilstein J. Nanotechnol., 2018, 9, 936–944 CrossRef CAS PubMed
.
- L. Sihem, B. Abderrahim, B. Brahim, M. Asma, T. Lahcene and S. Marzorati, Appl. Sci., 2019, 9, 4684 CrossRef
.
- E. D. Paul, A. F. Egbuniwe and P. A. Ekwumemgbor, ATBU J. Sci., Technol. Educ., 2018, 6, 176–182 Search PubMed
.
- M. M. Solomon, H. Gerengi and S. A. Umoren, ACS Appl. Mater. Interfaces, 2017, 9, 6376–6389 CrossRef CAS PubMed
.
- M. M. Solomon, H. Gerengi, T. Kaya and S. A. Umoren, ACS Sustainable Chem. Eng., 2016, 5, 809–820 CrossRef
.
- D. S. Chauhan, A. M. Kumar and M. A. Quraishi, Chem. Eng. Res. Des., 2019, 150, 99–115 CrossRef CAS
.
- I. B. Onyeachu, D. S. Chauhan, K. R. Ansari, I. Obot, M. A. Quraishi and A. H. Alamri, New J. Chem., 2019, 43, 7282–7293 RSC
.
- J. Haque, V. Srivastava, M. A. Quraishi, D. S. Chauhan, H. Lgaz and I.-M. Chung, Corros. Sci., 2020, 172, 108665 CrossRef CAS
.
- J. Haque, V. Srivastava, C. Verma and M. A. Quraishi, J. Mol. Liq., 2017, 225, 848–855 CrossRef CAS
.
- F. El-Hajjaji, R. Salim, M. Taleb, F. Benhiba, N. Rezki, D. S. Chauhan and M. Quraishi, Surf. Interfaces, 2021, 22, 100881 CrossRef
.
- F. El-Hajjaji, E. Ech-chihbi, N. Rezki, F. Benhiba, M. Taleb, D. S. Chauhan and M. Quraishi, J. Mol. Liq., 2020, 314, 113737 CrossRef CAS
.
-
M. Fisher, in Lehninger Principles of Biochemistry, ed. D. L. Nelson and M. M. Cox, 2001 Search PubMed
.
- G. Gece, Corros. Sci., 2011, 53, 3873–3898 CrossRef CAS
.
- C. Verma, D. S. Chauhan and M. A. Quraishi, J. Mater. Environ. Sci., 2017, 8, 4040–4051 CAS
.
- P. Dohare, D. S. Chauhan, A. A. Sorour and M. A. Quraishi, Mater. Discovery, 2017, 9, 30–41 CrossRef
.
- P. Dohare, D. S. Chauhan, B. Hammouti and M. A. Quraishi, Anal. Bioanal. Electrochem., 2017, 9, 762 CAS
.
- P. Singh, D. S. Chauhan, K. Srivastava, V. Srivastava and M. A. Quraishi, Int. J. Ind. Chem., 2017, 8, 363–372 CrossRef CAS
.
- P. Dohare, D. S. Chauhan and M. A. Quraishi, Int. J. Corros. Scale Inhib., 2018, 7, 25–37 CAS
.
- N. Vaszilcsin, V. Ordodi and A. Borza, Int. J. Pharm., 2012, 431, 241–244 CrossRef CAS PubMed
.
- J. Salimon, N. Salih and E. Yousif, Arabian J. Chem., 2012, 5, 135–145 CrossRef CAS
.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC
.
- R. Aslam, M. Mobin, J. Aslam, A. Aslam, S. Zehra and S. Masroor, Adv. Colloid Interface Sci., 2021, 102481 CrossRef CAS PubMed
.
- Y. Zhu, M. L. Free, R. Woollam and W. Durnie, Prog. Mater. Sci., 2017, 90, 159–223 CrossRef CAS
.
- M. Deyab, J. Mol. Liq., 2020, 309, 113107 CrossRef CAS
.
- S. Gurjar, S. K. Sharma, A. Sharma and S. Ratnani, Appl. Surf. Sci. Adv., 2021, 6, 100170 CrossRef
.
- M. A. Quraishi and J. Rawat, Corros. Rev., 2001, 19, 273–299 CAS
.
- M. Ajmal, J. Rawat and M. A. Quraishi, Anti-Corros. Methods Mater., 1998, 45, 419–425 CrossRef CAS
.
- A. Singh, Y. Lin, I. Obot, E. E. Ebenso, K. Ansari and M. A. Quraishi, Appl. Surf. Sci., 2015, 356, 341–347 CrossRef CAS
.
- F. Bentiss, M. Lebrini, H. Vezin, F. Chai, M. Traisnel and M. Lagrené, Corros. Sci., 2009, 51, 2165–2173 CrossRef CAS
.
- M. Lebrini, M. Lagrenée, H. Vezin, M. Traisnel and F. Bentiss, Corros. Sci., 2007, 49, 2254–2269 CrossRef CAS
.
- A. M. El-Shamy and S. M. Mouneir, J. Bio- Tribo-Corros., 2023, 9, 3 CrossRef
.
- N. Vaszilcsin, A. Kellenberger, M. L. Dan, D. A. Duca and V. L. Ordodi, Materials, 2023, 16, 5555 CrossRef CAS PubMed
.
- S. Tanwer and S. K. Shukla, Curr. Res. Green Sustainable Chem., 2022, 5, 100227 CrossRef CAS
.
- C. N. Njoku, T. U. Maduoma, W. Emori, R. E. Odey, B. M. Unimke, E. Yakubu, C. C. Anorondu, D. I. Udunwa, O. C. Njoku and K. B. Oyoh, Pigm. Resin Technol., 2023 DOI:10.1108/PRT-07-2023-0063
.
-
S. Sharma, R. Ganjoo, S. Kumar and A. Kumar, International Conference on Chemical, Bio and Environmental Engineering, Chm: Springer International Publishing, 2021, pp. 1071-1082 Search PubMed
.
- M. Ajiriyanto, S. Yudanto and F. Susetyo, Int. J. Corros. Scale Inhib., 2023, 12, 258–274 Search PubMed
.
- A. I. Ikeuba, J. E. Ntibi, P. C. Okafor, B. I. Ita, A. U. Agobi, F. C. Asogwa, B. J. Omang, E. A. Eno, H. Loius and S. A. Adalikwu, Results Chem., 2023, 5, 100909 CrossRef CAS
.
- F. Han, Z. Gong, R. Wang and S. Dang, Int. J. Electrochem. Sci., 2023, 18, 100319 CrossRef CAS
.
- H. Dong, L. Ding, B. Ran, Y. Song, R. Wang, L. Zhao and Y. Niu, Int. J. Electrochem. Sci., 2023, 100250 CrossRef CAS
.
- S. El Harrari, S. Ayoub, D. Takky and Y. Naimi, J. Electrochem. Sci. Eng., 2023 DOI:10.5599/jese.1867
.
- K. A. Alamry, A. Khan, J. Aslam, M. A. Hussein and R. Aslam, Sci. Rep., 2023, 13, 6724 CrossRef CAS PubMed
.
- A. N. Abd and E. S. Nasif, AIP Conf. Proc., 2023, 2475(1), 040003 CrossRef CAS
.
- C. Merimi, B. Hammouti, K. Zaidi, B. Hafez, H. Elmsellem, R. Touzani and S. Kaya, J. Mol. Struct., 2023, 1278, 134883 CrossRef CAS
.
- M. Abdallah, K. A. Soliman, M. Alfakeer, H. Hawsawi, A. M. Al-bonayan, S. S. Al-Juaid, S. Abd El Wanees and M. S. Motawea, ACS Omega, 2023, 8(38), 34516–34533 CrossRef CAS PubMed
.
- M. Hammi, C. Lazrak, Y. Ziat, O. Ifguis and H. Belkhanchi, S. Afr. J. Chem. Eng., 2023, 44, 265–275 Search PubMed
.
- G. Swetha and H. Sachin, Inorg. Chem. Commun., 2023, 155, 111082 CrossRef CAS
.
- G. Swetha, H. Sachin and J. R. Choudhuri, Emergent Mater., 2023, 1–20 Search PubMed
.
- L. C. Isaiah and N. B. Iroha, World Sci. News, 2023, 177, 51–67 CAS
.
- R. Narang, P. Vashishth, H. Bairagi, S. K. Shukla and B. Mangla, J. Mol. Liq., 2023, 384, 122277 CrossRef CAS
.
- M. M. Motawea and S. Melhi, J. Indian Chem. Soc., 2023, 100, 101013 CrossRef CAS
.
- A. Belafhaili, M. El Hawary, A. Bellaouchou, A. Guenbour, I. Warad and A. M. Zarrouk, Anal. Bioanal. Electrochem., 2023, 15, 118–136 CAS
.
- F. Abeng, B. Ita, V. Anadebe, V. Chukwuike, K. Etiowo, P. Nkom, O. Ekerenam, N. Iroha and I. Ikot, Results Eng., 2023, 17, 100924 CrossRef CAS
.
- N. B. Iroha, V. C. Anadebe, N. J. Maduelosi, L. A. Nnanna, L. C. Isaiah, O. Dagdag, A. Berisha and E. E. Ebenso, Colloids Surf., A, 2023, 660, 130885 CrossRef CAS
.
- A. I. Ikeuba, A. U. Agobi, L. Hitler, B. J. Omang, F. C. Asogwa, I. Benjamin, T. Unimuke and M. C. Udoinyang, Chem. Afr., 2023, 6, 983–997 CrossRef CAS
.
- S. Abd El Maksoud, A. el Aziz Fouda and H. Badawy, 2023, DOI:10.21203/rs.3.rs-3306604/vl.
- N. B. Iroha, N. J. Maduelosi and L. A. Nnanna, Emergent Mater., 2023, 6, 137–146 CrossRef CAS
.
- S. Sharma, R. Ganjoo, A. Thakur, H. Assad, S. Kaya and A. Kumar, AIP Conf. Proc., 2023, 2800, 020024 CrossRef
.
- L. Huang, W. Liu, J. Shen and Q. Liao, Thin Solid Films, 2023, 782, 140005 CrossRef CAS
.
- S. Eid, K. A. Soliman, A. El-Etre, E. Gad and H. Nady, J. Bio- Tribo-Corros., 2023, 9, 61 CrossRef
.
- A. A. Keshk, N. H. Elsayed, F. M. Almutairi, M. Al-Anazi, S. Said, H. M. Althurwi, R. K. Albalawi and M. El-Aassar, Biomass Convers. Biorefin., 2023, 1–14 Search PubMed
.
- M. Deyab, O. A. El-Shamy, H. K. Thabet and A. M. Ashmawy, Sci. Rep., 2023, 13, 8680 CrossRef CAS PubMed
.
- B. Nie, J. Yan, S. Shi, L.-J. Wang, Y.-C. Wu and H.-J. Li, J. Mater. Res. Technol., 2023, 23, 3665–3675 CrossRef CAS
.
- R. S. A. Hameed, S. Obeidat, M. Qureshi, S. Al-Mhyawi, E. H. Aljuhani and M. Abdallah, J. Mater. Res. Technol., 2022, 21, 2743–2756 CrossRef CAS
.
- R. Abdel Hameed, M. Faride, M. Othman, B. Huwaimel, S. Al-Mhyawi, A. Shamroukh, F. Alshammary, E. Aljuhani and M. Abdallah, Green Chem. Lett. Rev., 2022, 15, 847–862 CrossRef CAS
.
- R. K. Mehta and M. Yadav, Mater. Sci. Eng., B, 2023, 295, 116566 CrossRef CAS
.
- L. Feng, S. Zhang, Y. Zhou, R. Pan, H. Du, F. Liu and Y. Yang, Crystals, 2023, 13, 205 CrossRef CAS
.
- I. Martinović, Z. Pilić, G. Zlatić, V. Soldo and M. Šego, Int. J. Electrochem. Sci., 2023, 100238 CrossRef
.
- M. R. Barrodi, A. Mirzaee, A. Kafashan, S. Zahedifard, H. J. Majidi, A. Davoodi and S. Hosseinpour, Mater. Today Commun., 2023, 34, 105390 CrossRef CAS
.
- S. A. Mrani, N. Arrousse, R. Haldhar, A. A. Lahcen, A. Amine, T. Saffaj, S.-C. Kim and M. Taleb, Lubricants, 2022, 10, 43 CrossRef CAS
.
- C. Beltran-Perez, A. A. Serrano, G. Solís-Rosas, A. Martínez-Jiménez, R. Orozco-Cruz, A. Espinoza-Vázquez and A. Miralrio, Int. J. Mol. Sci., 2022, 23, 5086 CrossRef CAS PubMed
.
- F. E. Abeng and V. C. Anadebe, Comput. Theor. Chem., 2023, 114334 CrossRef CAS
.
- A. Ehsani and E. Kamali Ardakani, J. Bio- Tribo-Corros., 2022, 8, 93 CrossRef
.
- S. Hossain, S. Razzak and M. Hossain, Arab. J. Sci. Eng., 2020, 45, 7137–7159 CrossRef CAS
.
- A. Hamdouch, A. Anejjar, L. Bijla, S. Gharby, A. Asdadi, B. Chebli, R. Salghi and L. I. Hassani, Mor. J. Chem., 2023, 11(1), 105–118 CAS
.
- M. Kemel, D. Zama, S. Benayache, J.-C. Chalchat, G. Figueredo, P. Chalard and F. Benayache, Chem. Afr., 2022, 5, 1015–1025 CrossRef CAS
.
- S. Cherrad, A. A. Alrashdi, H.-S. Lee, H. Lgaz, B. Satrani, M. Ghanmi and A. Chaouch, Arabian J. Chem., 2022, 15, 103849 CrossRef CAS
.
- F. Simescu-Lazar, S. Slaoui, M. Essahli, F. Bohr, A. Lamiri, L. Vanoye and J. P. Chopart, Lubricants, 2023, 11, 56 CrossRef CAS
.
- K. Dahmani, M. Galai, A. Ech-chebab, M. Ouakki, L. Kadiri, A. Elgendy, R. Ez-Zriouli and M. Cherkaoui, J. Appl. Electrochem., 2022, 52, 1629–1646 CrossRef CAS
.
- L. Koursaoui, Y. Kerroum, M. Tabyaoui, A. Guenbour, A. Bellaouchou, A. M. Zarrouk, I. Warad, B. Satrani, M. Ghanmi and E. Aouane, Anal. Bioanal. Electrochem., 2023, 15, 198–213 CAS
.
- A. R. Simović, B. N. Grgur, J. Novaković, P. Janaćković and J. Bajat, Metals, 2023, 13, 508 CrossRef
.
- R. T. Loto, O. Osamudiame, A. C. Nissi, O. O. Oluwakayode, U. V. Oghoho, O. C. Daniel, I. P. Smart, P.-A. C. Lemuel and O. R. Nwabeze, J. Bio- Tribo-Corros., 2023, 9, 19 CrossRef
.
- R. Shanmugapriya, M. Ravi, S. Ravi, M. Ramasamy and A. Maruthapillai, Inorg. Chem. Commun., 2023, 154, 110958 CrossRef CAS
.
- G. Beniaich, M. Beniken, R. Salim, N. Arrousse, E. Ech-chihbi, Z. Rais, A. Sadiq, H.-A. Nafidi, Y. A. Bin Jardan and M. Bourhia, Separations, 2023, 10, 396 CrossRef CAS
.
- M. Damej, S. Skal, J. Aslam, M. Zouarhi, H. Erramli, A. A. Alrashdi, H.-S. Lee, Y. El aoufir and H. Lgaz, Colloids Surf., A, 2022, 643, 128745 CrossRef CAS
.
- W. Daoudi, A. El Aatiaoui, N. Falil, M. Azzouzi, A. Berisha, L. O. Olasunkanmi, O. Dagdag, E. E. Ebenso, M. Koudad and A. Aouinti, J. Mol. Liq., 2022, 363, 119839 CrossRef CAS
.
- R. Ihamdane, M. Tiskar, B. Outemsaa, L. Zelmat, O. Dagdag, A. Berisha, E. Berdimurodov, E. E. Ebenso and A. Chaouch, Arab. J. Sci. Eng., 2023, 1–17 Search PubMed
.
- A. Ansari, O. Ou-Ani, L. Oucheikh, Y. Youssefi, D. Chebabe, A. Oubair and M. Znini, Chem. Afr., 2022, 1–19 Search PubMed
.
- R. T. Loto, E. Alagbe and A. Busari, Mater. Today: Proc., 2023, 80, 1408–1412 Search PubMed
.
- R. T. Loto and M. M. Solomon, Sci. Afr., 2023, 19, e01489 CAS
.
- F. Laihemdi, A. Barhoumi, M. Zarri, M. Tahiri and M. Chafi, Environ. Sci. Pollut. Res., 2023, 1–27 Search PubMed
.
- K. Dahmani, M. Galai, M. Ouakki, A. Elgendy, R. Ez-Zriouli, R. Lachhab, S. Briche and M. Cherkaoui, J. Mol. Liq., 2022, 347, 117982 CrossRef CAS
.
- O. Sanni, S. A. Iwarere and M. O. Daramola, ACS Omega, 2022, 7, 40740–40749 CrossRef CAS PubMed
.
- A. Chraka, N. B. Seddik, I. Raissouni, J. Kassout, M. Choukairi, M. Ezzaki, O. Zaraali, H. Belcadi, F. Janoub and A. I. Mansour, J. Mol. Liq., 2023, 387, 122715 CrossRef CAS
.
- Q. Wang, Q. Zhang, H. Zheng, L. Liu, X. Wu, C. Zhao, X. Zhou, Y. Sun, Z. Yan and X. Li, Sustainable Chem. Pharm., 2023, 34, 101177 CrossRef CAS
.
- A. M. El-Azaly, Int. J. Electrochem. Sci., 2019, 14, 2714–2731 CrossRef CAS
.
- B. Liao, S. Ma, S. Zhang, X. Li, R. Quan, S. Wan and X. Guo, Int. J. Biol. Macromol., 2023, 239, 124358 CrossRef CAS PubMed
.
- M. Zunita, D. Wahyuningrum, I. G. Wenten and R. Boopathy, Bioresour. Technol. Rep., 2022, 17, 100973 CrossRef CAS
.
- K. Carla de Santana de Lima, V. Magno Paiva, M. Gustavo Araujo Schwarz, N. Martins Bomfim Barreto, D. Perrone and E. D'Elia, Sustainable Chem. Pharm., 2023, 35, 101174 CrossRef CAS
.
- O. Khlopyk, S. Korniy, I. Zin and M. Holovchuk, Chem. Eng. Commun., 2024, 211(1), 124–132 CrossRef CAS
.
- M. El Hawary, M. Khachani, F. Benhiba, G. Kaichouh, I. Warad, A. Guenbour, A. Zarrouk and A. Bellaouchou, Chem. Data Coll., 2022, 40, 100870 Search PubMed
.
- L. Cai, H.-R. Guo, Y.-Q. Zhu, F.-S. Du, J.-T. Qi, L.-Y. Cui, C.-B. Liu and R.-C. Zeng, Smart Mater. Manuf., 2023, 1, 100014 Search PubMed
.
- K. C. d. S. d. Lima, V. M. Paiva, D. Perrone, B. Ripper, G. Simões, M. L. M. Rocco, A. G. d. Veiga and E. D'Elia, J. Mater. Res. Technol., 2020, 9, 12756–12772 CrossRef CAS
.
-
M. Quraishi and D. S. Chauhan, in Sustainable Corrosion Inhibitors II: Synthesis, Design, and Practical Applications, ACS Publications, 2021, pp. 1–17 Search PubMed
.
- P. S. Umoren, D. Kavaz and S. A. Umoren, Sustainability, 2022, 14, 7981 CrossRef CAS
.
- S. R. Al-Mhyawi, Int. J. Electrochem. Sci., 2023, 100210 CrossRef CAS
.
- R. Aslam, M. Mobin, M. Shoeb, M. Parveen, S. Zehra and J. Aslam, Arab. J. Sci. Eng., 2021, 46, 5489–5503 CrossRef CAS
.
- R. Aslam, M. Mobin, M. Shoeb and J. Aslam, Sci. Rep., 2022, 12, 9274 CrossRef CAS PubMed
.
- M. Mobin, R. Aslam, S. Zehra and M. Ahmad, J. Surfactants Deterg., 2017, 20, 57–74 CrossRef CAS
.
- M. Mobin, R. Aslam and J. Aslam, Mater. Chem. Phys., 2017, 191, 151–167 CrossRef CAS
.
- M. Parveen, M. Mobin, S. Zehra and R. Aslam, Sci. Rep., 2018, 8, 7489 CrossRef PubMed
.
- M. Gobara, A. Baraka, R. Akid and M. Zorainy, RSC Adv., 2020, 10, 2227–2240 RSC
.
- X. Li, S. Deng, H. Fu and G. Mu, Corros. Sci., 2009, 51, 2639–2651 CrossRef CAS
.
- H. M. Abd El-Lateef, Res. Chem. Intermed., 2016, 42, 3219–3240 CrossRef CAS
.
- K. Mohammed, A. Hamdy, A. Abdel-Wahab and N. Farid, Life Sci. J., 2012, 9, 424–434 Search PubMed
.
- J. Hu, D. Huang, G.-L. Song and X. Guo, Corros. Sci., 2011, 53, 4093–4101 CrossRef CAS
.
- X. Li, L. Tang, H. Liu, G. Mu and G. Liu, Mater. Lett., 2008, 62, 2321–2324 CrossRef CAS
.
- A. Fouda, S. A. Abd El-Maksoud, A. El-Habab and A. R. Ibrahim, Biointerface Res. Appl. Chem., 2021, 11, 9382 CAS
.
- J. Aslam, J. Adhes. Sci. Technol., 2019, 33, 1989–2009 CrossRef CAS
.
- A. Khamis, M. M. Saleh, M. I. Awad and B. El-Anadouli, J. Adv. Res., 2014, 5, 637–646 CrossRef CAS PubMed
.
- D. Asefi, N. M. Mahmoodi and M. Arami, Colloids Surf., A, 2010, 355, 183–186 CrossRef CAS
.
- M. Mobin, R. Aslam and J. Aslam, Mater. Chem. Phys., 2019, 223, 623–633 CrossRef CAS
.
- M. Mobin, S. Zehra and M. Parveen, J. Mol. Liq., 2016, 216, 598–607 CrossRef CAS
.
- S. Zehra, M. Mobin, J. Aslam and M. Parveen, J. Adhes. Sci. Technol., 2018, 32, 317–342 CrossRef CAS
.
- M. Mobin, M. Parveen and M. Rafiquee, Arabian J. Chem., 2017, 10, S1364–S1372 CrossRef CAS
.
- M. Parveen, M. Mobin and S. Zehra, RSC Adv., 2016, 6, 61235–61248 RSC
.
- M. Mobin and M. Parveen, J. Dispersion Sci. Technol., 2014, 35, 29–37 CrossRef CAS
.
- M. Mobin, M. Parveen and M. Rafiquee, J. Mater. Eng. Perform., 2013, 22, 548–556 CrossRef CAS
.
- M. Motamedi, A. R. Tehrani-Bagha and M. Mahdavian, Electrochim. Acta, 2011, 58, 488–496 CrossRef CAS
.
- M. Mobin and M. A. Khan, Chem. Eng. Commun., 2013, 200, 1149–1169 CrossRef CAS
.
- M. Mobin, M. Khan and M. Parveen, J. Appl. Polym. Sci., 2011, 121, 1558–1565 CrossRef CAS
.
- M. Mobin and M. A. Khan, J. Dispersion Sci. Technol., 2013, 34, 1496–1506 CrossRef CAS
.
- M. Mobin and M. Rizvi, Carbohydr. Polym., 2016, 136, 384–393 CrossRef CAS PubMed
.
- M. Mobin and M. Rizvi, Carbohydr. Polym., 2017, 156, 202–214 CrossRef CAS PubMed
.
- R. Aslam, M. Mobin, J. Aslam, H. Lgaz, I.-M. Chung and S. Zehra, J. Mol. Struct., 2021, 1228, 129751 CrossRef CAS
.
- R. Aslam, M. Mobin, J. Aslam, H. Lgaz and I.-M. Chung, J. Mater. Res. Technol., 2019, 8, 4521–4533 CrossRef CAS
.
- M. A. Deyab and S. S. A. El-Rehim, Int. J. Electrochem. Sci., 2013, 8, 12613–12627 CrossRef CAS
.
- M. Parveen, M. Mobin, S. Zehra and R. Aslam, Sci. Rep., 2018, 8, 7489 CrossRef PubMed
.
- M. Mobin, S. Zehra and M. Parveen, J. Mol. Liq., 2016, 216, 598–607 CrossRef CAS
.
- S. Deng, X. Li and G. Du, Chin. J. Chem. Eng., 2021, 37, 222–231 CrossRef CAS
.
- D. Hou, W. Yang and S. Dong, Chem. Ind. Eng., 2022, 39, 100–107 CAS
.
- X. Li, S. Deng, T. Lin and X. Xie, J. Mol. Liq., 2019, 282, 499–514 CrossRef CAS
.
- Y. Wang, K. Wang, P. Gao, R. Liu, D. Zhao, I. Zhai and G. Qu, J. Chin. Soc. Corros. Prot., 2021, 41, 131–138 Search PubMed
.
- X. Li, S. Deng, T. Lin, X. Xie and G. Du, J. Mater. Res. Technol., 2020, 9, 2196–2207 CrossRef CAS
.
- D. K. Verma, R. Aslam, J. Aslam, M. Quraishi, E. E. Ebenso and C. Verma, J. Mol. Struct., 2021, 1236, 130294 CrossRef CAS
.
- E. E. Ebenso, C. Verma, L. O. Olasunkanmi, E. D. Akpan, D. K. Verma, H. Lgaz, L. Guo, S. Kaya and M. A. Quraishi, Phys. Chem. Chem. Phys., 2021, 23, 19987–20027 RSC
.
- I. Obot, D. Macdonald and Z. Gasem, Corros. Sci., 2015, 99, 1–30 CrossRef CAS
.
- C. Verma, H. Lgaz, D. Verma, E. E. Ebenso, I. Bahadur and M. Quraishi, J. Mol. Liq., 2018, 260, 99–120 CrossRef CAS
.
- H. Shao, X. Yin, K. Zhang, W. Yang, Y. Chen and Y. Liu, J. Mater. Res. Technol., 2022, 20, 916–933 CrossRef CAS
.
- C. A. R. Maestro, A. M. de Sousa Malafaia, C. F. Silva, C. S. Nascimento Jr., K. B. Borges, T. A. Simões, V. R. Capelossi and A. H. S. Bueno, Mater. Chem. Phys., 2023, 305, 127971 CrossRef CAS
.
- X. Wang, H. Zhao, L. Chang, Z. Yu, Z. Xiao, S. Tang, C. Huang, J. Fan and S. Yang, ACS Omega, 2022, 7, 39169–39180 CrossRef CAS PubMed
.
- H. Kumar, P. Yadav, R. Kumari, R. Sharma, S. Sharma, D. Singh, H. Dahiya, P. Kumar, S. Bhardwaj and P. Kaur, Colloids Surf., A, 2023, 675, 132039 CrossRef CAS
.
- S. Mandal, S. Zamindar, S. Sarkar, M. Murmu, L. Guo, S. Kaya, H. Hirani and P. Banerjee, J. Adhes. Sci. Technol., 2023, 37, 1649–1665 CrossRef CAS
.
- N. Asadi, M. Ramezanzadeh, G. Bahlakeh and B. Ramezanzadeh, J. Taiwan Inst. Chem. Eng., 2019, 95, 252–272 CrossRef CAS
.
- E. Alibakhshi, M. Ramezanzadeh, G. Bahlakeh, B. Ramezanzadeh, M. Mahdavian and M. Motamedi, J. Mol. Liq., 2018, 255, 185–198 CrossRef CAS
.
- S. K. Saha, M. Murmu, N. C. Murmu and P. Banerjee, J. Mol. Liq., 2022, 364, 120033 CrossRef CAS
.
- S. Mandal, S. Bej and P. Banerjee, J. Mol. Liq., 2023, 381, 121789 CrossRef CAS
.
- M. Mobin, S. Zamindar and P. Banerjee, J. Mol. Liq., 2023, 122403 CrossRef CAS
.
- D.-Y. Wang, J.-H. Wang, H.-J. Li and Y.-C. Wu, Ind. Crops Prod., 2022, 189, 115866 CrossRef CAS
.
- M. Palimi, Y. Tang, S. Mousavi, W. Chen, V. Alvarez, E. Kuru and D. Li, Tribol. Int., 2023, 187, 108728 CrossRef CAS
.
- A. Farhadian, A. Rahimi, N. Safaei, A. Shaabani, M. Abdouss and A. Alavi, Corros. Sci., 2020, 175, 108871 CrossRef CAS
.
- X. Chen, Y. Chen, J. Cui, Y. Li, Y. Liang and G. Cao, Comput. Mater. Sci., 2021, 188, 110229 CrossRef CAS
.
- S. Sengupta, M. Murmu, S. Mandal, H. Hirani and P. Banerjee, Colloids Surf., A, 2021, 617, 126314 CrossRef CAS
.
- S. Sengupta, M. Murmu, N. C. Murmu and P. Banerjee, J. Mol. Liq., 2021, 326, 115215 CrossRef CAS
.
- O. Dagdag, Z. Safi, H. Erramli, N. Wazzan, I. Obot, E. Akpan, C. Verma, E. Ebenso, O. Hamed and A. El Harfi, J. Mol. Liq., 2019, 287, 110977 CrossRef CAS
.
- X. Li, S. Deng, H. Fu and G. Mu, Corros. Sci., 2010, 52, 1167–1178 CrossRef CAS
.
- A. Khamis, M. M. Saleh, M. I. Awad and B. E. El-Anadouli, J. Adv. Res., 2014, 5, 637–646 CrossRef CAS PubMed
.
- O. Sanni, A. P. I. Popoola and O. S. I. Fayomi, Mater. Today: Proc., 2021, 43, 2215–2221 CAS
.
- O. Oyewole, J. B. Adeoye, V. C. Udoh and T. A. Oshin, Egypt. J. Pet., 2023, 32, 41–46 CrossRef
.
- N. Bhardwaj, P. Sharma, K. Singh, D. Rana and V. Kumar, Chem. Phys. Impact, 2021, 3, 100038 CrossRef
.
- Y. Fernine, R. Salim, N. Arrousse, R. Haldhar, F. El Hajjaji, S.-C. Kim, M. E. Touhami and M. Taleb, J. Mol. Liq., 2022, 355, 118867 CrossRef CAS
.
- A. Shahmoradi, N. Talebibahmanbigloo, A. Javidparvar, G. Bahlakeh and B. Ramezanzadeh, J. Mol. Liq., 2020, 304, 112751 CrossRef CAS
.
- Y. Wu, Y. Zhang, Y. Jiang, N. Li, Y. Zhang, L. Wang and J. Zhang, Colloids Surf., A, 2021, 626, 126969 CrossRef CAS
.
- M. M. El-Deeb, E. N. Ads and J. R. Humaidi, Int. J. Electrochem. Sci., 2018, 13, 4123–4138 CrossRef CAS
.
-
D. Asra, N. K. Othman and Z. Dasuki, AIP Conf. Proc., 2017, 1838 Search PubMed
.
- L. A. C. Matos, M. C. Taborda, G. J. T. Alves, M. T. da Cunha, E. do Prado Banczek, M. de Fátima Oliveira, E. D'Elia and P. R. P. Rodrigues, Int. J. Electrochem. Sci., 2018, 13, 1577–1593 CrossRef CAS PubMed
.
- N. Raghavendra and J. Ishwara Bhat, J. Bio- Tribo-Corros., 2018, 4, 44 CrossRef
.
- A. R. Shahmoradi, M. Ranjbarghanei, A. A. Javidparvar, L. Guo, E. Berdimurodov and B. Ramezanzadeh, J. Mol. Liq., 2021, 338, 116550 CrossRef CAS
.
- B.-l. Lin, J.-j. Shao, Y.-y. Xu, Y.-m. Lai and Z.-n. Zhao, Arabian J. Chem., 2021, 14, 103114 CrossRef CAS
.
- S. Paul and I. Koley, J. Bio- Tribo-Corros., 2016, 2, 6 CrossRef
.
- M. Sahraoui, M. Boulkroune, A. Chibani, Y. Larbah and A. Abdessemed, J. Bio- Tribo-Corros., 2022, 8, 54 CrossRef
.
- M. A. Chowdhury, M. M. S. Ahmed, N. Hossain, M. A. Islam, S. Islam and M. M. Rana, Results Eng., 2023, 17, 100996 CrossRef CAS
.
- F. Bouhlal, N. Labjar, F. Abdoun, A. Mazkour, M. Serghini-Idrissi, M. El Mahi, E. M. Lotfi and S. El Hajjaji, Int. J. Corros., 2020, 2020, 1–14 CrossRef
.
- N. A. Reza, N. H. Akhmal, N. A. Fadil and M. F. M. Taib, Metals, 2021, 11, 1062 CrossRef CAS
.
- A. A. Farag, A. S. Ismail and M. Migahed, Egypt. J. Pet., 2018, 27, 1187–1194 CrossRef
.
- C. M. Fernandes, P. C. de Oliveira, V. G. Pina, B. S. Peixoto, F. F. Massante, M. C. Veloso, G. A. Romeiro, M. C. de Moraes and E. A. Ponzio, Sustainable Chem. Pharm., 2022, 29, 100751 CrossRef CAS
.
- M. C. T. Larissa Aparecida Corrêa Matos, G. J. T. Alves, M. T. da Cunha, E. do Prado Banczek, M. d. F. Oliveira, E. D'Elia and P. R. P. Rodrigues, Int. J. Electrochem. Sci., 2018, 13, 1577–1593 CrossRef PubMed
.
-
D. Gusti, I. Lestari, E. Permana and R. Anggraini, IOP Conf. Ser: Earth Environ. Sci., 2020, 483 Search PubMed
.
- A. Ayoola, R. Babalola, B. Durodola, E. Alagbe, O. Agboola and E. Adegbile, Results Eng., 2022, 15, 100490 CrossRef CAS
.
- G. Salinas-Solano, J. Porcayo-Calderon, L. M. de la Escalera, J. Canto, M. Casales-Diaz, O. Sotelo-Mazon, J. Henao and L. Martinez-Gomez, Ind. Crops Prod., 2018, 119, 111–124 CrossRef CAS
.
- R. Vera, F. Figueredo, A. Díaz-Gómez and A. Molinari, Int. J. Electrochem. Sci., 2018, 13, 4139–4159 CrossRef CAS
.
- A. Cruz-Zabalegui, E. Vazquez-Velez, G. Galicia-Aguilar, M. Casales-Diaz, R. Lopez-Sesenes, J. Gonzalez-Rodriguez and L. Martinez-Gomez, Ind. Crops Prod., 2019, 133, 203–211 CrossRef CAS
.
- J. Halambek, I. Cindrić and A. Ninčević Grassino, Carbohydr. Polym., 2020, 234, 115940 CrossRef CAS PubMed
.
- I. Kusumaningrum, R. Soenoko, E. Siswanto and F. Gapsari, Case Stud. Chem. Environ. Eng., 2022, 6, 100223 CrossRef CAS
.
- N. Bhardwaj, P. Sharma and V. Kumar, Corros. Rev., 2021, 39, 27–41 CrossRef CAS
.
- M. H. Fekri, F. Omidali, M. M. Alemnezhad and A. Ghaffarinejad, Mater. Chem. Phys., 2022, 286, 126150 CrossRef CAS
.
- M. Radi, R. Melian, M. Galai, N. Dkhirche, M. Makha, C. Verma, C. Fernandez and M. EbnTouhami, J. Mol. Liq., 2021, 337, 116547 CrossRef CAS
.
- D. Payra, M. Naito, Y. Fujii, N. L. Yamada, S. Hiromoto and A. Singh, RSC Adv., 2015, 5, 15977–15984 RSC
.
-
S. Zehra, M. Mobin and R. Aslam, in Environmentally Sustainable Corrosion Inhibitors, ed. C. M. Hussain, C. Verma and J. Aslam, Elsevier, 2022, pp. 47–67. DOI:10.1016/B978-0-323-85405-4.00022-7
.
- A. A. Al-Amiery, W. N. R. W. Isahak and W. K. Al-Azzawi, Lubricants, 2023, 11, 174 CrossRef CAS
.
-
M. Rbaa, M. Galai, O. Dagdag, L. Guo, B. Tüzün, E. Berdimurodov, A. Zarrouk and B. Lakhrissi, in Eco-Friendly Corrosion Inhibitors, ed. L. Guo, C. Verma and D. Zhang, Elsevier, 2022, pp. 27–42. DOI:10.1016/B978-0-323-91176-4.00026-X
.
-
M. A. Quraishi and D. S. Chauhan, in Sustainable Corrosion Inhibitors II: Synthesis, Design, and Practical Applications, American Chemical Society, 2021, vol. 1404, ch. 1, pp. 1–17 Search PubMed
.
- C. Verma, M. A. Quraishi and K. Y. Rhee, J. Mol. Liq., 2022, 348, 118387 CrossRef CAS
.
- R. Bender, D. Féron, D. Mills, S. Ritter, R. Bäßler, D. Bettge, I. De Graeve, A. Dugstad, S. Grassini, T. Hack, M. Halama, E.-H. Han, T. Harder, G. Hinds, J. Kittel, R. Krieg, C. Leygraf, L. Martinelli, A. Mol, D. Neff, J.-O. Nilsson, I. Odnevall, S. Paterson, S. Paul, T. Prošek, M. Raupach, R. I. Revilla, F. Ropital, H. Schweigart, E. Szala, H. Terryn, J. Tidblad, S. Virtanen, P. Volovitch, D. Watkinson, M. Wilms, G. Winning and M. Zheludkevich, Mater. Corros., 2022, 73, 1730–1751 CrossRef CAS
.
- C. Verma, E. E. Ebenso, M. A. Quraishi and C. M. Hussain, Mater. Adv., 2021, 2, 3806–3850 RSC
.
- H. Wei, B. Heidarshenas, L. Zhou, G. Hussain, Q. Li and K. K. Ostrikov, Mater. Today Sustainability, 2020, 10, 100044 CrossRef
.
- A. Thakur, S. Sharma, R. Ganjoo, H. Assad and A. Kumar, J. Phys.: Conf. Ser., 2022, 2267, 012079 CrossRef
.
- C. Verma, A. Alfantazi and M. A. Quraishi, J. Mol. Liq., 2021, 343, 117648 CrossRef CAS
.
- B. R. Fazal, T. Becker, B. Kinsella and K. Lepkova, npj Mater. Degrad., 2022, 6, 5 CrossRef CAS
.
- M. Alimohammadi, M. Ghaderi, A. Ramazani S. A. and M. Mahdavian, Sci. Rep., 2023, 13, 3737 CrossRef CAS PubMed
.
-
M. Rizvi, in Organic Corrosion Inhibitors, 2021, pp. 411–434. DOI:10.1002/9781119794516.ch18
.
- S. A. Umoren, M. M. Solomon, A. Madhankumar and I. B. Obot, Carbohydr. Polym., 2020, 230, 115466 CrossRef CAS PubMed
.
- S. Marzorati, L. Verotta and S. P. Trasatti, Molecules, 2019, 24, 48 CrossRef PubMed
.
- A. N. Grassino, J. Halambek, S. Djaković, S. Rimac Brnčić, M. Dent and Z. Grabarić, Food Hydrocolloids, 2016, 52, 265–274 CrossRef CAS
.
-
K. Geiser, in Sustainability Science and Engineering, Elsevier, 2006, vol. 1, pp. 161–175 Search PubMed
.
- S. C. DeVito, Green Chem., 2016, 18, 4332–4347 RSC
.
- C. Verma, S. H. Alrefaee, M. Quraishi, E. E. Ebenso and C. M. Hussain, J. Mol. Liq., 2021, 321, 114484 CrossRef CAS
.
-
M. A. Usmani, I. Khan, A. Hasnat and A. Moheman, in Advanced Applications of Ionic Liquids, Elsevier, 2023, pp. 185–196 Search PubMed
.
- K. Ghandi, Green Sustainable Chem., 2014, 4, 44–53 CrossRef CAS
.
- L. Souza, E. Pereira, L. Matlakhova, V. A. Nicolin, S. N. Monteiro and A. R. de Azevedo, J. Mater. Res. Technol., 2022 DOI:10.1016/j.jmrt.2022.12.066
.
- J. Ye, R. Huang, X. Wang, Q. Bao, J. Mu, K. Pan, W. Ji, Z. Wei and H. Liu, Ind. Eng. Chem. Res., 2023, 62(36), 14427–14440 CrossRef CAS
.
- E. K. Ardakani, E. Kowsari, A. Ehsani and S. Ramakrishna, Microchem. J., 2021, 165, 106049 CrossRef CAS
.
- R. Haldhar, C. J. Raorane, V. Mishra, T. Periyasamy, A. Berisha and S.-C. Kim, J. Mol. Liq., 2023, 372, 121168 CrossRef CAS
.
- O. A. Al-Rashed and A. A. Nazeer, J. Mol. Liq., 2019, 288, 111015 CrossRef CAS
.
- C. Verma and M. A. Quraishi, e-Prime Advances in Electrical Engineering, Electronics and Energy, 2022, 2, 100070 CrossRef
.
- Y. Shi, Y. Fu, H. Huang, H. Li, S. Zhang, W. Li and F. Gao, J. Environ. Chem. Eng., 2021, 9, 106679 CrossRef CAS
.
- P. Singh, D. Singh Chauhan, S. Singh Chauhan, M. Ahmad Quraishi and S. Swarupa Tripathy, ChemistrySelect, 2021, 6, 11417–11430 CrossRef CAS
.
- M. Goyal, S. Kumar, L. Guo, S. H. Alrefaee and C. Verma, J. Taiwan Inst. Chem. Eng., 2021, 123, 21–33 CrossRef CAS
.
- T. W. Quadri, L. O. Olasunkanmi, O. E. Fayemi, E. D. Akpan, H.-S. Lee, H. Lgaz, C. Verma, L. Guo, S. Kaya and E. E. Ebenso, Comput. Mater. Sci., 2022, 214, 111753 CrossRef CAS
.
- R. Huang, H. Liu, Z. Wei, Y. Jiang, K. Pan, X. Wang and J. Kong, Environ. Sci. Pollut. Res., 2023, 1–23 Search PubMed
.
- A. Rathore, S. Sharma, A. Sharma and S. K. Sharma, J. Dispersion Sci. Technol., 2023, 1–13 CrossRef
.
- S. Monaci, D. Minudri, L. Guazzelli, A. Mezzetta, D. Mecerreyes, M. Forsyth and A. Somers, Molecules, 2023, 28, 5568 CrossRef CAS PubMed
.
- F. E. Hajjaji, R. Salim, M. Taleb, F. Benhiba, N. Rezki, D. S. Chauhan and M. Quraishi, Surf. Interfaces, 2021, 22, 100881 CrossRef CAS
.
- A. Singh, K. Ansari, I. H. Ali, Y. Lin, M. Murmu and P. Banerjee, J. Mol. Liq., 2023, 376, 121408 CrossRef CAS
.
- O. Al-Rashed and A. Abdel Nazeer, Materials, 2022, 15, 2326 CrossRef CAS PubMed
.
- E. E. El-Katori and A. S. Abousalem, RSC Adv., 2019, 9, 20760–20777 RSC
.
- G. Gómez-Sánchez, O. Olivares-Xometl, P. Arellanes-Lozada, N. V. Likhanova, I. V. Lijanova, J. Arriola-Morales, V. Díaz-Jiménez and J. López-Rodríguez, Int. J. Mol. Sci., 2023, 24, 6291 CrossRef PubMed
.
- E. K. Ardakani, E. Kowsari and A. Ehsani, Colloids Surf., A, 2020, 586, 124195 CrossRef
.
- E. Li, Y. Li, S. Liu and P. Yao, Colloids Surf., A, 2023, 657, 130541 CrossRef CAS
.
- A. Nahlé, R. Salim, F. E. Hajjaji, E. Ech-chihbi, A. Titi, M. Messali, S. Kaya, B. El Ibrahimi and M. Taleb, Arabian J. Chem., 2022, 15, 103967 CrossRef
.
- E. E. El-Katori, M. Nessim, M. Deyab and K. Shalabi, J. Mol. Liq., 2021, 337, 116467 CrossRef CAS
.
- J. Cai, J. Liu, S. Mu, J. Liu, J. Hong, X. Zhou, Q. Ma and L. Shi, Int. J. Electrochem. Sci., 2020, 15, 1287–1301 CrossRef CAS
.
- Y. Guo, J. Xue, J. Zhang, Q. Chen, L. Fan, C. Tang, K. Ren, A. Fu and Q. Bi, Colloids Surf., A, 2022, 129135 CrossRef CAS
.
- P. Kannan, A. Varghese, K. Palanisamy and A. S. Abousalem, Appl. Surf. Sci. Adv., 2021, 6, 100150 CrossRef
.
- Y. Jiang, Y. Liu, S. Gao, X. Guo and J. Zhang, J. Mol. Liq., 2022, 345, 116998 CrossRef CAS
.
- A. Singh, K. Ansari, P. Banerjee, M. Murmu, M. Quraishi and Y. Lin, Colloids Surf., A, 2021, 623, 126708 CrossRef CAS
.
- N. Kedimar, P. Rao and S. A. Rao, J. Appl. Electrochem., 2023, 1–17 Search PubMed
.
- S. Xu, Z. Luo, J. Zhang, B. Tan, S. Zhang and W. Li, J. Mol. Liq., 2021, 340, 117189 CrossRef CAS
.
- H. Huang, B. Li, X. Zheng, J. Fan and M. Gong, Int. J. Electrochem. Sci., 2022, 17, 220344 CrossRef CAS
.
- E. Berdimurodov, A. Kholikov, K. Akbarov, L. Guo, S. Kaya, K. P. Katin, D. K. Verma, M. Rbaa, O. Dagdag and R. Haldhar, J. Electroanal. Chem., 2021, 901, 115794 CrossRef CAS
.
- E. Berdimurodov, A. Kholikov, K. Akbarov, L. Guo, S. Kaya, K. P. Katin, D. K. Verma, M. Rbaa and O. Dagdag, Colloids Surf., A, 2022, 633, 127837 CrossRef CAS
.
- C. Verma, M. A. Quraishi, A. Alfantazi and K. Y. Rhee, Adv. Ind. Eng. Polym. Res., 2023, 6(4), 407–435 CAS
.
- S. Umoren and M. Solomon, The Open Mater. Sci. J., 2014, 8, 39–54 CrossRef
.
- M. A. Quraishi, D. S. Chauhan and V. S. Saji, J. Mol. Liq., 2021, 341, 117265 CrossRef CAS
.
- S. Nwanonenyi, I. Madufor, P. Uzoma and I. Chukwujike, Int. Res. J. Pure Appl. Chem., 2016, 1–15 CAS
.
-
T. W. Quadri, L. O. Olasunkanmi, O. E. Fayemi and E. E. Ebenso, Grafted Biopolymers as Corrosion Inhibitors: Safety, Sustainability, and Efficiency, 2023 Search PubMed
.
- S. Nwanonenyi, H. Obasi and I. Eze, Chem. Afr., 2019, 2, 471–482 CAS
.
- Y. Lu, H. Feng, H. Xia and W. H. Xia, Int. J. Electrochem. Sci., 2022, 17, 221180 CrossRef CAS
.
- P. P. Rahayu, C. D. D. Sundari and I. Farida, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 434, 012087 Search PubMed
.
- A. Peter, I. Obot and S. K. Sharma, Int. J. Ind. Chem., 2015, 6, 153–164 CrossRef CAS
.
- N. R. Vaidya, P. Aklujkar and A. R. Rao, J. Coat. Technol. Res., 2022, 19, 223–239 CrossRef CAS
.
- M. El Azzouzi, K. Azzaoui, I. Warad, B. Hammouti, S. Shityakov, R. Sabbahi, S. Saoiabi, M. Youssoufi, N. Akartasse and S. Jodeh, J. Mol. Liq., 2022, 347, 118354 CrossRef CAS
.
- C. Shen, V. Alvarez, J. D. Koenig and J.-L. Luo, Corros. Eng., Sci. Technol., 2019, 54, 444–454 CrossRef CAS
.
- A. Büyüksağiş, A. Baydır and M. Dilek, Prot. Met. Phys. Chem. Surf., 2021, 57, 211–221 CrossRef
.
- G. Palumbo, K. Berent, E. Proniewicz and J. Banaś, Materials, 2019, 12, 2620 CrossRef CAS PubMed
.
- N. B. Iroha and O. Akaranta, SN Appl. Sci., 2020, 2, 1514 CrossRef CAS
.
- A. Jmiai, B. El Ibrahimi, A. Tara, S. El Issami, O. Jbara and L. Bazzi, J. Mol. Struct., 2018, 1157, 408–417 CrossRef CAS
.
- K. Shamsheera, A. R. Prasad, M. Arshad and A. Joseph, J. Indian Chem. Soc., 2022, 99, 100271 CrossRef
.
- A. Toghan and A. Fawzy, Polymer, 2023, 15, 3144 CAS
.
- Y. Cao, C. Zou, C. Wang, W. Chen, H. Liang and S. Lin, J. Mol. Liq., 2021, 341, 117391 CrossRef CAS
.
- M. Mobin, I. Ahmad, M. Basik, M. Murmu and P. Banerjee, Sustainable Chem. Pharm., 2020, 18, 100337 CrossRef
.
- N. K. Gupta, P. Joshi, V. Srivastava and M. Quraishi, Int. J. Biol. Macromol., 2018, 106, 704–711 CrossRef CAS PubMed
.
- M. Srivastava, S. Srivastava, G. Ji and R. Prakash, Int. J. Biol. Macromol., 2019, 140, 177–187 CrossRef CAS PubMed
.
- A. Fawzy, A. Toghan, N. Alqarni, M. Morad, M. E. Zaki, M. M. Sanad, A. I. Alakhras and A. A. Farag, Polymers, 2023, 15, 891 CrossRef CAS PubMed
.
- M. Erna, H. Herdini and D. Futra, Int. J. Chem. Eng., 2019, 2019, 8514132 Search PubMed
.
- Q. Zhang, N. Xu, Z. Jiang, H. Liu and G. Zhang, J. Colloid Interface Sci., 2023, 640, 1052–1067 CrossRef CAS PubMed
.
- P. Kesari, G. Udayabhanu, A. Roy and S. Pal, Int. J. Biol. Macromol., 2023, 225, 1323–1349 CrossRef CAS PubMed
.
- A. Sun, G. Cui and Q. Liu, Colloids Surf., A, 2023, 664, 131106 CrossRef CAS
.
- W. Song, X. Zhao, Z. Jin, L. Fan, X. Ji, J. Deng and J. Duan, J. Cleaner Prod., 2023, 136390 CrossRef CAS
.
- T. A. Saleh, K. Haruna, M. M. Nur and B. Alharbi, Prog. Org. Coat., 2022, 170, 106974 CrossRef CAS
.
- N. Talat, A. Dahadha, M. Abunuwar, A. Hussien and W. a. Odeh, Int. J. Corros. Scale Inhib., 2023, 12, 215–243 CAS
.
- A. Fawzy, A. Al Bahir, N. Alqarni, A. Toghan, M. Khider, I. M. Ibrahim, H. H. Abulreesh and K. Elbanna, Sci. Rep., 2023, 13, 2585 CrossRef CAS PubMed
.
- X. Wang and W. Ding, J. Dispersion Sci. Technol., 2023, 44, 1288–1295 CrossRef CAS
.
- A. Yousefi, S. Javadian, M. Sharifi, N. Dalir and A. Motaee, J. Bio- Tribo-Corros., 2019, 5, 1–15 CrossRef
.
- S. Abd El-Maksoud, F. El-Dossoki, M. Abd-Elhamed and A. A. Farag, J. Bio- Tribo-Corros., 2023, 9, 1–16 CrossRef
.
- A. Elaraby, A. Elgendy, M. Abd-El-Raouf, M. Migahed, A. El-Tabei, A. M. Abdullah, N. H. Al-Qahtani, S. M. Alharbi, S. M. Shaban and D. H. Kim, Colloids Surf., A, 2023, 659, 130687 CrossRef CAS
.
- S. H. Shafek, E. A. Ghiaty, N. M. El Basiony, E. A. Badr and S. M. Shaban, Z. Phys. Chem., 2023, 237, 1–33 CrossRef CAS
.
- A. Elaraby, S. A. El-samad, E. A. Khamis and E. Zaki, Sci. Rep., 2023, 13, 942 CrossRef CAS PubMed
.
- R. Jalab, M. A. Saad, M. H. Sliem, A. M. Abdullah and I. A. Hussein, Molecules, 2022, 27, 6414 CrossRef CAS PubMed
.
- M. A. Bedair, H. M. Elaryian, A. H. Bedair, R. M. Aboushahba and A. E.-A. S. Fouda, Inorg. Chem. Commun., 2023, 148, 110304 CrossRef CAS
.
- H. M. Elaryian, M. A. Bedair, A. H. Bedair, R. M. Aboushahba and A. E.-A. S. Fouda, J. Mol. Liq., 2022, 346, 118310 CrossRef CAS
.
- A. M. Younis, T. H. Rakha, M. M. El-Gamil and G. M. A. El-Reash, J. Inorg. Organomet. Polym. Mater., 2022, 1–17 Search PubMed
.
- A. Groysman, Koroze Ochr. Mater., 2017, 61, 100–117 CrossRef
.
- D. Lopez, T. Perez and S. Simison, Mater. Des., 2003, 24, 561–575 CrossRef CAS
.
- J. Sun, C. Sun, X. Lin, X. Cheng and H. Liu, Materials, 2016, 9, 200 CrossRef PubMed
.
- J. Jiang, N. Li, Q. Li, Z. Jiang, B. Wang, Y. He, F. Liu and C. Liu, Metals, 2023, 13, 826 CrossRef CAS
.
- J. Feder, J. Pet. Technol., 2020, 72, 56–57 Search PubMed
.
- J. Ning, Y. Yoon, H. Li and S. Srinivasan, NACE CORROSION, 2019, NACE-2019-13400.
-
S.
A. Ali, L. J. Kalfayan and C. T. Montgomery, Acid Stimulation, Society of Petroleum Engineers, Richardson, Texas, 2016 Search PubMed
.
- B. Williams and D. Nierode, J. Pet. Technol., 1972, 24, 849–859 CrossRef CAS
.
- P. Rajeev, A. Surendranathan and C. S. Murthy, J. Mater. Environ. Sci., 2012, 3, 856–869 CAS
.
- P. Okafor, M. E. Ikpi, I. Uwah, E. Ebenso, U. Ekpe and S. Umoren, Corros. Sci., 2008, 50, 2310–2317 CrossRef CAS
.
- M. Finšgar and J. Jackson, Corros. Sci., 2014, 86, 17–41 CrossRef
.
- C. F. Smith, F. E. Dollarhide and N. J. Byth, J. Pet. Technol., 1978, 30, 737–746 CrossRef CAS
.
-
O. A. Thomas and A. P. Roberts, Production operations, Well completions, Work over and stimulation, 2007 Search PubMed
.
-
H. A. Nasr-El-Din, S. A. Ali, L. Kalfayan and C. T. Montgomery, in Acid Stimulation, Society of Petroleum Engineers, 2016, DOI:10.2118/9781613994269-09
.
- H. A. Nasr-El-Din, A. H. Al-Ghamdi, A. A. Al-Qahtani and M. M. Samuel, SPE J., 2008, 13, 35–47 CrossRef CAS
.
- S. D. Harms, J. M. Smith, G. E. King and K. Posey, Permian Basin Oil and Gas Recovery Conference, SPE, 1988 Search PubMed.
- D. Hill, Civil Engineering Magazine Archive, 2014, 84(1), 40–41 CrossRef
.
- H. Asaadian, P. Ahmadi, M. Z. Khormizi, S. Mohammadi, B. S. Soulgani, S. Baghersaei and B. Mokhtari, J. Pet. Sci. Eng., 2022, 218, 111009 CrossRef CAS
.
- N. Hossain, M. A. Islam and M. A. Chowdhury, Results Chem., 2023, 5, 100883 CrossRef
.
- U. Bharatiya, P. Gal, A. Agrawal, M. Shah and A. Sircar, J. Bio- Tribo-Corros., 2019, 5, 1–12 CrossRef
.
- M. Ghommem, W. Zhao, S. Dyer, X. Qiu and D. Brady, J. Pet. Sci. Eng., 2015, 131, 18–33 CrossRef CAS
.
- A. Shein and A. Denisova, Prot. Met., 2006, 42, 34–37 CrossRef CAS
.
- W. Frenier, F. Growcock and V. Lopp, SPE Prod. Eng., 1988, 3, 584–590 CrossRef CAS
.
- A. Singh and M. A. Quraishi, J. Mater. Environ. Sci., 2015, 6, 224–235 CAS
.
- K. Ansari, M. Quraishi and A. Singh, J. Assoc. Arab Univ. Basic Appl. Sci., 2017, 22, 45–54 Search PubMed
.
- S. Aribo, S. J. Olusegun, L. J. Ibhadiyi, A. Oyetunji and D. O. Folorunso, J. Assoc. Arab Univ. Basic Appl. Sci., 2017, 24, 34–38 Search PubMed
.
-
E. Ghali, V. S. Sastri and M. Elboujdaini, Corrosion prevention and protection: practical solutions, John Wiley & Sons, 2007 Search PubMed
.
- A. Marciales, T. Haile, B. Ahvazi, T.-D. Ngo and J. Wolodko, Corros. Rev., 2018, 36, 239–266 CrossRef CAS
.
- M. Abdallah, I. Zaafarany, K. Khairou and Y. Emad, Chem. Technol. Fuels Oils, 2012, 48, 234–245 CrossRef CAS
.
- A. Fouda, A. Emam, R. Refat and M. Nageeb, Surf. Eng. Appl. Electrochem., 2019, 55, 294–303 CrossRef
.
- K. Haruna, T. A. Saleh and M. Quraishi, J. Mol. Liq., 2020, 315, 113716 CrossRef CAS
.
- D. S. Chauhan, M. Quraishi, V. Srivastava and J. Haque, J. Mol. Struct., 2021, 1226, 129259 CrossRef CAS
.
- R. T. Loto, E. Okorie and T. Olukeye, S. Afr. J. Chem. Eng., 2019, 30, 28–41 Search PubMed
.
- R. T. Loto, Sustainable Chem. Pharm., 2020, 17, 100298 CrossRef
.
- G. Palumbo, M. Gorny and J. Banaś, J. Mater. Eng. Perform., 2019, 28, 6458–6470 CrossRef CAS
.
- L. B. Furtado, R. Nascimento, P. R. Seidl, M. J. O. Guimarães, L. M. Costa, J. Rocha and J. Ponciano, J. Mol. Liq., 2019, 284, 393–404 CrossRef CAS
.
- M. Quraishi, K. Ansari, D. S. Chauhan, S. A. Umoren and M. Mazumder, Cellulose, 2020, 27, 6425–6443 CrossRef CAS
.
- M. Palimi, Y. Tang, V. Alvarez, E. Kuru and D. Li, Mater. Chem. Phys., 2022, 288, 126406 CrossRef CAS
.
- M. Yadav, S. Kumar and D. Sharma, Anti-Corros. Methods Mater., 2014, 61, 129–138 CrossRef CAS
.
- I. B. Onyeachu, I. B. Obot, A. A. Sorour and M. I. Abdul-Rashid, Corros. Sci., 2019, 150, 183–193 CrossRef CAS
.
- H. A. Craddock, S. Caird, H. Wilkinson and M. Guzmann, SPE Proj., Fac. Const., 2007, 2, 1–8 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |