Heterointerface regulation of covalent organic framework-anchored graphene via a solvent-free strategy for high-performance supercapacitor and hybrid capacitive deionization electrodes†
Liming
Xu
a,
Yong
Liu
 *b,
Xiaoyang
Xuan
*c,
Xingtao
Xu
d,
Yuquan
Li
e,
Ting
Lu
a and
Likun
Pan
*b,
Xiaoyang
Xuan
*c,
Xingtao
Xu
d,
Yuquan
Li
e,
Ting
Lu
a and
Likun
Pan
 *a
*a
aShanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, Shanghai 200241, China. E-mail: lkpan@phy.ecnu.edu.cn
bSchool of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao, Shandong 266042, China. E-mail: yong.liu@qust.edu.cn
cCollege of Chemistry and Chemical Engineering, Taishan University, Taian, Shandong 271000, China. E-mail: xyxuan@tsu.edu.cn
dMarine Science and Technology College, Zhejiang Ocean University, Zhoushan, Zhejiang 316022, China
eCollege of Environmental Science and Engineering, Yangzhou University, Yangzhou, Jiangsu 225127, China
First published on 31st March 2024
Abstract
Covalent organic frameworks (COFs) with customizable geometry and redox centers are an ideal candidate for supercapacitors and hybrid capacitive deionization (HCDI). However, their poor intrinsic conductivity and micropore-dominated pore structures severely impair their electrochemical performance, and the synthesis process using organic solvents brings serious environmental and cost issues. Herein, a 2D redox-active pyrazine-based COF (BAHC-COF) was anchored on the surface of graphene in a solvent-free strategy for heterointerface regulation. The as-prepared BAHC-COF/graphene (BAHCGO) nanohybrid materials possess high-speed charge transport offered by the graphene carrier and accelerated electrolyte ion migration within the BAHC-COF, allowing ions to effectively occupy ion storage sites inside BAHC. As a result, the BAHCGO//activated carbon asymmetric supercapacitor achieves a high energy output of 61.2 W h kg−1 and a satisfactory long-term cycling life. More importantly, BAHCGO-based HCDI possesses a high salt adsorption capacity (SAC) of 67.5 mg g−1 and excellent long-term desalination/regeneration stability. This work accelerates the application of COF-based materials in the fields of energy storage and water treatment.
New conceptsThe challenges limiting the application of covalent organic framework (COF) materials in the fields of supercapacitors (SC) and capacitive deionization (CDI) are the poor intrinsic electronic conductivity and ion diffusion paths of COFs. In this work, we propose a strategy to spread two-dimensional redox-active pyrazine-based COF (BAHC-COF) on the graphene surface to construct a heterointerface (BAHCGO) through a solvent-free system. As a conductive substrate, graphene plays a heterogeneous nucleation role during the growth process of BAHC-COF, guiding BAHC-COF to spread evenly on its surface while inhibiting the stacking/aggregation of BAHC-COF. Furthermore, the π–π interaction between graphene and BAHC-COF enables BAHC-COF to capture the delocalized π electrons of graphene, thereby achieving the acceleration of charge transfer kinetics and high utilization of redox-active sites. As expected, BAHCGO-based SC and CDI achieved excellent energy output and desalination performance and outperformed most COF-based materials. This work not only provides COF-based hybrid materials with excellent performance but also promotes the advancement of practical applications of SC and CDI. |
Introduction
Supercapacitors (SC) and capacitive deionization (CDI) based on electric double layer capacitance (EDLC) and/or pseudocapacitance mechanisms exhibit the advantages of good reversibility, low maintenance cost, and green sustainability.1–3 Typical EDLC materials such as activated carbon (AC), carbon nanotubes, and graphene are widely used as electrode materials for SC and CDI devices due to their good stability, high conductivity, and fast/reversible ion adsorption/desorption responsiveness.4,5 However, the electrochemical properties of carbonaceous materials are excessively dependent on their specific surface area and porosity, and carbon is easily oxidized during long-term service.6,7 Pseudocapacitive materials (such as metal compounds, MXenes, MOFs, organic polymers, etc.) that store charge through a fast and reversible faradaic reaction occurring on the surface or near-surface redox-active sites have been rapidly developed in recent decades as candidates in the field of SC and CDI due to their ability to balance ion storage capacity vs. rate.8–10 Conventionally, pseudocapacitive materials were constructed by decorating redox-active materials (i.e., metal compounds or traditional conductive polymers) on the surface of the porous backbone (i.e., porous carbon) to enlarge the exposed redox surface area. However, this strategy is plagued by some serious intrinsic issues: (i) the surface decorated faradaic material is usually attached to the backbone via weak physical bonds like electrostatic attraction, and van der Waals forces, and could be easily washed off during cycling (this issue could only get worse with volumetric expansion);4,5 (ii) the contact between redox-active material and backbone is usually point-to-point that could lead to sluggish electron conductivity;6,7 (iii) the structural backbone used is usually porous carbon that suffers anodic-corrosion during long-term operation.8–12 To address the abovementioned issues, we hypothesize whether we could somehow lock the redox-active material inside (instead of loading it on the surface) a stable organic porous network scaffold structure, we might be able to achieve superior and ultrastable pseudocapacitance and prevent aggregation without causing severe anodic corrosion.As a special type of crystalline porous polymer, the covalent organic framework (COF) has a well-defined and adjustable chemical/nanostructure (to ensure sufficient exposure of the redox active sites), chemical stability, and permanent porosity.11,12 Moreover, specific redox active structures can be integrated into COFs by screening suitable monomer structures (instead of post-decoration), or a unique chemical/pore geometry can be customized to make COFs with a variable structure and flexible porosity, which could be beneficial to ensuring sufficient stability and uniform distribution of the redox-active sites triggering rapid and reversible multi-electron pseudocapacitance.11–13 For example, Haldar et al. proved that pyridyl-hydroxy-modified COFs incorporating keto–enol tautomerization and hydrogen bonding of hydroxyl units contributed to enhancing electrochemical stability.14 The solid-state device based on this COF produced a capacity of ∼92 mF cm−2 and a high power output of 98 μW cm−2. Moreover, COFs with redox activity were first introduced by Li et al. as a new faradaic cathode material for hybrid CDI (HCDI) applications, showing the highest SAC of 22.8 mg g−1 (at 500 mg L−1 NaCl).15 However, the practical application of COFs in SC and CDI devices still faces major challenges: the low intrinsic conductivity of COFs adds a charge transfer barrier and inhibits the improvement in electrochemical performance;16,17 the strong interlayer π–π interaction causes the COF layers to easily agglomerate/stack tightly, making it difficult to expose their porous structure for the ion to access the active sites in the COF skeleton, which leads to the capacitance of COF severely lower than its theoretical value.18,19 Recently, it was revealed that constructing COF-based hybrid materials can effectively adjust their microstructural characteristics to enhance conductivity and release porosity and active sites, especially when employing graphene with ultra-high theoretical specific surface area, extraordinary intrinsic electron mobility, and excellent chemical stability as a hybrid partner.20–39 However, the porosity of COF and the redox-active center on the skeleton are difficult to fully acheive because the agglomeration problem of COF is still severe at the hybrid interface, and the conductivity improvement is minimal and only occurs at the nodes. To overcome the above difficulties, there is an urgent need to optimize heterointerfaces to fully utilize the high conductivity of graphene and the ion storage sites on the COF skeleton.
Herein, we propose a design of anchoring 2D redox-active pyrazine-based COF (BAHC-COF) on the surface of graphene to construct heterointerfaces using a solvent-free strategy. The essence of this design is to use the multi-electron redox reaction of the high-density pyrazine species on the BAHC-COF skeleton to achieve high-capacity Na+ storage and the high conductivity of graphene to realize accelerated charge transport, while inhibiting the random π–π stacking/agglomeration slip of the BAHC layer and releasing its porosity, jointly triggering a fast reversible pseudocapacitance for Na+ storage. As a result, BAHC-COF/graphene (BAHCGO) with comprehensive advantages exhibits a typical pseudocapacitive behavior with a large specific capacitance of 458.6 F g−1. The BAHCGO//AC asymmetric SC achieves a high energy output of 61.2 W h kg−1 and a satisfactory long-term cycling life. Moreover, BAHCGO-based HCDI manifests an attractive SAC of 67.5 mg g−1 accompanied by excellent long-term adsorption/desorption stability.
Experimental
Preparation of GO
Graphene oxide (GO) was fabricated using a modified Hummers’ method.39Preparation of BAHCGO and BAHC
Firstly, GO was dispersed in 10 mL of deionized water (DIW) by sonication to obtain a homogeneous GO suspension. Then, hexaketocyclohexane octahydrate (HC, 104.4 mg), benzene-1,2,4,5-tetraamine tetrahydrochloride (BA, 284 mg), and p-toluenesulfonic acid monohydrate (PTSA, 1.0 g) were introduced into the GO suspension and stirring continued for 1 h. After that, the suspension was heated in a vacuum oven at 120 °C for 72 h. After the reaction was terminated, the BAHCGO was obtained through centrifuging and repeatedly washing (1-methyl-2-pyrrolidone, absolute ethanol, and DIW), while the as-prepared samples with the GO amounts of 50, 75, and 100 mg were denoted as BAHCGO-50, BAHCGO-57, and BAHCGO-100, respectively. For comparison, pristine BAHC-COF was prepared using the same procedure in the absence of GO.Computational method
CP2K quantum chemistry software package was employed to perform all theoretical calculations.40 Multiwfn program was employed to automatically generate CP2K input files, and perform post-processing analysis and electrostatic potential evaluation.41,42 VESTA was employed to draw the chemical structures.43 In detail, the theoretical method of PBEsol with DFT-D3(BJ) formation dispersion correction was employed to perform cell structure optimizing, and the theoretical method of HSE06 with ADMM was employed to calculate crystal orbital distribution, bandgap values, and electrostatic potential isosurface.Results and discussion
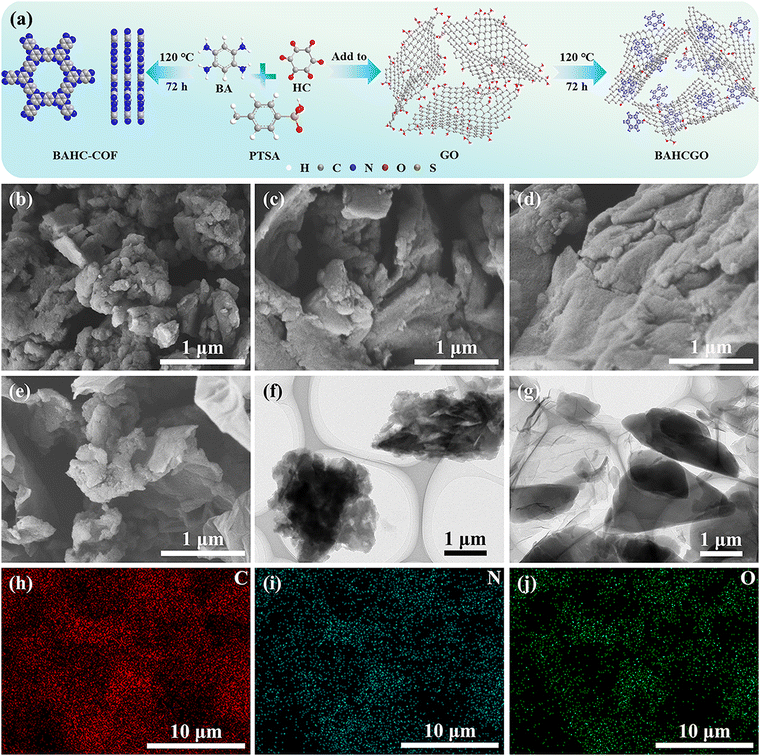
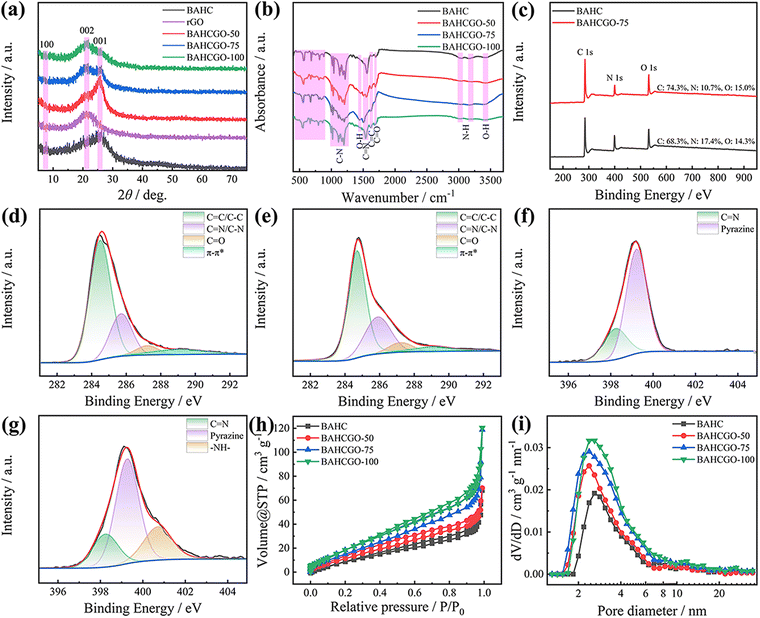
The BAHCGO nanohybrids were synthesized under a solvent-free system through stepwise reactions between organic units and graphene, as shown in Fig. 1a. Graphene as a conductive substrate to inhibit COF layers stacking/accumulation through amide bonds and π–π interactions. Scanning electron microscopy (SEM) images demonstrate that the pristine BAHC exhibits diverse sheet-like structures and are closely packed with each other (Fig. 1b and Fig. S1c, ESI†), consistent with transmission electron microscopy (TEM, Fig. 1f). Graphene (Fig. S1a and b, ESI†) presents a typical smooth two-dimensional sheet structure with surface wrinkles. BAHCGO inherits the two-dimensional layered structure of graphene and spreads multiple irregular BAHC sheets on the surface of graphene (Fig. 1c–e and Fig. S1d–f, ESI†). This nanostructure of graphene-loaded COF sheets can provide a “highway” for electron transfer from redox sites during electrochemical processes. Simultaneously, the graphene can act as an interlayer spacer to inhibit the aggregation between BAHCs (Fig. 1g). The element mapping image (Fig. 1h–j) shows that BAHCGO-75 is rich in C, N, and O elements and evenly distributed.X-ray diffraction (XRD, Fig. 2a) patterns of BAHC, rGO, and BAHCGO were used to study their crystal structures. The interlayer charge repulsion due to the electronegative N atoms in the pyrazine ring prevents the long-range ordered π–π stacking of BAHC, so the crystal diffraction peaks of BAHC and BAHCGO broaden.44 The weak and broad peaks of BAHC at 2θ = 7.6° and 26.2° correspond to the (100) and (001) planes, respectively.20,40–44 The (001) plane spacing of BAHC layers calculated from the (001) plane according to the Bragg equation is 3.40 Å. Pure rGO only shows an alone (002) plane around 21.4°.45,46 In addition to showing the (100) and (001) planes consistent with pristine BAHC, BAHCGO also displays the (002) plane consistent with graphene, proving that BAHC was successfully grown on the graphene surface. As the graphene content increases, the (001) plane of BAHC is gradually weakened and the (002) plane of graphene continues to strengthen. The (001) plane spacing of BAHCGO-50, BAHCGO-75, and BAHCGO-100 are 3.45 Å, 3.48 Å, and 3.49 Å, respectively, indicating that the presence of graphene delays the accumulation of BAHC. The chemical structure and functional group composition of BAHCGO-75 were investigated through Fourier transform infrared (FT-IR) spectroscopy and further compared with pristine BAHC (Fig. 2b). The FT-IR spectrum of BAHC shows absorption peaks at 3424.9, 3160.7/3027.7, 1693.2, 1631.9, 1514.3, 875.6–656.2, and 1209.1–1005.2 cm−1, corresponding to the stretching vibration of O–H, N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C–N, and the out-of-plane bending vibration of C–H.18,20,27,47 The additional peak at 1448.7 cm−1 was found for BAHCGO-75 representing the in-plane bending vibration of O–H.48–50 Moreover, compared with BAHC (1633.5 cm−1), the blue-shifts of the C
N, and C–N, and the out-of-plane bending vibration of C–H.18,20,27,47 The additional peak at 1448.7 cm−1 was found for BAHCGO-75 representing the in-plane bending vibration of O–H.48–50 Moreover, compared with BAHC (1633.5 cm−1), the blue-shifts of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of BAHCGO-50 (1626.9 cm−1), BAHCGO-75 (1625.2 cm−1), and BAHCGO-100 (1620.4 cm−1) originate from the π–π interaction between graphene and the BAHC framework.45,48,49
C stretching vibration of BAHCGO-50 (1626.9 cm−1), BAHCGO-75 (1625.2 cm−1), and BAHCGO-100 (1620.4 cm−1) originate from the π–π interaction between graphene and the BAHC framework.45,48,49
The chemical environment of BAHC and BAHCGO-75 was studied by X-ray photoelectron spectroscopy (XPS, Fig. 2c–g). The four peaks of the high-resolution C 1s XPS spectrum (Fig. 2d and e) at 284.5, 285.8, 287.2, and 289.1 eV are related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C bond, C
C/C–C bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–N bond, C
N/C–N bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and π–π* peaks,20,34,44,50 respectively. The high-resolution N 1s XPS spectrum of BAHC (Fig. 2f) shows only the C
O bond, and π–π* peaks,20,34,44,50 respectively. The high-resolution N 1s XPS spectrum of BAHC (Fig. 2f) shows only the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (398.3 eV) and the pyrazine species (399.2 eV) on the BAHC backbone.44 There is no additional pyridine-N, amino, and graphite-N, indicating that the bonding mode of N in BAHC is mainly dominated by the pyrazine-N species. Interestingly, in addition to the C
N bond (398.3 eV) and the pyrazine species (399.2 eV) on the BAHC backbone.44 There is no additional pyridine-N, amino, and graphite-N, indicating that the bonding mode of N in BAHC is mainly dominated by the pyrazine-N species. Interestingly, in addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and pyrazine species, an additional –NH– bond (400.7 e V) formed by acylation reaction of the –NH2 of BA with the –COOH of GO is also observed in the high-resolution N 1s XPS spectrum of BAHCGO-75 (Fig. 2g).27 The above results prove that π–π interactions and amide bonds jointly anchor BAHC to the graphene surface. The high-resolution O1s XPS spectrum in Fig. S2 (ESI†) shows that both BAHC and BAHCGO-75 display two peaks at 530.1 and 531.5 eV, corresponding to quinone O and C
N bond and pyrazine species, an additional –NH– bond (400.7 e V) formed by acylation reaction of the –NH2 of BA with the –COOH of GO is also observed in the high-resolution N 1s XPS spectrum of BAHCGO-75 (Fig. 2g).27 The above results prove that π–π interactions and amide bonds jointly anchor BAHC to the graphene surface. The high-resolution O1s XPS spectrum in Fig. S2 (ESI†) shows that both BAHC and BAHCGO-75 display two peaks at 530.1 and 531.5 eV, corresponding to quinone O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.46,51
O bonds.46,51
The surface pore structures of BAHC and BAHCGO were characterized by N2 adsorption/desorption (Fig. 2h). BAHCGO has a hybrid type II/IV isotherm with an obvious H3 type hysteresis loop, indicating the presence of a hierarchical porous structure comprising micropores and mesopores (verified through pore size distribution in Fig. 2i).20,45,48 In contrast, BAHC exhibits a typical type IV isotherm, indicating the solo existence of a mesoporous structure. The specific surface areas (SSA, calculated through the Brunauer–Emmett–Teller method) of BAHC, BAHCGO-50, BAHCGO-75, and BAHCGO-100 are 40.5, 60.6, 73.6, and 87.4 m2 g−1, respectively. The relatively low SSA of BAHC may be because the interlayer charge resistance of BAHC (supplied by the negatively charged pyrazine species) hinders the ordered interlayer stacking of BAHC, resulting in pore-clogging within BAHC.20,44 Notably, the graphene in BAHCGO could largely limit the random slip π–π stacking/agglomeration of BAHC through π–π interactions and amide bonds and subsequently release its blocked pores. As the graphene content increases, the pore volume of BAHCGO gradually increases and exposes more microporous structural features.
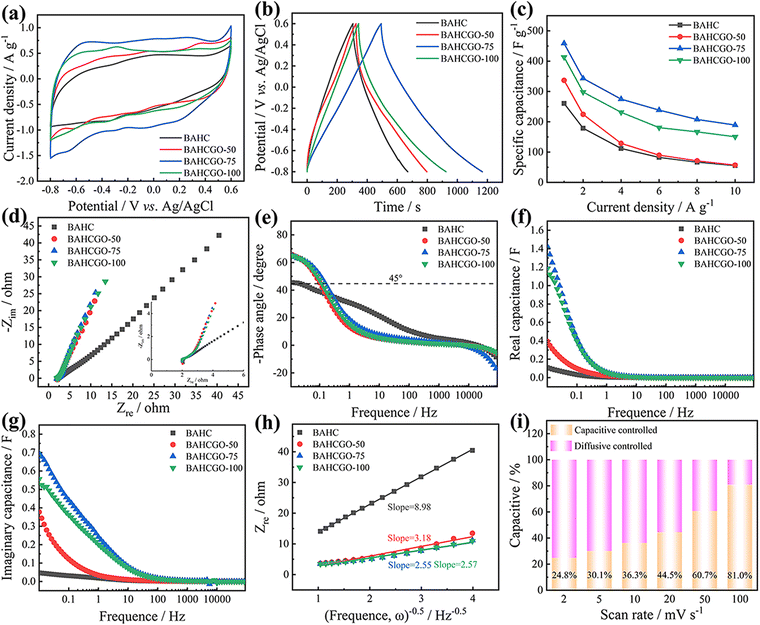
The Na+ storage properties of BAHC and BAHCGO were investigated by cyclic voltammetry (CV) testing. All CV curves in Fig. 3a present a twisted rectangular shape and contain three pairs of flat redox peaks, which is a typical pseudocapacitance feature of BAHC-COF (oriented from the reversible redox reaction of Na+ and pyrazine species in the COF skeleton, Fig. 4a).44,52,53 The specific capacitances of BAHC, BAHCGO-50, BAHCGO-75, and BAHCGO-100 at 2 mV s−1 were calculated to be 248.8, 302.7, 437.5, and 380.8 F g−1, respectively. The improved capacitances of BAHCGO compared to pristine BAHC could be ascribed to the high-speed charge transfer along the graphene layers and rapid Na+ diffusion through the BAHC layers, which increases the pyrazine species utilization on the BAHC skeleton. As the scan rate increases (Fig. S3, ESI†), the CV curves of BAHC and BAHCGO-50 gradually change to a fusiform shape, indicating limited charge transfer kinetics.45,46,51 However, the CV curve shape of BAHCGO-75 and BAHCGO-100 is unchanged, indicating that the increased graphene contents help to accelerate the charge transfer/ion diffusion kinetics. The quantitative capacitive was explored through the formula i(v) = k1v + k2v0.5.16,32,34 Due to the poor kinetic behavior of BAHC, the entire kinetic process is always dominated by diffusion control (Fig. S4b and c, ESI†), while BAHCGO-75 shows dual-mode capacitive control behavior (Fig. S4a and Fig. 3i, ESI†) and has a capacitance contribution close to AC (Fig. S6e, ESI†),16,18 indicating that the dual-mode capacitance behavior of BAHCGO-75 can not only unload the charge transfer barrier but also improve the utilization of active sites and accelerate the uptake rate of ions.18,34,37
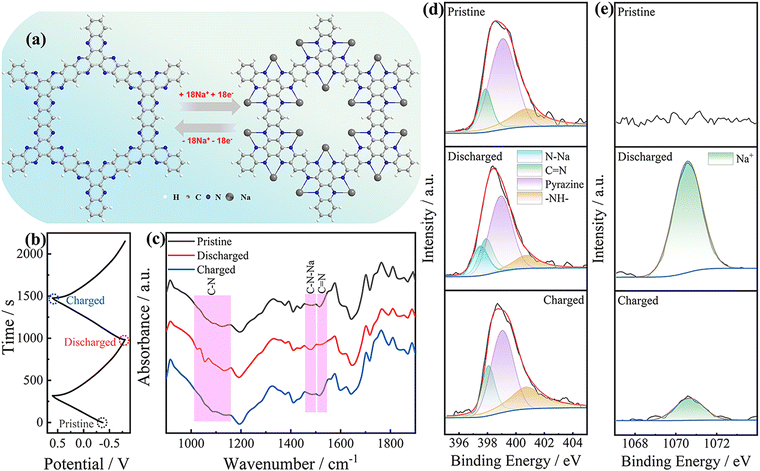
To explore the Na+ storage mechanism of BAHC, the evolution of the redox active sites of the BAHCGO-75 electrode in different potential states in the GCD test (Fig. 4b) was recorded by ex situ FT-IR spectroscopy and XPS spectra. The reversible adsorption/desorption of Na+ into BAHCGO-75 was recorded by ex-situ FT-IR spectroscopy (Fig. 4c). When discharged to −0.8 V, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond stretching vibration absorption peak of the pristine BAHCGO-75 electrode near 1514.6 cm−1 almost completely disappears. Instead, the C–N–Na bond stretching vibration peak appears at 1480.3 cm−1,54 and the C–N bond stretching vibration absorption peak in the range of 1158.3–1011.7 cm−1 is significantly enhanced. When charged to 0.6 V, the C–N–Na peak is absent and the C
N bond stretching vibration absorption peak of the pristine BAHCGO-75 electrode near 1514.6 cm−1 almost completely disappears. Instead, the C–N–Na bond stretching vibration peak appears at 1480.3 cm−1,54 and the C–N bond stretching vibration absorption peak in the range of 1158.3–1011.7 cm−1 is significantly enhanced. When charged to 0.6 V, the C–N–Na peak is absent and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–N peak is restored, indicating that BAHC has reversible adsorption/desorption behaviors of Na+ with the C
N/C–N peak is restored, indicating that BAHC has reversible adsorption/desorption behaviors of Na+ with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond as storage sites during charging/discharging. The Na+ adsorption/desorption on the BAHC skeleton was also recorded by ex situ XPS spectra. The reversible weakening/recovery of C
N bond as storage sites during charging/discharging. The Na+ adsorption/desorption on the BAHC skeleton was also recorded by ex situ XPS spectra. The reversible weakening/recovery of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and pyrazine species and the reversible appearance/disappearance of N–Na bonds (397.6 eV) are recorded during the discharge and charge process (Fig. 4d), further verifying the reversible adsorption/desorption of Na+ on the C
N bonds and pyrazine species and the reversible appearance/disappearance of N–Na bonds (397.6 eV) are recorded during the discharge and charge process (Fig. 4d), further verifying the reversible adsorption/desorption of Na+ on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.52,55 The reversible enhancement/weakening of the high-resolution Na 1s XPS spectra further verified the Na+ adsorption/desorption centered on the C
N bond.52,55 The reversible enhancement/weakening of the high-resolution Na 1s XPS spectra further verified the Na+ adsorption/desorption centered on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Fig. 4e).
N bond (Fig. 4e).
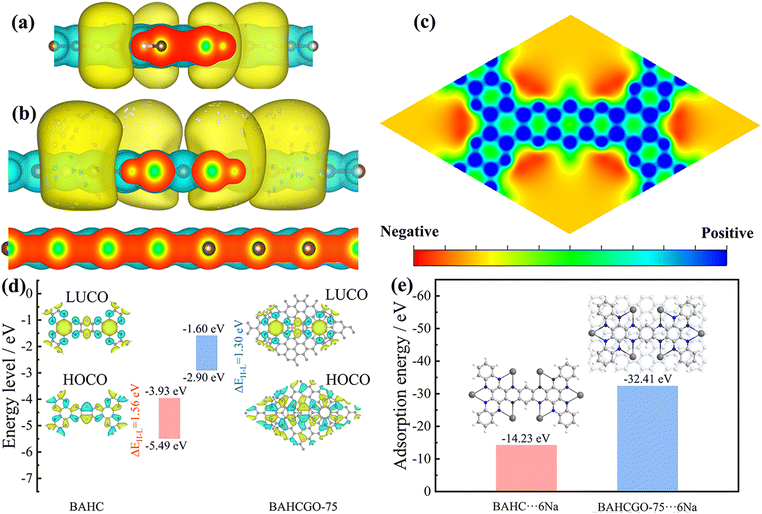
The Na+ storage mechanism in repeating units of BAHC and BAHCGO-75 electrodes was further understood through theoretical calculations based on density functional theory (DFT). Parallel views of the differential charge density of BAHC and BAHCGO-75 are shown in Fig. 5a and b. Compared with BAHC, charge depletion of the graphene layer and a large amount of charge accumulation around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N active area can be observed in BAHCGO-75. These results mean that the strong interlayer π–π interaction between the BAHC-COF and graphene interface leads to the delocalization of part of the electrons from graphene to the lowest unoccupied crystal orbital (LUCO) of the COF, which improves the stability of the COF layer and accelerates the charge transmission.36 The molecular electrostatic potential (MESP, Fig. 5c) shows that the electron-rich region is mainly concentrated near the pyrazine N atoms, implying that the best storage location of Na+ is near them.52 The highest occupied crystal orbital (HOCO) and the LUCO are shown in Fig. 5d. The LUCO energy level of BAHCGO-75 (1.60 eV) is much higher than that of BAHC (3.93 eV), indicating that BAHCGO-75 has a higher charge transfer rate and redox activity.36,37 In addition, the lower HOCO/LUCO energy gap (ΔE) of BAHCGO-75 (1.30 eV) compared with that of BAHC (1.56 eV) further verifies the efficient charge transfer of BAHCGO-75. The optimized structure of BAHC and BAHCGO-75 bonded with 6 Na+ during the electrochemical process is shown in Fig. 5e. The adsorption energy of BAHC and BAHCGO-75 bonding 6 Na+ are 14.23 and 32.41 eV, respectively, indicating that BAHCGO-75 has a stronger adsorption capacity for Na+. The above results indicate that the pyrazine N groups in BAHCGO-75 can store Na+ more efficiently.
N active area can be observed in BAHCGO-75. These results mean that the strong interlayer π–π interaction between the BAHC-COF and graphene interface leads to the delocalization of part of the electrons from graphene to the lowest unoccupied crystal orbital (LUCO) of the COF, which improves the stability of the COF layer and accelerates the charge transmission.36 The molecular electrostatic potential (MESP, Fig. 5c) shows that the electron-rich region is mainly concentrated near the pyrazine N atoms, implying that the best storage location of Na+ is near them.52 The highest occupied crystal orbital (HOCO) and the LUCO are shown in Fig. 5d. The LUCO energy level of BAHCGO-75 (1.60 eV) is much higher than that of BAHC (3.93 eV), indicating that BAHCGO-75 has a higher charge transfer rate and redox activity.36,37 In addition, the lower HOCO/LUCO energy gap (ΔE) of BAHCGO-75 (1.30 eV) compared with that of BAHC (1.56 eV) further verifies the efficient charge transfer of BAHCGO-75. The optimized structure of BAHC and BAHCGO-75 bonded with 6 Na+ during the electrochemical process is shown in Fig. 5e. The adsorption energy of BAHC and BAHCGO-75 bonding 6 Na+ are 14.23 and 32.41 eV, respectively, indicating that BAHCGO-75 has a stronger adsorption capacity for Na+. The above results indicate that the pyrazine N groups in BAHCGO-75 can store Na+ more efficiently.
The galvanostatic charge–discharge (GCD) curves of BAHC and BAHCGO at 1 A g−1 in Fig. 3b show nonlinearity and no obvious platform characteristics in the voltage range of −0.8–0.6 V. The specific capacitances of BAHC, BAHCGO-50, BAHCGO-75, and BAHCGO-100 at 1 A g−1 (Fig. 3c) are 260.7, 336.8, 458.6, and 413.3 F g−1, respectively. The compensation resistance gradually accumulates with the increase of current density, which causes the specific capacitance to gradually decrease, but BAHCGO-75 still exhibits the largest specific capacitance. Nyquist plots (Fig. 3d) were used to examine the charge transfer kinetics of BAHC and BAHCGO (the fitting equivalent circuit diagram of the EIS test is shown in Fig. S5, ESI†). The horizontal axis in the high-frequency range relates to the ohmic resistance (Rs).9,56 Both BAHC and BAHCGO show similar intercepts, indicating that the introduction of graphene does not change the Rs value. The twisted arc in the mid-frequency range is related to the charge transfer resistance (Rct).49,51 The Rct values of BAHC, BAHCGO-50, BAHCGO-75, and BAHCGO-100 are 1.41, 0.82, 0.52, and 0.76 Ω, respectively. The sloped line in the low-frequency range is related to the Warburg impedance (Zw).46,48 The smallest end and steepest slope of BAHCGO-75 in the low-frequency range indicates its fast ion migration rate. The relaxation time constant (τ0) is usually used to reflect the speed of electrochemical reactions on the electrode surface, defined as τ0 = 1/f0 (where f0 is the frequency at the angle of 45°).51,57 Compared with BAHC (54.95 s), BAHCGO-50 (9.32 s), and BAHCGO-100 (8.41 s), the lowest τ0 value of BAHCGO-75 (6.55 s) (Fig. 3e) indicates that BAHCGO-75 has faster reaction kinetics. In addition, a typical composite capacitance model (C(ω) = Cre(ω) − jCim(ω)) is used to evaluate the impedance behavior of the electrode, and the real capacitance (Cre(ω)) and imaginary capacitance (Cim(ω)) are determined according to the formula, Cre(ω) = −Zim/2πf/(Zre2 + Zim2), Cim(ω) = Zre/2πf/(Zre2 + Zim2).57,58 BAHCGO-75 has a higher Cre value (1.43 F) and Cim value (0.70 F) (Fig. 3f and g), further confirming that BAHCGO-75 has fast charge transport and ion response dynamics. The Warburg coefficient (σ) also verifies that BAHCGO-75 has faster reaction kinetics.59,60 As shown in Fig. 3h, the calculated σ values are 8.98 Ω s−1/2 for BAHC, 3.18 Ω s−1/2 for BAHCGO-50, 2.55 Ω s−1/2 for BAHCGO-75, and 2.57 Ω s−1/2 for BAHCGO-100. The best electrochemical performance of BAHCGO-75 benefits from the combination of graphene and BAHC. The highly conductive framework of graphene can serve as the growth matrix of BAHC and weaken the agglomeration and stacking of BAHC, thus supplying efficient electron transfer and highly accessible ion storage sites.
An aqueous Na+ asymmetric SC (BAHCGO-75//AC) was constructed in a 1 M NaCl electrolyte to evaluate the practicability of BAHCGO-75. The mass ratio of BAHCGO-75 to the AC electrode was calculated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
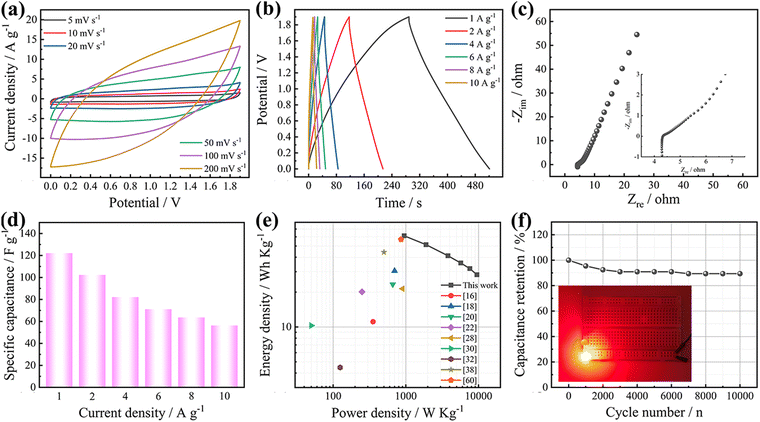
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to maintain charge balance (Fig. S6, ESI†). Fig. 6a and b show the CV and GCD curves of BAHCGO-75//AC under the operating voltage of 0–1.9 V. They have obvious pseudocapacitive properties, with a maximum specific capacitance of 122.1 F g−1 at 1 A g−1 (Fig. 6d). Nyquist (Fig. 6c) shows that BAHCGO-75//AC possesses excellent charge transfer/ion migration kinetics. The energy/power output of BAHCGO-75//AC calculated based on the GCD curve is shown in Fig. 6e, achieving a high energy output of 61.2 W h kg−1 at a power density of 950 W kg−1. Fig. 6f shows that the capacitance retention rate of BAHCGO-75//AC after 10
2 to maintain charge balance (Fig. S6, ESI†). Fig. 6a and b show the CV and GCD curves of BAHCGO-75//AC under the operating voltage of 0–1.9 V. They have obvious pseudocapacitive properties, with a maximum specific capacitance of 122.1 F g−1 at 1 A g−1 (Fig. 6d). Nyquist (Fig. 6c) shows that BAHCGO-75//AC possesses excellent charge transfer/ion migration kinetics. The energy/power output of BAHCGO-75//AC calculated based on the GCD curve is shown in Fig. 6e, achieving a high energy output of 61.2 W h kg−1 at a power density of 950 W kg−1. Fig. 6f shows that the capacitance retention rate of BAHCGO-75//AC after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles is 89.4%. The performance decline after cycling may be due to the degradation of the COF skeleton caused by long-term ion doping/dedoping and the continued accumulation of polarization resistance.15,22,34 The electrochemical properties of BAHCGO-75 are compared with the reported organic/graphene composites (Fig. 6e). Among them, the good electrochemical properties of BAHCGO-75 grant it a potential value in high-performance energy storage devices. The excellent performance of BAHCGO-75//AC stems from the cooperation between the conductive networks supported by graphene and the abundant Na+ storage sites on the BAHC. In this hybrid system, the formed hierarchical porous structure not only accelerates electron transfer but also exposes more Na+ storage sites. Furthermore, two BAHCGO-75//AC connected in series can make a red light-emitting diode (LED) light up for up to 35 minutes after charging (inset in Fig. 6f and Fig. S7, ESI†), further verifying its practical application.
000 cycles is 89.4%. The performance decline after cycling may be due to the degradation of the COF skeleton caused by long-term ion doping/dedoping and the continued accumulation of polarization resistance.15,22,34 The electrochemical properties of BAHCGO-75 are compared with the reported organic/graphene composites (Fig. 6e). Among them, the good electrochemical properties of BAHCGO-75 grant it a potential value in high-performance energy storage devices. The excellent performance of BAHCGO-75//AC stems from the cooperation between the conductive networks supported by graphene and the abundant Na+ storage sites on the BAHC. In this hybrid system, the formed hierarchical porous structure not only accelerates electron transfer but also exposes more Na+ storage sites. Furthermore, two BAHCGO-75//AC connected in series can make a red light-emitting diode (LED) light up for up to 35 minutes after charging (inset in Fig. 6f and Fig. S7, ESI†), further verifying its practical application.
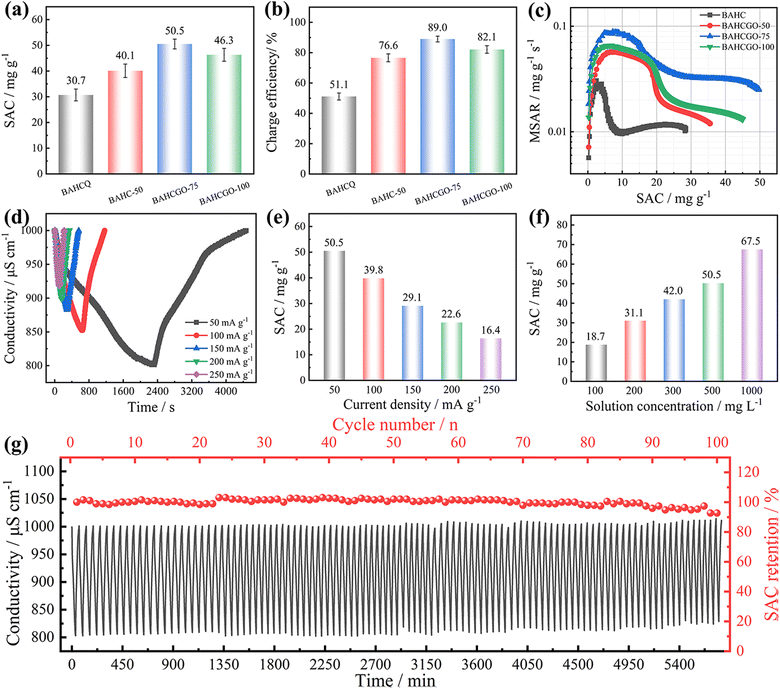
To evaluate the seawater desalination performance of BAHC and BAHCGO, HCDI tests were conducted in batch mode with a BAHC/BAHCGO cathode and AC anode. The desalination performances of BAHC, BAHCGO-50, BAHCGO-75, and BAHCGO-100 were evaluated using the constant current mode with a cut-off voltage of ±1.6 V. The concentration and conductivity of the circulating fluid drop sharply once the current is applied, and the concentration gradually returns to the initial value when the current is reversed (Fig. 7d and Fig. S8, S9, ESI†). Compared with BAHC (30.7 mg g−1/51.5%), the SACs and charge efficiencies of BAHCGO-50, BAHCGO-75, and BAHCGO-100 at 50 mA g−1 are greatly improved, reaching 40.1 mg g−1/76.6%, 50.5 mg g−1/89.0%, and 46.3 mg g−1/82.1%, respectively (Fig. 7a and b). The excellent SAC/charge efficiency of BAHCGO-75 benefits from the charge transfer highway provided by graphene and the sufficient Na+ adsorption sites within BAHC. In Ragone plots (Fig. 7c), BAHCGO-75 occupies the highest point, further confirming the excellent desalination ability and fastest desalination rate of BAHCGO-75.61–64 In addition, even compared with previously reported carbon-based CDI (Table S1, ESI†), BAHCGO-75 still has a far superior SAC and mean salt adsorption rate (MSAR). This observation shows that the addition of graphene not only increases the utilization of active sites, but its inhibitory effect on the self-stacking of the COF layer also helps accelerate the diffusion rate of Na+ and enhance the capacitive contribution to the total capacitance of BAHCGO-75, thereby triggering higher ion absorption rates. Furthermore, BAHCGO-75 remains the most outstanding at higher current densities (Fig. 7e and Fig. S8, ESI†).
The SAC of BAHCGO-75 in various salt concentration solutions is shown in Fig. 7f. Apparently, the SAC of BAHCGO-75 is significantly affected by the initial solubility of the NaCl solution, reaching 67.5 mg g−1 in 1000 mg L−1 NaCl solution. Surprisingly, the SAC can also reach 18.7 mg g−1 in 100 mg L−1 NaCl solution, demonstrating its potential application in desalinating water with low salinity. Compared with the reported organic polymer-based CDI electrode materials (Table 1), BAHCGO-75 still exhibits excellent desalination performance. The excellent desalination ability of BAHCGO-75 can be attributed to the synergistic effect between BAHC and graphene. The graphene as the substrate in BAHCGO-75 provides a shortcut to accelerate charge migration, thereby improving the reversible Na+ adsorption/desorption kinetics centered on the pyrazine species in the BAHC. Furthermore, graphene inhibits the aggregation/packing of BAHC layers and increases the Na+ accessibility of pyrazine species in the BAHC framework through π–π interactions and amide bonds.
| Material | Voltage (V) | Initial concentration (mg L−1) | SAC (mg g−1) | Cycling stability | Ref. |
|---|---|---|---|---|---|
| DAAQ-TFP-COF | 1.4 | 500 | 22.8 | 90.1% (20 cycles) | 15 |
| MXene@DAAQ-TFPCOF | 1.6 | 1000 | 53.1 | 92.2% (100 cycles) | 26 |
| TFPDQGO | ±1.6 | 1000 | 58.4 | 84.5% (50 cycles) | 39 |
| Porous activated carbons/PANI | 1.2 | 500 | 35.3 | 37.0% (100 cycles) | 62 |
| Poly[N,N′-(ethane-1,2-diyl)-1,4,5,8-naphthalenetetracarboxiimide] | 1.8 | 1000 | 54.2 | 37.0% (100 cycles) | 65 |
| Mesoporous polydopamine/MXene | 1.5 | 1000 | 37.72 | 98.6% (200 cycles) | 66 |
| Poly (anthraquinonyl sulfide) | 1.0 | 29221.4 | 53.0 | — | 67 |
| Covalent triazine-based frameworks | 1.2 | 1000 | 29.34 | 100% (12 cycles) | 68 |
| Poly-p-phenylene | ±1.8 | 3500 | 52.5 | 85.1% (40 cycles) | 69 |
| BAHCGO-75 | ±1.6 | 1000 | 67.5 | 92.7% (100 cycles) | This work |
Excellent CDI electrodes require reversible cycling regeneration capabilities, so long-term cycling stability is also one significant parameter for evaluating the HCDI performance. The desalination/regeneration performance of BAHCGO-75 was explored in 100 desalination/regeneration cycles through repeated charge/discharge processes in constant current mode, as shown in Fig. 7g. Although the SAC fluctuates significantly in the first 70 cycles, there is no obvious downward trend, and even the SAC retention exceeds 100% in some cycles. This may be because the salt solution penetrated the BAHCGO-75 interlayer, increasing the interlayer spacing and the exposure of active sites. After 100 cycles, BAHCGO-75 achieves a SAC retention of 92.7%, superior to the 83.2% retention of BAHC (Fig. S10, ESI†). In addition, the well-matched SEM images, XRD patterns, and chemical structures of the BAHCGO-75 electrode before and after 100 desalination/regeneration cycles further verified the excellent electrochemical reversibility of BAHCGO-75 (Fig. S11, ESI†). The improved desalination/regeneration stability of BAHCGO-75 benefits from the delocalized π-electrons generated by the π–π interaction between graphene and BAHC, which accelerates the charge transfer kinetics of pyrazine species on the BAHC skeleton, mitigates the agglomeration of BAHC and improves the reversibility of Na+ adsorption/desorption.63 Simultaneously, the presence of graphene also helps alleviate the stress/strain caused by long-term Na+ adsorption/desorption and prevents degradation of the COF skeleton.37 The decreased SAC in the late cycles might be oriented from the following points: (i) some ions cannot completely desorb and occupy the pyrazine species (even if a reverse voltage is applied), blocking the ion diffusion channel, and increasing the co-ion effect;62 (ii) reverse voltage leads to partial oxidation of graphene and BAHC during regeneration, triggering the destruction of the interconnection network structure and surface chemical charges, destruction and dissolution of BAHC skeleton, and the degradation of pseudocapacitive ion storage.64
Conclusions
In summary, we reported a heterogeneous interface regulation strategy for grafting redox-active BAHC-COF on graphene surfaces to prepare high-performance SC and CDI cathodes. The essence of this work is that graphene anchors the BAHC layer through π–π interactions and amide bonds to suppress random sliding π–π stacking/agglomeration of BAHC and release its porosity. Moreover, more delocalized π-electrons in BAHCGO accelerate charge transport. Benefiting from the excellent electrochemical reaction kinetics, the BAHCGO-75-based asymmetric SC and HCDI systems have exciting energy storage and desalination capacity with a high energy output of 61.2 W h kg−1, exciting SAC of 67.5 mg g−1 and excellent long-term cycling stability. This study reflects the unique advantages of COF-based hybrid materials, which could offer a valuable template for the future development of energy storage and environmental science.Author contributions
L. X. and L. P. conceived the concept. L. X. designed the experiments, constructed the devices, and performed data measurements. Y. L., X. X., Y. L., and T. L. analysed the data. L. X. and L. P. wrote the manuscript. Y. L. and T. L. helped with the experiments. Y. L. and L. P. provided some useful suggestions. Y. L., X. X., and L. P. supervised this work. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (52009057), and the Natural Science Foundation of Shandong Province (ZR201910240303) is gratefully acknowledged.Notes and references
- Y. L. Shao, M. F. El-Kady, J. Y. Sun, Y. G. Li, Q. H. Zhang, M. F. Zhu, H. Z. Wang, B. Dunn and R. B. Kaner, Chem. Rev., 2018, 118, 9233–9280 CrossRef CAS PubMed.
- X. H. Wang, T. S. Mathis, K. Li, Z. F. Lin, L. Vlcek, T. Torita, N. C. Osti, C. Hatter, P. Urbankowski, A. Sarycheva, M. Tyagi, E. Mamontov, P. Simon and Y. Gogotsi, Nat. Energy, 2019, 4, 241–248 CrossRef CAS.
- Z. Y. Zhao, K. Q. Xia, Y. Hou, Q. H. Zhang, Z. Z. Yead and J. G. Lu, Chem. Soc. Rev., 2021, 50, 12702 RSC.
- P. Srimuk, X. Su, J. Yoon, D. Aurbach and V. Presser, Nat. Rev. Mater., 2020, 5, 517–538 CrossRef CAS.
- K. G. Sun, M. Tebyetekerwa, C. Wang, X. F. Wang, X. W. Zhang and X. S. Zhao, Adv. Funct. Mater., 2023, 33, 2213578 CrossRef CAS.
- L. M. Xu, W. Q. Zhou, S. X. Chao, Y. M. Liang, X. Q. Zhao, C. C. Liu and J. K. Xu, Adv. Energy Mater., 2022, 12, 2200101 CrossRef CAS.
- F. Y. Meng, X. B. Tu, Y. Liu, K. Wang, X. T. Xu, X. J. Li, Z. W. Gong, T. Lu and L. K. Pan, Sep. Purif. Technol., 2024, 329, 125155 CrossRef CAS.
- P. Zhang, M. M. He, F. K. Li, D. Z. Fang, C. Li, X. P. Mo, K. X. Li and H. Wang, Environ. Sci. Technol., 2024, 58, 2112–2122 CrossRef CAS PubMed.
- L. M. Xu, Z. B. Ding, Y. Y. Chen, X. T. Xu, Y. Liu, J. B. Li, T. Lu and L. K. Pan, J. Colloid Interface Sci., 2023, 630, 372–381 CrossRef CAS PubMed.
- P. Zhang, M. M. He, Z. K. Xu, F. K. Li, D. Z. Fang, C. Li, C. C. Lv, X. P. Mo, K. X. Li and H. Wang, Chem. Eng. J., 2024, 481, 148518 CrossRef CAS.
- Q. C. Yu, Z. H. Xue, M. C. Li, P. M. Qiu, C. G. Li, S. P. Wang, J. X. Yu, H. Nara, J. Na and Y. Yamauchi, Adv. Energy Mater., 2021, 11, 2002523 CrossRef CAS.
- Y. K. An, S. S. Tan, Y. Liu, K. Zhu, L. Hu, Y. G. Rong and Q. Y. An, Energy Storage Mater., 2021, 41, 354–379 CrossRef.
- J. H. Sun, A. Klechikov, C. Moise, M. Prodana, M. Enachescu and A. V. Talyzin, Angew. Chem., Int. Ed., 2018, 57, 1034 CrossRef CAS PubMed.
- S. Haldar, R. Kushwaha, R. Maity and R. Vaidhyanathan, ACS Mater. Lett., 2019, 1, 490–497 CrossRef CAS.
- Y. Q. Li, Z. B. Ding, X. L. Zhang, J. L. Li, X. J. Liu, T. Lu, Y. F. Yao and L. K. Pan, J. Mater. Chem. A, 2019, 7, 25305 RSC.
- D. Cui, W. Xie, S. R. Zhang, Y. H. Xu and Z. M. Su, Polym. Chem., 2023, 14, 803–810 RSC.
- S. Patial, P. Raizada, A. A. P. Khan, A. Singh, Q. V. Le, V. H. Nguyen, R. Selvasembian, C. M. Hussain and P. Singh, Chem. Eng. J., 2022, 433, 134594 CrossRef CAS.
- N. An, Z. Guo, J. Xin, Y. Y. He, K. F. Xie, D. M. Sun, X. Y. Dong and Z. G. Hu, J. Mater. Chem. A, 2021, 9, 16824–16833 RSC.
- Z. Meng, R. M. Stolz and K. A. Mirica, J. Am. Chem. Soc., 2019, 141, 11929–11937 CrossRef CAS PubMed.
- S. Kandambeth, J. T. Jia, H. Wu, V. S. Kale, P. T. Parvatkar, J. Czaban-Jóźwiak, S. Zhou, X. M. Xu, Z. O. Ameur, E. Abou-Hamad, A. H. Emwas, O. Shekhah, H. N. Alshareef and M. Eddaoudi, Adv. Energy Mater., 2020, 10, 2001673 CrossRef CAS.
- D. Pakulski, V. Montes-Garcia, A. Gorczynski, W. Czepa, T. Chudziak, P. Samori and A. Ciesielski, J. Mater. Chem. A, 2022, 10, 16685 RSC.
- C. J. Wang, F. Liu, S. J. Yan, C. Liu, Z. X. Yu, J. S. Chen, R. Lyu, Z. Y. Wang, M. Y. Xu, S. L. Dai, Y. Chen and L. Wei, Carbon, 2022, 190, 412–421 CrossRef CAS.
- C. X. Li, J. Yang, P. Pachfule, S. Li, M. Y. Ye, J. Schmidt and A. Thomas, Ultralight covalent organic framework/graphene aerogels with hierarchical porosity, Nat. Commun., 2020, 11, 4712 CrossRef CAS PubMed.
- X. J. Zhao, P. Pachfule and A. Thomas, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC.
- Q. H. Geng, H. C. Wang, Y. Wu, L. P. Lv, S. Q. Chen, W. W. Sun and Y. Wang, ChemElectroChem, 2022, 9, e202200340 CrossRef CAS.
- S. H. Zhang, X. T. Xu, X. H. Liu, Q. Yang, N. Z. Shang, X. X. Zhao, X. H. Zang, C. Wang, Z. Wang, J. G. Shapter and Y. Yamauchi, Mater. Horiz., 2022, 9, 1708–1716 RSC.
- X. Y. Kong, S. Y. Zhou, M. Strømme and C. Xu, Carbon, 2021, 171, 248–256 CrossRef CAS.
- X. Zhao, M. Sajjad, Y. Q. Zheng, M. M. Zhao, Z. J. Li, Z. Y. Wu, K. Kang and L. Qiu, Carbon, 2021, 182, 144–154 CrossRef CAS.
- H. C. Yang, Y. Y. Chen, S. Y. Suen and R. H. Lee, Polymer, 2023, 273, 125853 CrossRef CAS.
- C. J. Wang, F. Liu, J. S. Chen, Z. W. Yuan, C. Liu, X. S. Zhang, M. Y. Xu, L. Wei and Y. Chen, Energy Storage Mater., 2020, 32, 448–457 CrossRef.
- Z. J. Xu, Y. N. Liu, Z. T. Wu, R. T. Wang, Q. F. Wang, T. Li, J. H. Zhang, J. Cheng, Z. H. Yang, S. F. Chen, M. H. Miao and D. H. Zhang, Chem. Eng. J., 2020, 387, 124071 CrossRef CAS.
- Y. Y. Dong, Y. L. Wan, X. F. Zhang, Q. Lai and Y. K. Yang, Chem. Eng. J., 2022, 449, 137858 CrossRef CAS.
- Y. Shi, Y. Liu, X. Huang and X. Qian, Mater. Today Chem., 2023, 31, 101617 CrossRef CAS.
- Y. Q. Jiang, Z. Y. Zhang, D. Chen, J. G. Du, Y. H. Yang, S. Wang, F. Guo, X. Y. Chen, C. Gao, W. J. Wang and P. W. Liu, Adv. Mater., 2022, 34, 2204250 CrossRef CAS PubMed.
- P. Y. Wang, Q. Wu, L. F. Han, S. Wang, S. M. Fang, Z. H. Zhang and S. M. Sun, RSC Adv., 2015, 5, 27290 RSC.
- J. J. Zhao, M. M. Zhou, J. Chen, L. Y. Wang, Q. Zhang, S. W. Zhong, H. J. Xie and Y. T. Li, Small, 2023, 19, 2303353 CrossRef CAS PubMed.
- X. L. Liu, Y. C. Jin, H. L. Wang, X. Y. Yang, P. P. Zhang, K. Wang and J. Z. Jiang, Adv. Mater., 2022, 34, 2203605 CrossRef CAS PubMed.
- M. Y. Yao, C. F. Guo, Q. H. Geng, Y. F. Zhang, X. Zhao, X. Zhao and Y. Wang, Ind. Eng. Chem. Res., 2022, 61, 7480–7488 CrossRef CAS.
- L. M. Xu, Y. Liu, Z. B. Ding, X. T. Xu, X. J. Liu, Z. W. Gong, J. B. Li, T. Lu and L. K. Pan, Small, 2023, 2307843 Search PubMed.
- T. D. Kühne, M. Iannuzzi, M. D. Ben, V. V. Rybkin, P. Seewald, F. Stein, T. Laino, R. Z. Khaliullin, O. Schütt, F. Schiffmann, D. Golze, J. Wilhelm, S. Chulkov, M. H. Bani-Hashemian, V. Weber, U. Borštnik, M. Taillefumier, A. S. Jakobovits, A. Lazzaro, H. Pabst, T. Müller, R. Schade, M. Guidon, S. Andermatt, N. Holmberg, G. K. Schenter, A. Hehn, A. Bussy, F. Belleflamme, G. Tabacchi, A. Glöβ, M. Lass, I. Bethune, C. J. Mundy, C. Plessl, M. Watkins, J. VandeVondele, M. Krack and J. Hutter, J. Chem. Phys., 2020, 152, 194103 CrossRef PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- J. Zhang and T. Lu, Phys. Chem. Chem. Phys., 2021, 23, 20323–20328 RSC.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- Z. Q. Lin, J. Xie, B. W. Zhang, J. W. Li, J. N. Weng, R. B. Song, X. Huang, H. Zhang, H. Li, Y. Liu, Z. C. J. Xu, W. Huang and Q. C. Zhang, Nano Energy, 2017, 41, 117–127 CrossRef CAS.
- L. M. Xu, Y. Y. Zhang, W. Q. Zhou, F. X. Jiang, H. Zhang, Q. L. Jiang, Y. H. Jia, R. Wang, A. Q. Liang, J. K. Xu and X. M. Duan, ACS Appl. Mater. Interfaces, 2020, 12, 45202–45213 CrossRef CAS PubMed.
- L. M. Xu, D. H. Zhu, W. Q. Zhou, F. X. Jiang, Y. L. Wu, Y. Cai, H. Kang and J. K. Xu, J. Energy Storage, 2021, 43, 103303 CrossRef.
- Y. Zhao, Y. X. Huang, F. Wu, R. J. Chen and L. Li, Adv. Mater., 2021, 33, 2106469 CrossRef CAS PubMed.
- L. M. Xu, Y. Cai, F. X. Jiang, H. Kang, J. K. Xu, W. Q. Zhou and X. M. Duan, Mater. Chem. Front., 2021, 5, 3073–3084 RSC.
- Z. Q. Chen, X. T. Xu, K. Wang, D. Jiang, F. Y. Meng, T. Lu, Y. Yamauchi and L. K. Pan, Sep. Purif. Technol., 2023, 315, 123628 CrossRef CAS.
- J. Xu, M. J. Shi, J. He, H. Ye, H. T. Zhu, C. Yang, L. P. Zhao and C. Yan, Composites, Part B, 2022, 235, 109750 CrossRef CAS.
- L. M. Xu, J. Wu, W. Q. Zhou, F. X. Jiang, H. Zhang, R. Wang, A. Q. Liang, J. K. Xu and X. M. Duan, Electrochim. Acta, 2020, 354, 136703 CrossRef CAS.
- Y. L. Lin, H. L. Cui, C. Liu, R. Li, S. P. Wang, G. M. Qu, Z. Q. Wei, Y. H. Yang, Y. X. Wang, Z. J. Tang, H. F. Li, H. Y. Zhang, C. Y. Zhi and H. M. Lv, Angew. Chem., Int. Ed., 2023, 62, e202218745 CrossRef CAS PubMed.
- C. X. Peng, G. H. Ning, J. Su, G. M. Zhong, W. Tang, B. B. Tian, C. L. Su, D. Y. Yu, L. H. Zu, J. H. Yang, M. F. Ng, Y. S. Hu, Y. Yang, M. Armand and K. P. Loh, Nat. Energy, 2017, 2, 17074 CrossRef CAS.
- T. T. Shi, G. F. Li, Y. Han, Y. J. Gao, F. Wang, Z. J. Hu, T. T. Cai, J. Chu and Z. P. Song, Energy Storage Mater., 2022, 50, 265–273 CrossRef.
- K. K. Jia, H. T. Liu, G. M. Huang, J. W. Zhang, X. R. Liu, L. Li, L. N. Zhu and F. Wu, J. Mater. Chem. A, 2022, 10, 14917 RSC.
- L. M. Xu, G. D. Pan, C. Y. Yu, J. B. Li, Z. W. Gong, T. Lu and L. K. Pan, Inorg. Chem. Front., 2023, 10, 1748–1757 RSC.
- C. J. Raj, R. Manikandan, P. Thondaiman, P. Sivakumar, A. D. Savariraj, W. J. Cho, B. C. Kim and H. Jung, Carbon, 2021, 184, 266–276 CrossRef CAS.
- C. Zheng, M. Yoshio, L. Qi and H. Y. Wang, J. Power Sources, 2014, 260, 19–26 CrossRef CAS.
- K. Li, X. H. Wang, X. F. Wang, M. Y. Liang, V. Nicolosi, Y. X. Xu and Y. Gogotsi, Nano Energy, 2020, 75, 104971 CrossRef CAS.
- T. Li, X. D. Yan, W. D. Zhang, W. K. Han, Y. Liu, Y. X. Li, H. Y. Zhu, Z. J. Li and Z. G. Gu, Chem. Commun., 2020, 56, 14187 RSC.
- F. Y. Meng, Z. B. Ding, X. T. Xu, Y. Liu, T. Lu and L. K. Pan, Sep. Purif. Technol., 2023, 317, 123899 CrossRef CAS.
- B. F. Li, Q. Cao, Y. Liu, Y. K. Sun, X. L. Ma, X. G. Duan, C. M. Chen and Y. X. Wang, J. Mater. Chem. A, 2022, 10, 24905–24914 RSC.
- S. Kandambeth, V. S. Kale, O. Shekhah, H. N. Alshareef and M. Eddaoudi, Adv. Energy Mater., 2022, 12, 2100177 CrossRef CAS.
- J. Ma, Y. J. Cheng, L. Wang, X. H. Dai and F. Yu, Chem. Eng. J., 2020, 384, 123329 CrossRef CAS.
- Y. Q. Li, Z. B. Ding, J. F. Li, J. B. Li, T. Lu and L. K. Pan, Desalination, 2019, 469, 114098 CrossRef CAS.
- Q. Li, X. T. Xu, J. R. Guo, J. P. Hill, H. S. Xu, L. X. Xiang, C. Li, Y. Yamauchi and Y. Y. Mai, Angew. Chem., Int. Ed., 2021, 60, 26528–26534 CrossRef CAS PubMed.
- W. F. Wei, W. S. Zou, D. Z. Yang, R. J. Zheng, R. H. Wang and H. Chen, J. Mater. Chem. A, 2020, 8, 11811–11817 RSC.
- D. H. Liu, X. A. Ning, Y. X. Hong, Y. Li, Q. S. Bian and J. P. Zhang, Electrochim. Acta, 2019, 296, 327–334 CrossRef CAS.
- W. Q. Kong, X. Ge, M. Q. Yang, Q. G. Zhang, J. Y. Lu, H. K. Wen, H. Y. Wen, D. S. Kong, M. Zhang, X. Zhu and Y. Y. Feng, Desalination, 2023, 553, 116452 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mh00161c |
| This journal is © The Royal Society of Chemistry 2024 |