Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Revolutionizing eye care: the game-changing applications of nano-antioxidants in ophthalmology
Yuhang
Cheng†
ab,
Shundong
Cai†
ab,
Han
Wu†
b,
Jintao
Pan
a,
Min
Su
*c,
Xingyuan
Wei
ab,
Jinfa
Ye
b,
Lang
Ke
b,
Gang
Liu
 *a and
Chengchao
Chu
*a and
Chengchao
Chu
 *ab
*ab
aShen Zhen Research Institute of Xiamen University, Shenzhen 518057, China. E-mail: chuchengchao@xmu.edu.cn; gangliu.cmitm@xmu.edu.cn
bXiamen University affiliated Xiamen Eye Center, Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, School of Medicine, Xiamen University, Xiamen, 361102, China
cDepartment of Pharmacy, Xiamen Medical College, Xiamen 361023, China. E-mail: sumin@xmmc.edu.cn
First published on 11th March 2024
Abstract
Since the theory of free radical-induced aging was proposed in 1956, it has been constantly proven that reactive oxygen species (ROS) produced by oxidative stress play a vital role in the occurrence and progression of eye diseases. However, the inherent limitations of traditional drug therapy hindered the development of ophthalmic disease treatment. In recent years, great achievements have been made in the research of nanomedicine, which promotes the rapid development of safe theranostics in ophthalmology. In this review, we focus on the applications of antioxidant nanomedicine in the treatment of ophthalmology. The eye diseases were mainly classified into two categories: ocular surface diseases and posterior eye diseases. In each part, we first introduced the pathology of specific diseases about oxidative stress, and then presented the representative application examples of nano-antioxidants in eye disease therapy. Meanwhile, the nanocarriers that were used, the mechanism of function, and the therapeutic effect were also presented. Finally, we summarized the latest research progress and limitations of antioxidant nanomedicine for eye disease treatment and put forward the prospects of future development.
1 Introduction
Oxidation/reduction mechanisms are an important regulatory mechanism in organisms and they are usually in equilibrium.1 Since the theory of free radical-induced aging was proposed in 1956, various studies have confirmed that oxidative stress disorder is a key factor in many pathological processes and development,2 such as cancer, cardiovascular disease, neurodegenerative diseases, diabetes, and eye degenerative diseases.3–5 Oxidative stress is associated with the expansion of reactive oxygen species (ROS) or decreased concentrations of antioxidants. ROS consist of several key components, including superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (˙OH), singlet oxygen (1O2), and nitric oxide (NO). These species act like a double-edged sword, being harmful to biological systems at high concentrations and preventing infection at moderate concentrations.6 When excess ROS continuously accumulates, it can cause damage to cellular components such as DNA, proteins, and lipids, leading to structural and functional changes, thereby contributing to aging, carcinogenesis, neurodegenerative diseases, autoimmune diseases, and other conditions. Meanwhile, the continuous imbalance between the production and scavenging capacities of ROS can lead to the system being subjected to increasing oxidative stress, and the constant vicious cycle produces more free radicals. The eyes are the “ruins” for ROS damage. ROS production in the eye can be divided into exogenous and endogenous types according to the source. Corneal and ocular surface epithelial cells are exposed to atmospheric and high concentrations of oxygen with a partial pressure of oxygen of about 20%, therefore it is more susceptible to exogenous ROS interference. The posterior structure of the eye, such as the retina and optic nerve, need to be extremely active to produce vision, which is one of the most active parts of the mitochondria in the body. Mitochondria produce large amounts of ROS, so the posterior part of the eye is highly vulnerable to endogenous ROS attack. ROS can directly damage ocular tissues, leading to cell death, tissue degeneration, and visual impairment, which can result in various ocular diseases, such as age-related macular degeneration (AMD), cataract, and glaucoma. With the destruction of the stratospheric ozone layer and the popularization of LED electronic products, more and more people suffer from the oxidative stress on the eye, which is bound to aggravate the inflammatory response of the eye.7 Therefore, management of ocular oxidative stress has become high priority and urgent.Therefore, the utilization of antioxidants as a therapeutic intervention holds significant promise in addressing ROS-related diseases, rendering them an appealing avenue for clinical research and implementation. Clinical practice involves two main categories of antioxidant drugs, as outlined in Table 1. The first category encompasses direct free radical scavengers, including vitamin C, vitamin E, and beta-carotene. These compounds contribute electrons to counteract free radicals, thereby mitigating oxidative stress within the body. The second category of antioxidant drugs functions by enhancing the body's inherent antioxidant defenses, employing natural enzymes such as superoxide dismutase, catalase, glutathione peroxidase, etc.8 These enzymes work to convert ROS into less harmful substances, and by upregulating their activity, the body is better able to neutralize free radicals and reduce oxidative stress.
| Antioxidant molecule | Potential applications in ophthalmology | Ref. |
|---|---|---|
| Vitamin E | Prevention or slowing of AMD | 9 |
| Vitamin C | Reduces the ability of IFN-γ to increase CFH expression in RPE | 10 |
| Glutathione | Maintenance of the antioxidant defense system | 11 |
| Alpha-lipoic acid | α-LA reduced retinal cell death partly through AMPK activation or OGT inhibition in diabetic mice | 12 |
| Curcumin | Curcumin modulation of CaMKII and/or ser/thr phosphatases activities as a mechanism involved in GluN2A expression and neuroprotection against excitotoxicity | 13 |
| Resveratrol | Reduction of oxidative damage and inflammation | 14 |
| Quercetin | Quercetin demonstrated protection in an in vitro model of early AMD | 15 |
| Delphinidin | Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells | 16 |
| Lutein and zeaxanthin | Prevention of age-related macular degeneration and cataracts | 17 |
| Melatonin | Ameliorate retinal degeneration through potentially attenuating apoptosis, reactive gliosis, and microglial activation | 18 |
| Magnesium acetyltaurate | Protection against NMDA-induced retinal damage | 19 |
| Coenzyme Q10 | Neuroprotective effects on retinal ganglion cells | 20 |
| Epigallocatechin gallate | Reduced the loss of visual function in P23H rats and improved the levels of antioxidant enzymes and reduced oxidative damage | 21 |
Reducing ROS levels as an underlying therapeutic approach has subsequently been proposed for the treatment of eye diseases. Antioxidants have been shown their effectiveness in mitigating damage and even inhibiting the progression of eye diseases. Numerous studies have demonstrated that antioxidants can improve immune function, modulate gene expression, and reduce inflammation, which are all critical factors in the development and progression of eye diseases.22,23 Despite the proven benefits of antioxidants in ocular disease therapy, practical application faces limitations due to factors including low solubility, poor bioavailability, and a short half-life. Due to the unique physiological characteristics of the eye, drugs are difficult to realize effective penetration and target enrichment, making the efficiency and therapeutic effect unsatisfactory, especially the eye drops.24 When the eye drops drop into the eye, the drug is hindered by the tear film, whose outer and inner layers are composed of lipid layers, mucins, and metabolic enzymes, while the middle layer is a hydrophobic layer containing salt. The stromal layer is hydrophilic, while the inner cortex and outer cortex are lipophilic. Regular eye drops can hardly penetrate the cornea because most drugs are just lipid- or water soluble.25 At the same time, tear washes and blinking make it more difficult to stay on the ocular surface. Therefore, it is difficult for drugs to achieve a durable therapeutic effect and a stable therapeutic concentration. Furthermore, in retinal and optic nerve diseases, drugs need to penetrate the anterior chamber to reach the posterior segment of the eye. One barrier after another like cornea, conjunctiva, sclera and vitreous, it is almost impossible for drugs to reach the posterior segment of the eye. In addition to the basic barrier properties, the cornea also has various transmembrane transport pumps such as P-glycoprotein26 and multidrug resistance proteins at the same time,27 which further reduce the therapeutic effect of regular antioxidant drugs.
Another clinical administration mode is intravitreal injection. Although the poor bioavailability of drugs can be improved by injecting the solution or suspension directly into the tissue site, the pain and complications associated with frequent injections also preclude it from becoming a satisfactory treatment. Hence, the current limitation in the therapeutic efficacy of antioxidant drugs is predominantly attributed to their distinctive chemical properties and the challenges associated with achieving effective delivery.
In recent years, with the continuous development of nanomedicine, various nanomaterials have been used to deliver drugs to the target site. By encapsulating drugs in nanomaterials or modifying them on the surface, effective nano-drug delivery systems can be constructed.28 Nanocarriers enhance the bioavailability of antioxidant drugs by improving their solubility and absorption in the body. This elevation in bioavailability prolongs the circulation time of the drugs, thereby extending their therapeutic impact. Furthermore, nanocarriers contribute to enhanced target specificity by surface modification or functionalization, enabling more effective drug release in specific cells or tissues while minimizing effects on normal cells. In addition, nanocarriers serve to overcome physiological barriers encountered by drugs in vivo, such as metabolism and excretion, ensuring prolonged drug stability and effective delivery to the intended therapeutic targets. They not only exhibit advantages that are difficult to achieve with conventional antioxidants, but also can realize a variety of effects through continuous modification. Nanomaterials can use their own properties to achieve synergistic anti-oxidation, diagnostic therapeutics and other functions.29 For example, specific cell membranes can be modified on the surface of NPs to improve biocompatibility and targeting,30 simultaneous gene therapy can be achieved by siRNA loading,31 photothermal therapy can make use of the photo responsiveness of materials,32 and the imaging function can be used to realize the integration of diagnosis and treatment of diseases.33 All of these prompt nano-antioxidants as a promising approach for treating ocular diseases, offering exciting opportunities to enhance clinical outcomes and improve patients’ life quality.
At present, many studies have introduced nanocarriers into ophthalmic antioxidant therapy and nanocarriers loaded with antioxidant drugs to exert synergistic antioxidant effects are referred to as “nano-antioxidants” (summarized in Table 2). Using these nanoparticles (NPs) is gradually becoming the most promising therapeutic strategy for ophthalmic diseases like AMD,34 diabetic retinopathy,35 and glaucoma.36 Accordingly, in this review, we first classified eye diseases into two categories: ocular surface diseases and posterior eye diseases. In each part, we analyzed the pathology of specific diseases about oxidative stress and discussed the possibility and necessity of nano-antioxidants. Second, we introduced the current application of nanomedicine for antioxidant therapy. At the same time, the nanocarriers that were used, the mechanism of function, and the therapeutic effect were presented. Finally, we summarized the latest research progress and limitations of antioxidant nanomedicine for eye disease treatment and also put forward the prospects of future development (Fig. 1).
| Nanomaterial | Application | Benefits | Ref. |
|---|---|---|---|
| Gold | Drug delivery, imaging, gene therapy, and tissue engineering | Biocompatible, easily functionalized, and efficient drug carriers | 37 |
| Iron oxide | Imaging, drug delivery, and hyperthermia | Good contrast agents for MRI imaging. Efficient drug delivery and hyperthermia agents | 38 |
| Liposomes | Drug delivery | Biocompatible and biodegradable drug carriers with high drug loading capacity | 39 |
| Polymeric | Drug delivery, imaging, and tissue engineering | Biocompatible and biodegradable drug carriers with high drug loading capacity. Can be engineered for targeted delivery and imaging | 40 |
| Carbon dots | Drug delivery, imaging, and neuroprotection | Optical properties for imaging. Can cross the blood–retinal barrier and provide neuroprotection in retinal diseases | 41 |
| Fullerenes | Drug delivery, imaging, and neuroprotection | Antioxidant and neuroprotective properties. Can cross the blood–retinal barrier and provide neuroprotection in retinal diseases | 42 |
| Quantum dots | Imaging, drug delivery and a sensor | Optical properties for imaging. Can be engineered for specific imaging applications | 43 |
| Cerium oxide | Antioxidant therapy, and tissue engineering | Protect cells from oxidative damage and promote tissue regeneration | 44 |
| Zinc oxide | Antioxidant therapy, imaging, and drug delivery | Good antioxidant properties | 45 |
| Selenium | Antioxidant therapy, imaging, and drug delivery | Good antioxidant properties. Can be used for imaging and drug delivery | 46 |
| Silica | Imaging, drug delivery, and gene therapy | Biocompatible and easily functionalized drug carriers. Good optical properties for imaging | 47 |
| Chitosan | Drug delivery and gene therapy | Biocompatible and biodegradable drug carriers with high drug loading capacity. Can also be used for gene delivery | 48 |
| Gelatin | Drug delivery | Biocompatible and biodegradable drug carriers with high drug loading capacity | 49 |
| Titanium dioxide | Imaging and drug delivery | Good optical properties for imaging. Can be used for drug delivery | 50 |
2 Ocular surface diseases
Ocular surface diseases (OSDs) are a group of disorders that affect the surface of the eye, including the cornea and conjunctiva. OSDs are characterized by inflammation and oxidative stress, which can lead to tissue damage and visual impairment.51 Antioxidant therapy has been proposed as a potential treatment for OSDs due to their ability to counteract oxidative stress and reduce inflammation. However, conventional antioxidants have limited therapeutic efficacy because of their low bioavailability and poor tissue penetration. Nanomaterials, such as liposomes, polymeric NPs, and dendrimers, have been developed to improve the stability, bioavailability, and tissue penetration of antioxidants.52 These nano-antioxidants can effectively reduce oxidative stress and inflammation in OSDs. In addition, the use of nano-antioxidants in combination with other treatments, such as anti-inflammatory drugs and lubricants, has been proved to provide a synergistic effect. In this section, we briefly summarize the association of ocular surface diseases with oxidative stress and introduce typical application examples of nano-antioxidants in ocular surface disease treatment.2.1 Dry eye syndrome
Dry eye syndrome (DES), a multifactorial ailment predominantly caused by hyperosmolarity of the tear film, can cause ocular discomfort and potentially impair vision.53 Inflammation, the result of both the early innate immune response and subsequent adaptive response, has been recognized as a significant factor that might start the vicious cycle of dry eye. Oxidative stress usually plays a key role in the inflammatory response and it is well accepted that ROS production is the cumulative result of oxidative stress.54 Intracellular ROS have high chemical reactivity, making them unusually reactive with practically all cellular elements and capable of inducing cell death. It has been demonstrated that ROS can cause dry eye by activating cytosolic NLRP3 inflammasomes, indicating that the inflammatory response is crucial in the ROS-induced cell damage.55 Cortisol hormone is the most widely used drug for DES treatment, but its long-term use has the risk of causing glaucoma and cataract.56 Immunomodulators, such as cyclosporine A, have been widely approved by the US FDA for the treatment of inflammatory ocular surface diseases. However, studies have shown that at least long-term follow-up is needed to prove the positive effect of immunomodulators on dry eye and the incidence of adverse reactions such as burning sensation and stinging will be significantly increased.57 Drugs for the treatment of DES usually require high concentrations and long-term use, so the risks caused by these drugs should not be overlooked. Therefore, new therapeutic strategies are urgently needed.There is usually a wide range of antioxidant active components in nature. Lee et al.44 extracted an active agent from Camellia japonica (CJ) which could promote antioxidative protein expression and suppress apoptosis in HCE cells. They used eye drops with a nano CJ extract or balanced salt solution on dry eye models, which showed that HCE cell apoptosis was reduced under the treatment with CJ extracts. When compared the control groups to the mice treated with 0.1% CJ extract, clinical parameters were significantly improved. The CJ extract groups showed a marked reduction in the levels of inflammatory markers and intracellular ROS production. It suggested that the nano CJ extract eye drops could be utilized as an approach for dry eye treatment.
In addition to natural antioxidant components, loading existing drugs onto nanocarriers is also an attractive strategy. Li et al.58 developed a novel treatment for dry eye disease using poly(catechin)-capped gold nanoparticles (Au@Poly-CH NPs) carrying amfenac (AF) to reduce ocular surface tissue damage in dry eye (Fig. 2A). A dual-target strategy based on ocular therapy was used to block cyclooxygenase-induced inflammation and ROS-induced oxidative stress simultaneously (Fig. 2B). The experimental results showed that Au@Poly-CH NPs function synergistically to reduce inflammation in addition to acting as an antioxidant to inhibit ROS-mediated activities. First, the researchers found that Au@Poly-CH NPs possess significant superoxide anion scavenging activity. This activity may be ascribed to the inhibition of enzyme XO which produces ROS through the formation of hydroxyl radicals and urea. Then biocompatibility studies of AF/Au@Poly-CH NPs demonstrated their high tolerability for the treatment of ocular surface diseases. In a rabbit model of DES, the dual-targeted therapeutic actions of AF/Au@Poly-CH NPs resulted in quick recovery. HE staining showed that the corneal normality and thickness were improved compared with the control group (Fig. 2C and D), and the number of goblet cells was also significantly increased (Fig. 2E and F). Therefore, Au@Poly-CH NPs loaded with nonsteroidal anti-inflammatory drugs is a potential multifunctional nanocomposite for treating DES, and it may also be useful for other oxidative stress-related diseases.
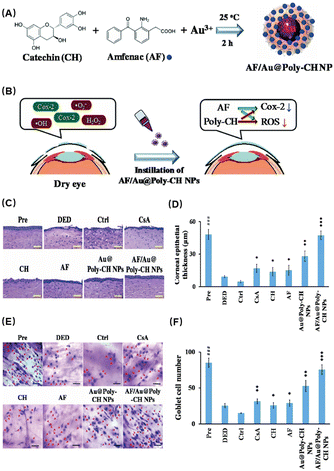 | ||
| Fig. 2 (A) Schematic diagram of the preparation process of AF/Au@Poly-CH NPs. (B) Schematic diagram of AF/Au@Poly-CH NP administration. (C) Representative images of H&E stain after different treatments. (D) Corneal epithelial thickness values after different treatments. (E) Conjunctival impression cytological image. (F) Goblet cell numbers. Values are the mean ± standard deviation (n = 6). Asterisks indicate statistically significant differences as compared treated groups to Ctrl (*p < 0.05, **p < 0.005, ***p < 0.001) and DED groups to the Pre group (###p < 0.001). Reproduced with permission.58 Copyright 2019, The Royal Society of Chemistry. | ||
Huang et al. investigated the effectiveness of gelatin–epigallocatechin gallate NPs (GEH NPs) with hyaluronic acid (HA) decoration as eye drops for the treatment of DES in rabbits (Fig. 3A).59 This study found that the GEH NPs with HA decoration exhibited notable efficacy in alleviating the symptoms of DES by exerting anti-inflammatory effects. First, they used small animal imaging to observe the drug and found that compared with the control group, the GEH group had better enrichment in the eye region and reduced systemic reactions (Fig. 3B). Subsequently, slit lamp observation showed that GEH had a significant therapeutic effect (Fig. 3C). These findings were also confirmed by corneal thickness and the number of apoptotic cells detected by immunofluorescence staining (Fig. 3D and E). The results indicated a significant decrease in the inflammatory response and an improvement in ocular surface damage induced by DES. Compared with traditional drugs, it can significantly improve the retention time and treatment effect while ensuring safety.
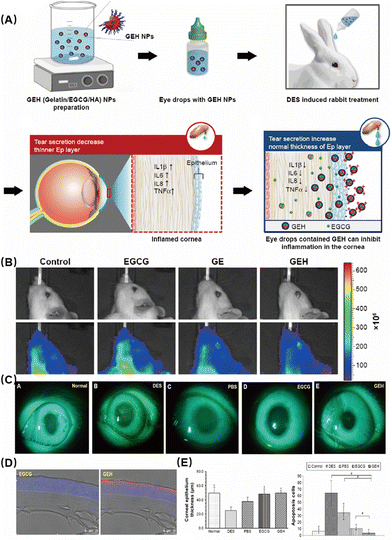 | ||
| Fig. 3 (A) Schematic diagram of the preparation of GEH NPs. (B) Accumulation of fluorescent particles on rat eye after 5 min dosing with different formulations. (C) Slit lamp photography after fluorescein staining. (D) Confocal microscopy of dye or NP distribution in the corneal epithelium from a cryosection of the rabbit cornea in images at low magnification. (E) Thickness of corneal epithelium layers and the number of apoptotic cells in the cornea. (*P < 0.05 compared with DES, #P < 0.05 compared with GEH). Reproduced with permission.59 Copyright 2020, Elsevier Inc. | ||
Hu et al. rationally designed and developed a pterostilbene-peptide amphiphile (PS-GA-RGD), which can self-assemble into prodrug nanomedicine as a potential ophthalmic agent for the treatment of dry eye disease.60 After esterase treatment, active pterostilbene (PS) was sustainably released from PS-GA-RGD nanomedicine within 48 h. The PS-GA-RGD nanodrugs showed minimal cytotoxicity against RAW 0.20 and HCCEC cells in the range of 264–7 μM compared to native PS and did not delay wound healing of HCEC monolayers within 6 h. In addition, PS-GA-RGD nanomedicine effectively reduced H2O2-stimulated RAW264.7 macrophages and significantly inhibited the secretion of inflammatory cytokines such as NO, TNF-α and IL-7 in lipopolysaccharide (LPS)-activated RAW6.264 macrophages.
Dry eye is a disease highly related to oxidative stress. Although many multifunctional NPs have been investigated for anti-inflammatory and anti-oxidative synergistic therapy, a few antioxidant drugs have been used in clinical practice. New research advances are expected to promote clinical trials and provide more effective and safe methods for dry eye patients.
2.2 Allergic conjunctivitis
Allergic conjunctivitis is a common ocular disease caused by an exaggerated immune response to environmental allergens such as pollen, dust, and animal dander, which leads to the release of inflammatory mediators and ultimately results in a spectrum of clinical manifestations, including itching, redness, and tearing.61 ROS are highly reactive molecules that are produced by various metabolic and cellular processes, including the immune response to allergens. When the production of ROS in the conjunctival region exceeds the antioxidant defense capacity, it leads to cell damage and inflammation, and allergic conjunctivitis ensues. Studies have shown that the increase of intracellular ROS levels in allergic keratitis leads to the infiltration and recruitment of inflammatory cells.62 Although topical or systemic medications, such as antihistamines, mast cell stabilizers, and corticosteroids, are widely used to alleviate symptoms and manage the disease, their therapeutic effect is limited due to their poor bioavailability, rapid clearance, and side effects. Therefore, the development of nanodrugs has emerged as a promising strategy to relieve symptoms of conjunctivitis. Several examples of nanomedicine-based approaches of conjunctivitis have been reported, such as polymeric NPs loaded with anti-inflammatory agents,63 liposomes encapsulating immunomodulatory drugs,64 and nanofibers incorporating anti-allergy molecules.65 These nano drugs have shown significant improvements in pharmacokinetics, bioactivity, and therapeutic outcomes in preclinical and clinical studies, indicating the great potential of nanomedicine in the management of allergic conjunctivitis.Due to its high biocompatibility and high biodegradability, PLGA (poly (D,L-lactide-co-glycolide)) has been licensed by the FDA for use in ophthalmic products, and PLGA-NP systems have been hailed as promising for the prolonged and regulated delivery of medications.67 Cao et al. found that using NPs loaded with rAmb-a-1, a major allergen of ragweed pollen, was effective in reducing the symptoms of allergic conjunctivitis in a murine model.66 The NPs were made of a biocompatible polymer, PLGA-PEG, and were able to efficiently deliver the allergen to the site of inflammation. First, they characterized the drug and found that uniform NPs of about 100–200 nm could be prepared (Fig. 4A-a and b). The release speed of the NPs was evaluated and the efficacy of slow release was proved (Fig. 4A-c). In addition, the NPs exhibited antioxidant effects, further reducing inflammation and oxidative stress. By inducing allergic keratitis in Balb/c mice (Fig. 4B), the symptoms of the treatment group were significantly better than those of the control group, as assessed by the slit lamp and clinical scores after treatment (Fig. 4C). Subsequently, the detection of the tissue morphology (Fig. 4D), IgE, IL-13 and other allergy-related factors (Fig. 4E) also found that the NPs not only relieved the symptoms of allergic keratitis, but also played a protective role in the tissues. This indicates that regulating oxidative stress can also obtain good therapeutic benefits for immune-related inflammatory diseases. At the same time, PLGA as an FDA-approved material is expected to be used for subsequent ophthalmic treatment.
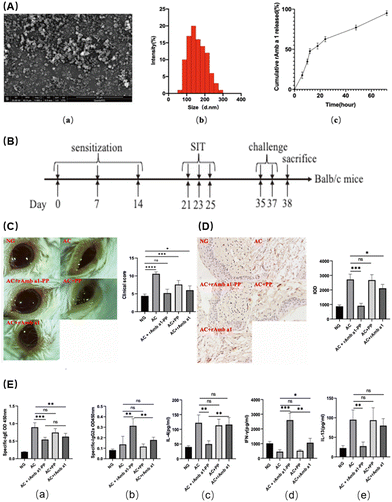 | ||
| Fig. 4 (A) Characterization of rAmb a 1-loaded PLGA-PEG nanoparticles: (a) field emission scanning electron microscopy image; (b) dynamic light-scattering spectra; and (c) in vitro cumulative protein release of rAmb a 1-loaded PLGA-PEG nanoparticles. (B) Experimental design. (C) Ocular signs of allergic conjunctivitis and eye feature score. (D) Formalin-fixed conjunctival tissue section with mast cells stained with toluidine blue and degranulation rate of mast cells. (E) Serum-specific IgE (a), IgG2a (b), IL-4 (c), IFN-γ (d) and IL-13 (e) in each group. The data are shown as the mean ± SD from five individual mice (***p < 0.001, **p < 0.01, *p < 0.05. ns: no significant difference). Abbreviations: NG, naive group; AC, allergic conjunctivitis group; AC + rAmb a 1-PP, allergic conjunctivitis + rAmb a 1-PLGA-PEG treatment group; AC + PP, allergic conjunctivitis + PLGA-PEG treatment group; and AC + rAmb a 1, allergic conjunctivitis + rAmb a 1 treatment group. Reproduced with permission.66 Copyright 2022, MDPI. | ||
2.3 Cornea disease
Wu et al. developed and attenuated effective MMC@MSNs-LDL NPs to effectively treat pterygium.71 Mitomycin C (MMC) has been used for a long time as an adjuvant therapy to reduce the recurrence of pterygium, but it is associated with many adverse reactions. In an effort to deliver MMC to activated pterygium fibroblasts in a targeted manner, MMC-loaded mesoporous silica NPs conjugated with LDL (MMC@MSNs-LDL) were synthesized (Fig. 5A). Characterization of the NPs showed that they were spherical particles with uniform stability and excellent physical properties (Fig. 5B). Previous studies have shown that activated pterygium subconjunctival fibroblasts overexpress LDL receptors. The addition of LDL enables better enrichment of the drug in the targeted region. The uptake of targeted NPs in fibroblasts was time dependent and saturated at 6 h, with VEGF-activated pterygium fibroblasts showing an increased uptake of MMC@MSNs-LDL compared to normal fibroblasts with or without VEGF activation (Fig. 5C). Furthermore, MMC@MSNs-LDL played an effective antiproliferative role in activated pterygium fibroblasts, with reduced toxicity to normal fibroblasts when compared to traditional MMC application (Fig. 5D). Therefore, LDL-mediated drug delivery using MMC@MSNs-LDL have the effect of controlling pterygium recurrence. This targeted therapeutic approach may also minimize the adverse effects associated with traditional treatment methods, thereby highlighting the therapeutic benefits of nano-antioxidant in the management of ocular diseases.
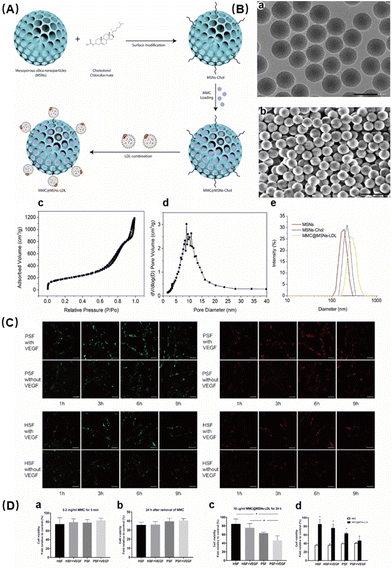 | ||
| Fig. 5 (A) Schematic illustration of MMC@MSNs-LDL preparation. (B) Characterization of MMC@MSNs-LDL: (a) transmission electron microscopy (TEM) image, bar = 200 nm; (b) scanning electron microscopy (SEM) image, bar = 500 nm; (c) nitrogen adsorption–desorption isotherm; (d) pore size distribution; and (e) diameter distribution. (C) Uptake of Dil/FITC-labeled MMC@MSNs-LDL (10 μg mL−1) by PSFs and HSFs with or without VEGF (600 pg ml−1) stimulation. (D) Cell viability changes. Reproduced with permission.71 Copyright 2020, Elsevier Inc. | ||
Zheng et al. investigated the potential of cerium oxide NPs (CeNPs) as a nanodrug to inhibit CNV, which is associated with ocular inflammation.73 First, they found that the particles were spherical and on the scale of about 10 nanometers (Fig. 6A). They evaluated the properties of Ce ions using X-ray photoelectron spectroscopy spectra and found that Ce ions are located in the trivalent and tetravalent multi-energy levels, which are closely related to the antioxidant properties of Ce NPs (Fig. 6B). Next, the researchers demonstrated that CeNPs could effectively scavenge ROS and protect human corneal epithelial cells from oxidative stress-induced cell death. They evaluated the effects of oxidized cerium on intracellular oxygen free radicals induced by hydrogen peroxide and found excellent antioxidant properties (Fig. 6C). HUVEC cell tube formation assay showed that CeNPs significantly inhibited the proliferation of vascular epithelial cells, which verified their ability to resist neovascularization (Fig. 6D). In addition, in vivo studies using a CNV mouse model showed that CeNPs significantly reduced neovascularization by preventing inflammatory cell infiltration and reducing proinflammatory cytokine levels (Fig. 6E and F). Therefore, the antioxidative stress capabilities of CeNPs may have a therapeutic effect in treating ocular neovascularization linked to inflammation.
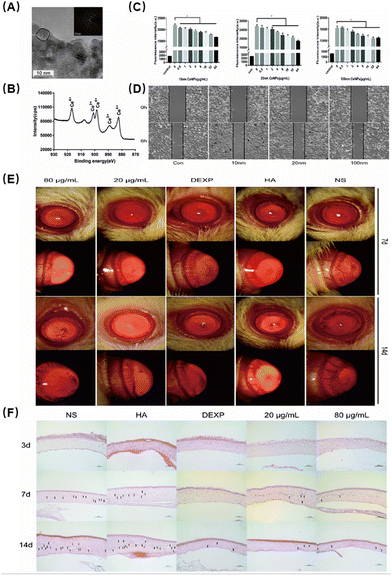 | ||
| Fig. 6 (A) Representative high-resolution TEM images of CeNPs. (B) X-ray photoelectron spectroscopy spectra of CeNPs. Peaks at 882, 898 and 916 eV are related to Ce4+. The peak at 889 eV indicates the presence of Ce3+. (C) Effects of oxidized cerium on intracellular oxygen free radicals induced by hydrogen peroxide. Data were analysed using one-way ANOVA, *p > 0.05, n = 3. (D) Migration assays. (E) The inhibitory effect of CeNPs in the corneal neovascularization model. (F) Corneal H&E staining to evaluate the therapeutic effect (200×). Reproduced with permission.73 Copyright 2019, The Royal Society of Chemistry. | ||
Pradhan et al. prepared a potential candidate for the prevention of CNV using curcumin.74 The researchers prepared and characterized MePEG-PCL NPs containing curcumin and found that the NPs containing curcumin were more efficient in preventing angiogenic sprouting compared to those without curcumin. In vitro studies demonstrated that the prepared nanodrug significantly reduced the viability and migration of human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner. In vivo experiments on a rat model of CNV showed that the topical application of CNPs significantly reduced the area and length of blood vessels, as well as the expression of pro-inflammatory cytokines and angiogenic markers in the cornea. Topical delivery of curcumin NPs to the eye showed enhanced retention of curcumin in the cornea and a significant improvement in the prevention of corneal neovascularization. The researchers also observed a suppression in the expressions of VEGF, inflammatory cytokines, and MMP in the treated cornea. Curcumin was found to inhibit NF-κB in LPS-induced corneal cells.
Gelatin/epigallocatechin-3-gallate (EGCG) as a natural component of green tea has long been used for research because of its anti-inflammatory and antioxidant activities.75 Miyagawa et al. formulated an eye drop incorporating EGCG NPs for CNV therapy.76 They assembled EGCG and gelatin into NPs and then loaded RGD (arginine–glycine–aspartic)-modified HA onto the surface to form nano-eye drops (Fig. 7A). The successful synthesis of NPs with a spherical shape was verified by atomic force microscopy images (Fig. 7B). First, they evaluated the performance of the nano-eye drops in vitro. The western blot results showed that GEH-RGD NP treatment reduced endothelial tube formation and suppressed the activities of metalloproteinase (MMP)-2 and MMP-9 in HUVECs (Fig. 7C and D). The inhibition of HUVEC cell tube formation indicated that they had excellent anti-angiogenesis ability. Next, they tested the therapeutic efficacy in an animal corneal neovascularization model. Corneal neovascularization was induced by the attachment of sodium hydroxide to the central region of the cornea. In vivo experiments demonstrated that topical administration of GEH-RGD NPs could dramatically reduce the growth of diseased blood vessels in mouse corneas (Fig. 7E). H&E staining also showed that the GEH-RGD group had the least inflammatory cell infiltration and a more complete recovery compared with the other control groups (Fig. 7F). Vascular endothelial growth factor (VEGF) and MMP-9 are usually highly expressed in the microenvironment of neovascularization. In cauterized corneas treated with GEH-RGD NPs, the levels of VEGF and MMP-9 protein were significantly lower than the control group (Fig. 7G), which may be due to their antioxidant and anti-inflammatory effects to alleviate local inflammation.
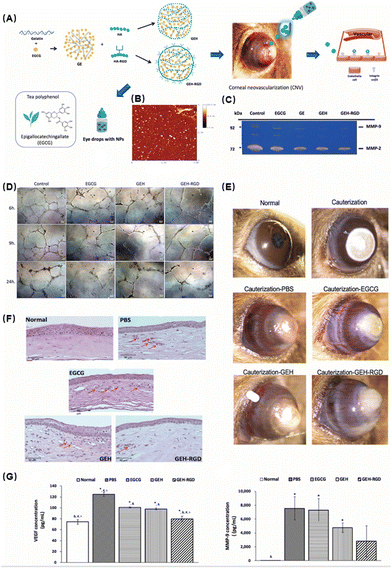 | ||
| Fig. 7 (A) Schematic illustration of the preparation of GEH-RGD NPs with EGCG loading. (B) Atomic force microscopy image of GEH-RGD NPs. (C) EGCG NPs inhibit the activity of MMPs in endothelial cells. The results of gelatin zymography from the culture medium after 24 h of incubation with a variant formulation to confirm the MMP activity. (D) Representative images of HUVECs cultured on Matrigel at different time points (EGCG: 20 μg mL−1, 100×). (E) EGCG NPs inhibit neovessel formation in the chemical cauterization-induced CNV model. (F) Histological assessment of corneal sections after different treatments. (G) GEH-RGD NPs inhibit the expression of angiogenic factors in the cauterized cornea. The corneas were harvested and homogenized and the protein levels of (G) VEGF and (F) MMP-9 were assayed by ELISA. Reproduced with permission.76 Copyright 2020, MDPI. | ||
Zhu et al. recently reported a biocompatible shell with excellent antioxidant and anti-vascularity prepared by the co-assembly of EGCG and Cu(II).77 After loading glucose oxidase (GOx) inside the shell, it was modified with a DPA–Zn dimer to co-transport vascular endothelial growth factor (VEGF) small interfering RNA (VEGF-siRNA). At the same time, the cell membrane modified with RGD peptides improved the targeting and biocompatibility of angiogenesis. They cleverly exploited the targeting of the eye drop and the internal GOx lowering the local pH by consuming glucose, resulting in the release of EGCE and VEGF-siRNA. The results show that nanodrugs exert synergistic antioxidant and anti-vascular effects by down-regulating the expression of differentiation cluster 31 and VEGF through the combined antioxidant/gene effects, thereby significantly reducing angiogenesis and inhibiting CNV formation. Compared with the traditional single treatment, the multifunctional eye drops achieved a simple and efficient combination therapy of gene therapy and antioxidant therapy. This will provide new guiding significance for clinical treatment and research development.
Singh et al. discussed a potential therapeutic use of lactoferrin in KC.84 Lactoferrin (LF) is an iron-binding glycoprotein that influences the innate and adaptive immune responses. The LF structure shares 60% of its similarity with other iron-transporting protein members and is connected to a wide range of biological processes in the human body, including antitumor activity, antioxidant activity, antimicrobial activity, cell proliferation, differentiation regulation, and antibacterial activity.85 It is categorized as an iron chelator as a member of the transferrin family due to its ability to bind Fe3+ ions. Chelation might therefore be an effective strategy for treating inflammatory illnesses by eliminating free iron and reducing redox reactions. LF has been shown to promote corneal epithelial wound healing. By promoting changes in humoral and cellular components and inducing extracellular and intracellular signaling pathways involving toll-like receptors (TLRS), LF may affect both innate and adaptive responses of the immune system. Various nano-formulations of lactoferrin were developed to enhance its therapeutic efficacy, including lactoferrin-loaded NPs, liposomes, and hydrogels. It is reported that LF NPs significantly improve bioavailability and permeability, and their ability to cross the blood–retinal barrier makes them potential therapeutic agents for retinal and corneal diseases. Therefore, lactoferrin and its nano-formulations could potentially be used as a therapeutic strategy for KC, although further research is needed to fully understand their efficacy and safety.
3 Posterior eye diseases
In recent decades, great progress has been made in the treatment of ocular surface diseases by improving the dosage form of nanomedicine.86 Topical administration, such as eye drops, is the most commonly used treatment for ocular surface diseases. However, the time of contact between the drug and the eye surface is limited, and the corneal epithelium and the blood–aqueous humor barrier also limit the drug from entering the eye. At present, the commonly used treatment for posterior eye diseases is periocular injection or intravitreal injection. The drug is injected directly into the posterior tissue of the eye to overcome the complex physiological barriers such as the blood, retina, choroid, and sclera. Although this strategy is effective to some extent, injection not only causes pain and reduces patient compliance, but also may be accompanied by many complications such as cataract and retinal detachment.87 Due to the special physiological role of the retina, which is the area of high mitochondrial metabolism, the oxidative stress in the posterior part of the eye is more obvious than that in other parts due to the high amount of ROS production accompanied by visual production. Therefore, a drug delivery system with sustained release, strong penetration and a long retention time in the eye is needed to improve the therapeutic efficacy of posterior segment diseases. Advances in nanotechnology have made it possible to overcome eye-related barriers. Nano-drug delivery systems have shown the following advantages that make them potentially applicable to these diseases: (1) improved drug efficacy, stability and biocompatibility; (2) better penetration may allow drugs to reach the posterior segment of the eye by non-invasive means (e.g., eye drops and transdermal delivery); and (3) the retention time prolonged so that the drugs could stay in the target area for a long time to reduce the frequency of administration, especially injection. In this section, we summarized the pathological correlation between oxidative stress and posterior eye diseases and illustrated how antioxidant nanomedicines exert their functions in posterior eye disease treatment with representative examples.3.1 Diabetic cataract
Diabetic cataract (DC) is one of the major causes of blindness in diabetic patients. Hyperglycemia can cause ocular pathological damage including retinal capillary basement membrane thickening, tissue ischemia, and lens protein degeneration. Lens protein degeneration and opacity are the main causes of vision loss in cataract patients and oxidative stress is an important pathological factor in the progression of DC.88 The degree of membrane lipid peroxidation and the amount of ROS in DC patients are much higher than those in normal people. Lipid peroxidation caused by free radicals is the initial mechanism leading to the occurrence of DC. It can affect the permeability of the cell membrane and further change its internal composition and cell configuration, leading to the loss of protein function. ROS also promote the decrease of the solubility of crystallin and stimulate the formation of aggregations89 and induce the down-regulation of Na-K-ATPase in the lens epithelial cell membrane, causing the retention of water and sodium. At the moment, surgical is the mainstream treatment because almost no effective drugs could be used. The operation is relatively complicated and has related complications such as posterior capsular opacification, increased intraocular pressure, and corneal edema.CeO2 NPs have been shown to be a promising biomaterial with catalytic properties in redox reactions. Zhou et al. coated CeO2 NPs with PEG-PLGA to develop self-regenerating redox NPs (PCNPs) against oxidative stress in lens epithelial cells (Fig. 8A).90 Glycosylation can lead to lens aggregation and eventually lens opacity. Oxidative stress caused by excessive ROS is undoubtedly an important mitigation of glycosylation. Based on this, they developed PCNPs with antioxidant capacity and self-regenerating redox properties, which can be applied to combat lens’ oxidative stress. They first characterized PCNPs and found that the size of CeO2 was about 5 nm and formed spherical aggregates of about 200 nm when loaded with PEG-PLGA (Fig. 8B and C). Confocal microscopy observation showed that the ROS levels were significantly reduced in the PCNP-treated group compared with other control groups, demonstrating their excellent antioxidant capacity (Fig. 8D). Subsequently, the safety of PCNPs was tested. The results showed that PCNPs had no obvious inflammation and pathological abnormalities in conjunctiva and other tissues and organs. Then, the sustained release properties of the nanomedicine in animals were tested. After labeling with Cy5.5, the ocular pharmacokinetics found that the administered drug rapidly enriched in the eye and remained for more than 20 days (Fig. 8F). Glycosylation is one of the important pathological mechanisms of cataract. In an STZ-induced rat diabetic cataract model, a high concentration of PCNPs presented an excellent therapeutic effect (Fig. 8G). Compared with the other control groups, the corneal edema of the high concentration PCNP group basically disappeared. Histological examination also confirmed that PCNPs had a significant protective effect on the lens of the diabetic rat. Therefore, in addition to acting as an antioxidant to protect lens epithelial cells from oxidative stress based on the repeated elimination of ROS, PCNPs may also function as a glycation inhibitor to prevent crystallin glycation and crosslinking.
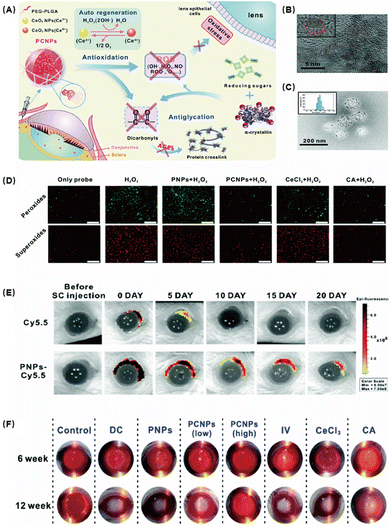 | ||
| Fig. 8 (A) Schematic of CeO2 NPs with the polymer PEG-PLGA (PCNPs) alleviating DCs. (B) TEM image of CeO2 NPs. (C) TEM image of PCNPs. (D) Fluorescence images of the antioxidative activity in HLE-B3 in vitro incubated with different samples. (E) Fluorescence images of the retention and distribution of NPs in the eyes. (F) Photographs of rats’ eyes after treatment. Reproduced with permission.90 Copyright 2019, The Royal Society of Chemistry. | ||
Yang et al. also reported that Ce NPs loaded with mesoporous silica (CeCl3@mSiO2) could reduce oxidative stress in lens epithelial cells and delay the progression of DC.91 The in vivo experimental results showed that CeCl3@mSiO2 could not only significantly abolish hyperglucose-mediated advanced glycation end products, lipid peroxidation, but also upregulate protein carbonylation in animal lenses, and finally effectively slowing the DC progress. Therefore, Ce NPs are worthy of further investigation as a potential nanocarrier for DC management.
3.2 Age-related macular degeneration
Age-related macular degeneration (AMD) is characterized by structural changes in the macular area. The main manifestations are that the ability of retinal pigment epithelial cells to phagocytose and digest the outer segment disc membrane of the optic cell is decreased and the incompletely digested disc membrane remains retained in the protoplasm of the basal cell, and then discharged to the outside of the cell and deposited in Bruch's membrane.92 Although the cornea and lens absorb most of the UV light, a small amount of UV light can still reach the retina, and this continuous oxidative damage plays an important role in the pathogenesis of retina-related eye diseases. ROSs are usually produced in large quantities in highly metabolic mitochondria.93 The retina is made up of ten layers, with the internal limiting membrane being the innermost layer and the retinal pigment epithelium (RPE) being the outermost layer. To generate and transmit visually evoked potential signals, the retina must maintain a high metabolic rate and retinal tissue cells experience higher levels of oxidative stress than other tissues. The retinal pigment epithelium maintains the normal function of the retina. In RPE cells, oxidative stress induces inflammation, autophagic cell death, and apoptosis, resulting in an impaired retinal and optic nerve function. There are a number of studies that hypothesize or demonstrate that the pathogenesis of AMD begins in RPE cells.94 Oxidative stress-induced RPE and choriocapillaris damage are associated with AMD.95Melanin is a natural component widely present in the body such as retinal pigment epithelium cells and is considered as a potential free radical scavenger and antioxidant.96 With the increase of age, the protective effect of melanin is gradually weakened and the pathogenesis of AMD is closely related to it. Kwon et al. designed a therapeutic strategy for the replenishment of melanin using PEGylated synthetic melanin-like NPs (MNPs) in the RPE for the treatment of AMD.97 They synthesized MNPs with thiol-terminated methoxy polyethylene glycol (mPEG-SH) attached to the NPs to improve the stability. MNPs could target mitochondria-specific ROS and had excellent ROS scavenging ability. Mitochondrial ROS can overflow into mitochondria when the balance is broken, while MNPs are only found in the cytoplasmic matrix, not in the mitochondria and nucleus. MNPs were only found in the RPE and retina, indicating that MNPs had good targeting properties. Subsequently, in vivo experiments showed that MNP treatment significantly alleviated photodamage, reduced inflammation caused by ROS, and reduced the thickness of neovascularization, demonstrating that MNPs can be used as a natural antioxidant defense platform for AMD therapy.
In recent years, the water solubility and biocompatibility of CNPs have been improved by the modification of polyhydroxy compounds such as chitosan, glucose and heparin, and a variety of multifunctional antioxidants with anti-oxidation and anti-neovascularization properties have been developed. Mitra et al. developed GCCNPs encapsulated with biocompatible ethylene glycol chitosan (GC) to construct a robust antioxidant system (Fig. 9A).98 They observed by TEM that they successfully synthesized nanoparticles at ultra-small scales (Fig. 9B). In order to verify the self-regeneration ability of GCCNPs, they incubated the drug with the NPs and continuously added hydrogen peroxide. The results showed that the NPs had long lasting antioxidant ability. The toxicity of GCCNPs on ARPE19 cells was assessed by the MTT assay and the NPs were found to have almost no effect on cell viability at low concentrations (≤1 μM). At 10 μM concentration, 80% of the cells survived on the third and fourth days, proving that GCCNPs were less toxic. GCCNPs also inhibited H2O2-induced EC migration and tube formation in vitro and pro-angiogenic VEGF, oxidative marker protein (4-HNE adduct), and chemokine CXCR4 receptor expressions. After that, they explored the inhibition of corneal neovascularization in a laser-induced AMD model. Compared with the control group, GCCNPs significantly reduced the area of neovascularization in the fundus (Fig. 9C). It was also found that GCCNPs tend to accumulate at the laser-induced AMD lesion, proving that they had good targeting ability.
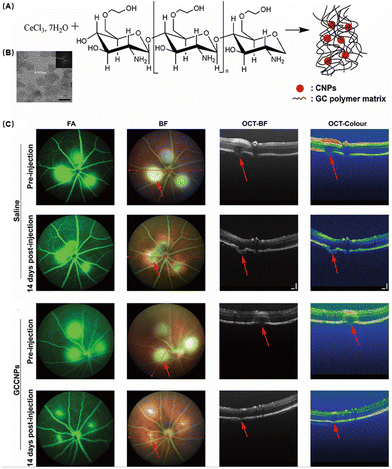 | ||
| Fig. 9 (A) Schematic illustration of the synthesis of GCCNPs. (B) Representative transmission electron microscopy image. (C) Representative images of the GCCNP treatment effect on AMD. Reproduced with permission.98 Copyright 2017, American Chemical Society. | ||
3.3 Glaucoma
Glaucoma is another common disease of the posterior eye associated with the optic nerve which is characterized by a specific structural change in the head of the optic nerve, as well as progressive damage to the visual field. The loss of retinal ganglion cells (RGCs) and their axons, leading to an excavated optic nerve head and related vision field abnormalities, characterizes a heterogeneous collection of illnesses. Although increased intraocular pressure is the major risk for glaucoma, other related factors such as increased glutamate levels,99 vascular changes100 and oxidative damage are also independent risk factors. According to Alvarado et al.,101 the progressive loss of trabecular meshwork (TM) cellularity in glaucoma patients may be attributed to the long-term effects of oxidative damage caused by free radicals. This hypothesis has been supported by experimental studies conducted continuously.102 Saccà et al. demonstrated in vivo that both IOP increase and visual field damage are significantly related to the amount of oxidative DNA damage.103 Furthermore, Izzotti et al. demonstrated that oxidative DNA damage is significantly more abundant in glaucoma patients’ TM cells than in unaffected controls.104 These results all suggest that oxidative stress plays an important role in the pathogenesis of glaucoma.RGCs and their axons damage are a leading cause of irreversible blindness of Glaucoma. However, the existing drugs can hardly enter the retina and have a short residence time. Rong et al. developed a drug carrier polymer that responds to the glaucomatous milieu and is distinguished by the presence of thioketal linkages, a 1,4-dithione unit in the main chain for reducing ROS, and pendant cholesterols for specifically targeting cell membranes (Fig. 10).105 This polymer was used to create NPs that contained the necrostatin-1 necroptosis inhibitor (designated as NP1). In an acute pathological glaucomatous damage model, NP1 with higher biosafety could scavenge ROS in RGCs both in vitro and in vivo. In addition, it was discovered that NP1 successfully inhibited the activation of the necroptosis pathway, hence lowering the mortality of RGCs. The results of this study provide an excellent illustration of the potential for using nano-antioxidants to cure glaucoma.
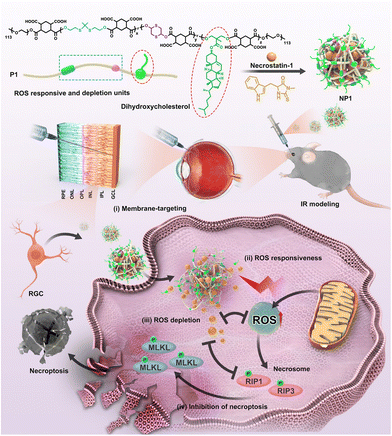 | ||
| Fig. 10 Schematic illustration of the design of NPs and mechanisms of the protection of RGCs. Reproduced with permission.105 Copyright 2022, American Chemical Society. | ||
4 Other eye diseases
Commonly, the onset and development of many eye diseases are multifactorial. For eye diseases such as trauma and bacterial keratitis, anti-inflammatory and antioxidant treatments are necessary to restore tissue normality and promote wound healing. Multifunctional nano-antioxidants may have advantages over conventional multi-drug treatment at the same time in large doses for a long time. Therefore, Lei et al. fabricated a novel nano-phytochemical ophthalmic eye drop DG-PAL to promote corneal wound healing. In the previous work, the authors found that HMGB1 signaling plays an important role in the development of diabetic keratopathy, and when this signal is inhibited, the treatment outcome could be improved. The FDA-approved drug glycyrrhizin is a common HMGB1-specific inhibitor in clinical use. Since it was authorized for use as an anti-inflammatory drug, dipotassium glycyrrhizinate (DG), a dipotassium salt of glycyrrhizin with high aqueous solubility, has been widely used in clinical practice. Palmatine (PAL) is a natural phytochemical with pharmacological properties that include strong antioxidant and anti-inflammatory properties. However, low water solubility and poor permeability limit the use of PAL. The authors prepared DG-PAL nano-eye drops by encapsuling PAL with DG and the results showed that the solubility and the corneal permeability were significantly improved. At the same time, corneal re-epithelialization and nerve regeneration were assessed as two key assessment indices in the efficacy evaluation experiment. Both corneal re-epithelialization and nerve regeneration were significantly sped up by the DG-PAL ophthalmic solution. DG-PAL has an excellent promoting effect on corneal epithelial/nerve wound healing in both healthy and diabetic mice models, demonstrating that DG-PAL is a novel marked improvement of corneal wound healing.106Mesenchymal stem cell-derived exosomes (mExo) have attracted more and more attention due to their anti-inflammatory and repair promoting mechanisms, due to some key proteins such as CD73, Wnt4, etc. mExo is also frequently used in eye therapy to regulate autoimmune uveitis, promote wound healing, and inhibit neovascularization. Ascorbic acid (AA) has significant biosafety and may efficiently maintain the balance of free radicals in the human body by scavenging ROS or producing stable molecules when reacting with ROS. Ma and coworkers reported on the development and application of an innovative eye drop containing AA-coupled mExo (mExo@AA) for the treatment of DES.107 In order to create mExo@AA, gold NPs (AuNPs) were in situ-deposited onto the exosomal phospholipid membrane. The results from both in vitro and in vivo experiments showed that mExo@AA reduced DED through a variety of mechanisms, including healing ocular surface damage, scavenging ROS, and lowering inflammation.
5 Summary and prospects
Traditional ophthalmic preparations such as eye drops have their disadvantages such as low bioavailability, frequent use of drugs, poor permeability, and ineffectiveness in the treatment of posterior eye diseases due to rapid clearance by the eye. For posterior ocular diseases, intravitreal injection of drugs has a certain therapeutic effect on overcoming the ocular barrier, but the high risk of surgery, various complications and adverse reactions bring great challenges to the treatment of the disease.To address these challenges, scientists are actively exploring various nanomedicines and the advancements in nanotechnology in ophthalmology hold great promise. Emerging trends such as long-acting drug delivery systems and combination therapies offer new possibilities for the treatment of eye diseases that may potentially preserve vision and enhance patients’ quality of life. New nano-antioxidants are a revolutionary breakthrough in the field of nanomedicine for eye disease therapy. These nano-formulated antioxidants have the advantages of traditional nanomedicine such as improved drug delivery precision, reduced toxicity and enhanced therapeutic efficacy. At the same time, some nanocarriers themselves have strong antioxidant effects and synergize to produce antioxidant effects while loading therapeutic drugs. By targeting oxidative stress, which plays a pivotal role in various eye diseases, nano-antioxidants can help in mitigating cellular damage and inflammation, offering potential treatments for conditions like AMD, glaucoma, and diabetic retinopathy. They also hold the promise of preventing ocular damage and vision loss, improving the quality of life for patients. Therefore, nano-antioxidants are bound to bring new and powerful prospects for ophthalmology.
At present, a large number of ophthalmic nano-agents are being tested in clinical trials and marketed. For example, on April 11, 2023, the phase II study and two phase III studies of APP13007 in the United States reached clinical end points. APP13007 is an anti-inflammatory and analgesic nano-suspension eye drop. Its unique nano-preparation process effectively solves the low bioavailability and safety risks caused by the low water solubility of the product. However, the nano delivery system has its unresolved issues, such as poor stability, low drug loading, excipient irritation, and incomplete drug release. Although nano-antioxidants require further studies to optimize formulations and to evaluate the long-term safety and efficacy in clinical settings, we can still expect increasingly personalized and novel approaches to control eye problems as nano-antioxidants develop. It can be solved from the following aspects: (1) broad clinical medication scope, ophthalmic delivery of other clinical agents that have not yet been used for ophthalmic treatment could be attempted; (2) increase the retention time of eye drops, improving the drug dosage form can delay the time of drug release, enhance the adhesion of the drug to the targeted area, reduce the frequency of administration, and increase the concentration of the drug; (3) improve the penetration of drugs, the drug could administered simply by eye drops to avoid invasive methods of administration such as injections; and (4) improve therapeutic efficacy and achieve multi-function. Photodynamic, sonodynamic, gene therapy, gas therapy and other advanced methods can be combined to achieve multifunction efficient treatment. These improvements are anticipated to include tailored treatments that address the specific oxidative stress profiles of individual patients and have the potential to broaden the scope of ocular health care. The development of non-invasive nano-antioxidant delivery systems will further improve the efficacy of ophthalmic treatment. We look forward to more research studies and clinical translations to make a contribution to the eye diseases in the world!
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Shenzhen Science and Technology Program (JCYJ20210324121801004), the Natural Science Foundation of Xiamen (3502Z202371026), the National Natural Science Foundation of China (NSFC) (32271447), the Guangdong Basic and Applied Basic Research Foundation (2021A1515012541), the National College Student Innovation and Entrepreneurship Training Program (202012631001), and the Science Foundation of Fujian Province (2022J01021).References
- P. Ghezzi and A. D. Mooradian, Handb. Exp. Pharmacol., 2021, 264, 3–26 CrossRef CAS PubMed.
- U. G. Knaus, Handb. Exp. Pharmacol., 2021, 264, 27–47 CrossRef CAS PubMed.
- S. K. Wattanapitayakul and J. A. Bauer, Pharmacol. Ther., 2001, 89, 187–206 CrossRef CAS PubMed.
- F. J. Jiménez-Jiménez, H. Alonso-Navarro, L. Ayuso-Peralta and T. Jabbour-Wadih, Rev. Neurol., 2006, 42, 419–427 Search PubMed.
- Y. J. Hsueh, Y. N. Chen, Y. T. Tsao, C. M. Cheng, W. C. Wu and H. C. Chen, Int. J. Mol. Sci., 2022, 23, 1255 CrossRef CAS PubMed.
- J. Barar, A. R. Javadzadeh and Y. Omidi, Expert Opin. Drug Delivery, 2008, 5, 567–581 CrossRef CAS PubMed.
- I. Jaadane, P. Boulenguez, S. Chahory, S. Carré, M. Savoldelli, L. Jonet, F. Behar-Cohen, C. Martinsons and A. Torriglia, Free Radicals Biol. Med., 2015, 84, 373–384 CrossRef CAS PubMed.
- L. Flohé, S. Toppo and L. Orian, Free Radicals Biol. Med., 2022, 187, 113–122 CrossRef PubMed.
- Arch. Ophthalmol., 2001, 119, 1417–1436, DOI:10.1001/archopht.119.10.1417.
- L. I. Lau, S. H. Chiou, C. J. Liu, M. Y. Yen and Y. H. Wei, Invest. Ophthalmol. Visual Sci., 2011, 52, 6832–6841 CrossRef CAS PubMed.
- S. A. Marchitti, Y. Chen, D. C. Thompson and V. Vasiliou, Eye Contact Lens., 2011, 37, 206–213 CrossRef PubMed.
- Y. S. Kim, M. Kim, M. Y. Choi, D. H. Lee, G. S. Roh, H. J. Kim, S. S. Kang, G. J. Cho, E. K. Hong and W. S. Choi, Biochem. Biophys. Res. Commun., 2018, 503, 1307–1314 CrossRef CAS PubMed.
- C. Mallozzi, M. Parravano, L. Gaddini, M. Villa, F. Pricci, F. Malchiodi-Albedi and A. Matteucci, Cell. Mol. Neurobiol., 2018, 38, 1315–1320 CrossRef CAS PubMed.
- D. Delmas, C. Cornebise, F. Courtaut, J. Xiao and V. Aires, Int. J. Mol. Sci., 2021, 22, 1295 CrossRef CAS PubMed.
- Q. Zhu, M. Liu, Y. He and B. Yang, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 2010–2015 CrossRef PubMed.
- T. Ni, W. Yang and Y. Xing, Biosci. Rep., 2019, 39, BSR20190689 CrossRef CAS PubMed.
- M. Mrowicka, J. Mrowicki, E. Kucharska and I. Majsterek, Nutrients, 2022, 14, 827 CrossRef CAS PubMed.
- X. J. Xu, S. M. Wang, Y. Jin, Y. T. Hu, K. Feng and Z. Z. Ma, J. Pineal Res., 2017, 63, e12428 CrossRef PubMed.
- J. A. Whitson, P. A. Wilmarth, J. Klimek, V. M. Monnier, L. David and X. Fan, Free Radicals Biol. Med., 2017, 113, 84–96 CrossRef CAS PubMed.
- R. H. Sim, S. R. Sirasanagandla, S. Das and S. L. Teoh, Nutrients, 2022, 14, 534 CrossRef CAS PubMed.
- L. Perdices, L. Fuentes-Broto, F. Segura, A. Cavero, E. Orduna-Hospital, G. Insa-Sánchez, A. I. Sánchez-Cano, L. Fernández-Sánchez, N. Cuenca and I. Pinilla, Neural Regener. Res., 2022, 17, 625–631 CrossRef CAS PubMed.
- J. Mares, Annu. Rev. Nutr., 2016, 36, 571–602 CrossRef CAS PubMed.
- S. C. Saccà, C. A. Cutolo, D. Ferrari, P. Corazza and C. E. Traverso, Nutrients, 2018, 10, 668 CrossRef PubMed.
- V. Gote, S. Sikder, J. Sicotte and D. Pal, J. Pharmacol. Exp. Ther., 2019, 370, 602–624 CrossRef CAS PubMed.
- B. A. Nichols, M. L. Chiappino and C. R. Dawson, Invest. Ophthalmol. Visual Sci., 1985, 26, 464–473 CAS.
- M. Barot, M. R. Gokulgandhi, M. Haghnegahdar, P. Dalvi and A. K. Mitra, J. Ocul. Pharmacol. Ther., 2011, 27, 553–559 CrossRef CAS PubMed.
- P. K. Karla, D. Pal and A. K. Mitra, Exp. Eye Res., 2007, 84, 53–60 CrossRef CAS PubMed.
- M. Su, Q. Dai, C. Chen, Y. Zeng, C. Chu and G. Liu, Nano-Micro Lett., 2020, 12, 96 CrossRef CAS PubMed.
- Y. Min, J. M. Caster, M. J. Eblan and A. Z. Wang, Chem. Rev., 2015, 115, 11147–11190 CrossRef CAS PubMed.
- R. H. Fang, W. Gao and L. Zhang, Nat. Rev. Clin Oncol., 2023, 20, 33–48 CrossRef PubMed.
- J. A. Kulkarni, D. Witzigmann, S. Chen, P. R. Cullis and R. van der Meel, Acc. Chem. Res., 2019, 52, 2435–2444 CrossRef CAS PubMed.
- Z. Chen, P. Zhao, Z. Luo, M. Zheng, H. Tian, P. Gong, G. Gao, H. Pan, L. Liu, A. Ma, H. Cui, Y. Ma and L. Cai, ACS Nano, 2016, 10, 10049–10057 CrossRef CAS PubMed.
- V. K. Chaturvedi, A. Singh, V. K. Singh and M. P. Singh, Curr. Drug Metab., 2019, 20, 416–429 CrossRef CAS PubMed.
- S. Kumar Dubey, R. Pradhan, S. Hejmady, G. Singhvi, H. Choudhury, B. Gorain and P. Kesharwani, Int. J. Pharm., 2021, 600, 120499 CrossRef CAS PubMed.
- J. Hanaguri, N. Nagai, H. Yokota, A. Kushiyama, M. Watanabe, S. Yamagami and T. Nagaoka, Pharmaceutics, 2022, 14, 384 CrossRef CAS PubMed.
- N. Mohan, A. Chakrabarti, N. Nazm, R. Mehta and D. P. Edward, Indian J. Ophthalmol., 2022, 70, 1920–1930 CrossRef PubMed.
- P. P. P. Kumar and D. K. Lim, Pharmaceutics, 2021, 14, 70 CrossRef PubMed.
- A. Adam and D. Mertz, Nanomaterials, 2023, 13, 1342 CrossRef CAS PubMed.
- D. Guimarães, A. Cavaco-Paulo and E. Nogueira, Int. J. Pharm., 2021, 601, 120571 CrossRef PubMed.
- B. Pelaz, C. Alexiou, R. A. Alvarez-Puebla, F. Alves, A. M. Andrews, S. Ashraf, L. P. Balogh, L. Ballerini, A. Bestetti, C. Brendel, S. Bosi, M. Carril, W. C. Chan, C. Chen, X. Chen, X. Chen, Z. Cheng, D. Cui, J. Du, C. Dullin, A. Escudero, N. Feliu, M. Gao, M. George, Y. Gogotsi, A. Grünweller, Z. Gu, N. J. Halas, N. Hampp, R. K. Hartmann, M. C. Hersam, P. Hunziker, J. Jian, X. Jiang, P. Jungebluth, P. Kadhiresan, K. Kataoka, A. Khademhosseini, J. Kopeček, N. A. Kotov, H. F. Krug, D. S. Lee, C. M. Lehr, K. W. Leong, X. J. Liang, M. Ling Lim, L. M. Liz-Marzán, X. Ma, P. Macchiarini, H. Meng, H. Möhwald, P. Mulvaney, A. E. Nel, S. Nie, P. Nordlander, T. Okano, J. Oliveira, T. H. Park, R. M. Penner, M. Prato, V. Puntes, V. M. Rotello, A. Samarakoon, R. E. Schaak, Y. Shen, S. Sjöqvist, A. G. Skirtach, M. G. Soliman, M. M. Stevens, H. W. Sung, B. Z. Tang, R. Tietze, B. N. Udugama, J. S. VanEpps, T. Weil, P. S. Weiss, I. Willner, Y. Wu, L. Yang, Z. Yue, Q. Zhang, Q. Zhang, X. E. Zhang, Y. Zhao, X. Zhou and W. J. Parak, ACS Nano, 2017, 11, 2313–2381 CrossRef CAS PubMed.
- Z. X. Wang, Z. Wang and F. G. Wu, ChemMedChem, 2022, 17, e202200003 CrossRef CAS PubMed.
- S. Dhiman, A. Kaur and M. Sharma, Mini-Rev. Med. Chem., 2022, 22, 2864–2880 CrossRef PubMed.
- C. T. Matea, T. Mocan, F. Tabaran, T. Pop, O. Mosteanu, C. Puia, C. Iancu and L. Mocan, Int. J. Nanomed., 2017, 12, 5421–5431 CrossRef CAS PubMed.
- H. S. Lee, J. H. Choi, L. Cui, Y. Li, J. M. Yang, J. J. Yun, J. E. Jung, W. Choi and K. C. Yoon, Invest. Ophthalmol. Visual Sci., 2017, 58, 1196–1207 CrossRef CAS PubMed.
- M. Murali, N. Kalegowda, H. G. Gowtham, M. A. Ansari, M. N. Alomary, S. Alghamdi, N. Shilpa, S. B. Singh, M. C. Thriveni, M. Aiyaz, N. Angaswamy, N. Lakshmidevi, S. F. Adil, M. R. Hatshan and K. N. Amruthesh, Pharmaceutics, 2021, 13, 1662 CrossRef CAS PubMed.
- M. P. Purohit, A. K. Kar, M. Kumari, D. Ghosh and S. Patnaik, ACS Appl. Mater. Interfaces, 2023, 15, 19904–19920 CrossRef CAS PubMed.
- H. A. Hussein, M. S. Nazir, N. Azra, Z. Qamar, A. Seeni, T. A. D. A. Tengku Din and M. A. Abdullah, Mar. Drugs, 2022, 20, 480 CrossRef CAS PubMed.
- S. Rashki, K. Asgarpour, H. Tarrahimofrad, M. Hashemipour, M. S. Ebrahimi, H. Fathizadeh, A. Khorshidi, H. Khan, Z. Marzhoseyni, M. Salavati-Niasari and H. Mirzaei, Carbohydr. Polym., 2021, 251, 117108 CrossRef CAS PubMed.
- A. C. Trindade, P. de Castro, B. Pinto, J. A. R. Ambrósio, B. M. de Oliveira Jr., M. Beltrame Jr., E. P. Gonçalves, J. G. Pinto, J. Ferreira-Strixino and A. R. Simioni, J. Biomater. Sci., Polym. Ed., 2022, 33, 551–568 CrossRef PubMed.
- S. Bhullar, N. Goyal and S. Gupta, Int. J. Nanomed., 2022, 17, 3147–3161 CrossRef PubMed.
- X. Zhang, S. Vadoothker, W. M. Munir and O. Saeedi, Eye Contact Lens., 2019, 45, 11–18 CrossRef PubMed.
- A. L. Focsan, N. E. Polyakov and L. D. Kispert, Molecules, 2019, 24, 3947 CrossRef CAS PubMed.
- E. C. O'Neil, M. Henderson, M. Massaro-Giordano and V. Y. Bunya, Curr. Opin. Ophthalmol., 2019, 30, 166–178 CrossRef PubMed.
- M. L. Circu and T. Y. Aw, Free Radicals Biol. Med., 2010, 48, 749–762 CrossRef CAS PubMed.
- Y. Nakazawa, M. Oka, A. Mitsuishi, M. Bando and M. Takehana, Biochem. Biophys. Res. Commun., 2011, 415, 125–130 CrossRef CAS PubMed.
- E. M. Messmer, Dtsch. Arztebl. Int., 2015, 112, 71–81 Search PubMed , quiz 82.
- C. S. de Paiva, S. C. Pflugfelder, S. M. Ng and E. K. Akpek, Cochrane Database Syst. Rev., 2019, 9, Cd010051 Search PubMed.
- Y. J. Li, L. J. Luo, S. G. Harroun, S. C. Wei, B. Unnikrishnan, H. T. Chang, Y. F. Huang, J. Y. Lai and C. C. Huang, Nanoscale, 2019, 11, 5580–5594 RSC.
- H. Y. Huang, M. C. Wang, Z. Y. Chen, W. Y. Chiu, K. H. Chen, I. C. Lin, W. V. Yang, C. C. Wu and C. L. Tseng, Int. J. Nanomed., 2018, 13, 7251–7273 CrossRef CAS PubMed.
- L. Hu, Z. Hu, Y. Yu, X. Ding, K. Li, Q. Gong, D. Lin, M. Dai, F. Lu and X. Li, Int. J. Pharm., 2020, 588, 119683 CrossRef CAS PubMed.
- B. V. Villegas and J. M. Benitez-Del-Castillo, Turk. J. Ophthalmol., 2021, 51, 45–54 CrossRef PubMed.
- A. Bacsi, N. Dharajiya, B. K. Choudhury, S. Sur and I. Boldogh, J. Allergy Clin. Immunol., 2005, 116, 836–843 CrossRef CAS PubMed.
- W. Muhammad, J. Zhu, Z. Zhai, J. Xie, J. Zhou, X. Feng, B. Feng, Q. Pan, S. Li, R. Venkatesan, P. Li, H. Cao and C. Gao, Acta Biomater., 2022, 148, 258–270 CrossRef CAS PubMed.
- W. Badri, K. Miladi, S. Robin, C. Viennet, Q. A. Nazari, G. Agusti, H. Fessi and A. Elaissari, Pharm. Res., 2017, 34, 1773–1783 CrossRef CAS PubMed.
- C. B. Chesson, M. Huante, R. J. Nusbaum, A. G. Walker, T. M. Clover, J. Chinnaswamy, J. J. Endsley and J. S. Rudra, Sci. Rep., 2018, 8, 12519 CrossRef PubMed.
- H. Cao, L. Liu, J. Wang, M. Gong, R. Yuan, J. Lu, X. Xiao and X. Liu, Molecules, 2022, 27, 598 CrossRef CAS PubMed.
- A. Vasconcelos, E. Vega, Y. Pérez, M. J. Gómara, M. L. García and I. Haro, Int. J. Nanomed., 2015, 10, 609–631 Search PubMed.
- H. C. Kau, C. C. Tsai, C. F. Lee, S. C. Kao, W. M. Hsu, J. H. Liu and Y. H. Wei, Eye, 2006, 20, 826–831 CrossRef CAS PubMed.
- A. M. Cimpean, M. P. Sava and M. Raica, Mol. Vision, 2013, 19, 348–356 Search PubMed.
- R. Sebastiá, M. P. Ventura, H. P. Solari, E. Antecka, M. E. Orellana and M. N. Burnier Jr., Diagn. Pathol., 2013, 8, 32 CrossRef PubMed.
- M. Wu, S. Wang, Y. Wang, F. Zhang and T. Shao, Exp. Eye Res., 2020, 197, 108124 CrossRef CAS PubMed.
- M. P. Nicholas and N. Mysore, Exp. Eye Res., 2021, 202, 108363 CrossRef CAS PubMed.
- Q. Zheng, Y. Fang, L. Zeng, X. Li, H. Chen, H. Song, J. Huang and S. Shi, J. Mater. Chem. B, 2019, 7, 6759–6769 RSC.
- N. Pradhan, R. Guha, S. Chowdhury, S. Nandi, A. Konar and S. Hazra, J. Mol. Med., 2015, 93, 1095–1106 CrossRef CAS PubMed.
- B. N. Singh, S. Shankar and R. K. Srivastava, Biochem. Pharmacol., 2011, 82, 1807–1821 CrossRef CAS PubMed.
- T. Miyagawa, Z. Y. Chen, C. Y. Chang, K. H. Chen, Y. K. Wang, G. S. Liu and C. L. Tseng, Pharmaceutics, 2020, 12, 404 CrossRef CAS PubMed.
- H. Zhu, J. Ye, Y. Wu, Y. Cheng, M. Su, Q. Dai, Y. Han, J. Pan, Z. Wu, C. Chen, C. Qiu, W. Li, G. Liu and C. Chu, Adv. Healthc. Mater., 2024, 13, e2302192 CrossRef PubMed.
- M. Romero-Jiménez, J. Santodomingo-Rubido and J. S. Wolffsohn, Cont. Lens Anterior Eye, 2010, 33, 157–166 CrossRef PubMed , quiz 205.
- S. M. Ahmadi Hosseini, N. Mohidin, F. Abolbashari, B. Mohd-Ali and C. T. Santhirathelagan, Int. Ophthalmol., 2013, 33, 139–145 CrossRef PubMed.
- M. Chwa, S. R. Atilano, V. Reddy, N. Jordan, D. W. Kim and M. C. Kenney, Invest. Ophthalmol. Visual Sci., 2006, 47, 1902–1910 CrossRef PubMed.
- R. Buddi, B. Lin, S. R. Atilano, N. C. Zorapapel, M. C. Kenney and D. J. Brown, J. Histochem. Cytochem., 2002, 50, 341–351 CrossRef CAS PubMed.
- G. L. Squadrito and W. A. Pryor, Free Radicals Biol. Med., 1998, 25, 392–403 CrossRef CAS PubMed.
- H. Cui, Y. Kong and H. Zhang, J. Signal Transduction, 2012, 2012, 646354 Search PubMed.
- J. Singh, M. Sharma, N. Jain, I. Aftab, N. Vikram, T. P. Singh, P. Sharma and S. Sharma, Indian J. Ophthalmol., 2022, 70, 2328–2334 CrossRef PubMed.
- B. Wang, Y. P. Timilsena, E. Blanch and B. Adhikari, Crit. Rev. Food Sci. Nutr., 2019, 59, 580–596 CrossRef CAS PubMed.
- Z. Tang, X. Fan, Y. Chen and P. Gu, Adv. Sci., 2022, 9, e2003699 CrossRef PubMed.
- J. Bjerager, E. H. C. van Dijk, L. M. Holm, A. Singh and Y. Subhi, Acta Ophthalmol., 2022, 100, 614–623 CrossRef PubMed.
- E. Altomare, I. Grattagliano, G. Vendemaile, T. Micelli-Ferrari, A. Signorile and L. Cardia, Eur. J. Clin. Invest., 1997, 27, 141–147 CrossRef CAS PubMed.
- Z. Kyselová, S. J. Garcia, A. Gajdosíková, A. Gajdosík and M. Stefek, Physiol. Res., 2005, 54, 49–56 Search PubMed.
- Y. Zhou, L. Li, S. Li, S. Li, M. Zhao, Q. Zhou, X. Gong, J. Yang and J. Chang, Nanoscale, 2019, 11, 13126–13138 RSC.
- J. Yang, X. Gong, L. Fang, Q. Fan, L. Cai, X. Qiu, B. Zhang, J. Chang and Y. Lu, Nanomedicine, 2017, 13, 1147–1155 CrossRef CAS PubMed.
- C. J. Thomas, R. G. Mirza and M. K. Gill, Med. Clin. North Am., 2021, 105, 473–491 CrossRef PubMed.
- L. Ung, U. Pattamatta, N. Carnt, J. L. Wilkinson-Berka, G. Liew and A. J. R. White, Clin. Sci., 2017, 131, 2865–2883 CrossRef CAS PubMed.
- S. Datta, M. Cano, K. Ebrahimi, L. Wang and J. T. Handa, Prog. Retinal Eye Res., 2017, 60, 201–218 CrossRef CAS PubMed.
- S. G. Jarrett and M. E. Boulton, Mol. Aspects Med., 2012, 33, 399–417 CrossRef CAS PubMed.
- A. K. Rimpelä, M. Reinisalo, L. Hellinen, E. Grazhdankin, H. Kidron, A. Urtti and E. M. Del Amo, Adv. Drug Delivery Rev., 2018, 126, 23–43 CrossRef PubMed.
- Y. S. Kwon, M. Zheng, A. Y. Zhang and Z. Han, ACS Nano, 2022, 16, 19412–19422 CrossRef CAS PubMed.
- R. N. Mitra, R. Gao, M. Zheng, M. J. Wu, M. A. Voinov, A. I. Smirnov, T. I. Smirnova, K. Wang, S. Chavala and Z. Han, ACS Nano, 2017, 11, 4669–4685 CrossRef CAS PubMed.
- F. Shen, B. Chen, J. Danias, K. C. Lee, H. Lee, Y. Su, S. M. Podos and T. W. Mittag, Invest. Ophthalmol. Visual Sci., 2004, 45, 3107–3112 CrossRef PubMed.
- H. S. Chung, A. Harris, D. W. Evans, L. Kagemann, H. J. Garzozi and B. Martin, Surv. Ophthalmol., 1999, 43(Suppl 1), S43–S50 CrossRef PubMed.
- J. Alvarado, C. Murphy and R. Juster, Ophthalmology, 1984, 91, 564–579 CrossRef CAS PubMed.
- L. Zhou, Y. Li and B. Y. Yue, J. Cell Physiol., 1999, 180, 182–189 CrossRef CAS.
- S. C. Saccà, A. Pascotto, P. Camicione, P. Capris and A. Izzotti, Arch. Ophthalmol., 2005, 123, 458–463 CrossRef PubMed.
- A. Izzotti, S. C. Saccà, C. Cartiglia and S. De Flora, Am. J. Med., 2003, 114, 638–646 CrossRef CAS PubMed.
- R. Rong, X. Zhou, G. Liang, H. Li, M. You, Z. Liang, Z. Zeng, H. Xiao, D. Ji and X. Xia, ACS Nano, 2022, 16, 21225–21239 CrossRef CAS PubMed.
- Q. Li, M. Xin, X. Wu and B. Lei, Nanomedicine, 2022, 17, 151–165 CrossRef CAS PubMed.
- F. Ma, J. Feng, X. Liu, Y. Tian, W. J. Wang, F. X. Luan, Y. J. Wang, W. Q. Yang, J. Y. Bai, Y. Q. Zhang and Y. Tao, Nanoscale, 2023, 15, 1890–1899 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |