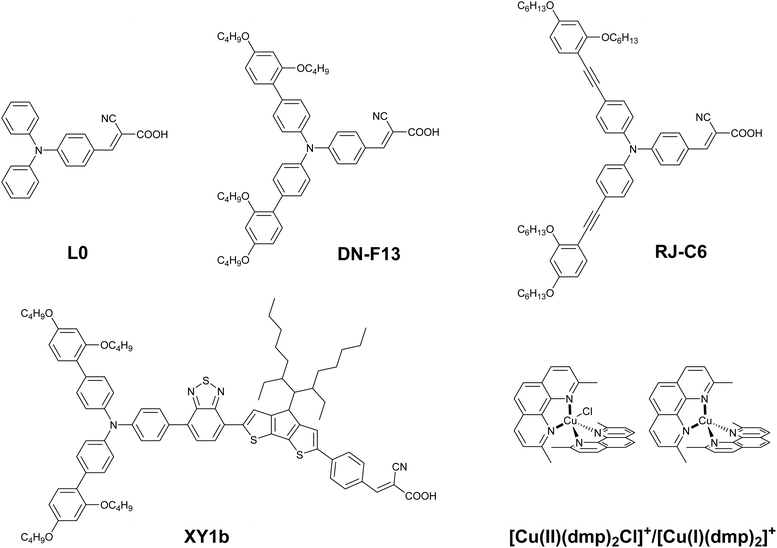
Enhanced indoor photovoltaic efficiency of 40% in dye-sensitized solar cells using cocktail starburst triphenylamine dyes and dual-species copper electrolyte†‡
Paravakkal R.
Jebin
ac,
Andrew Simon
George
ac,
Rakesh K.
Mishra
 d,
Jubi
John
d,
Jubi
John
 *bc and
Suraj
Soman
*bc and
Suraj
Soman
 *ac
*ac
aCentre for Sustainable Energy Technologies, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram 695019, India. E-mail: suraj@niist.res.in
bChemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram 695019, India
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
dDepartment of Chemistry, National Institute of Technology Uttarakhand (NITUK), Garhwal, Srinagar, 246174, India
First published on 5th November 2024
Abstract
Recombination is the most critical process that controls the photovoltaic performance in dye-sensitized solar cells (DSCs). Herein, we successfully introduced a new triphenylamine-based starburst photosensitizer, RJ-C6 [3-(4-(bis(4-((2,4-bis(hexyloxy)phenyl)ethynyl)phenyl)amino)phenyl)-2-cyanoacrylic acid] grafted with extended alkyl groups and phenylethynyl bridges capable of arresting recombination more effectively and realizing enhanced light harvesting through improved conjugation and rigidity. Using a cocktail mixture of RJ-C6 with XY1b dye and asymmetric dual-species copper(II/I) electrolyte, we realized an efficiency of 10.40% under standard AM 1.5G, 100 mW cm−2 irradiation. Strikingly, the same co-sensitized devices exhibited panchromatic absorption overlapping the entire fluorescent light spectra, delivering efficiencies of 35% under 100 lux, 37% under 1000 lux and a record PCE of 40% under 4000 lux, taking DSCs one step closer to being used as an attractive candidate for indoor photovoltaic applications.
1 Introduction
Molecular photovoltaic technologies like dye-sensitized solar cells (DSCs) hold great promise towards our effort to realize a carbon-neutral society.1–5 These emerging photovoltaic technologies can supplement the ever-increasing energy demand in niche areas like Indoor Photovoltaics (IPV) and Building Integrated Photovoltaics (BIPV).4–8 Even though perovskite solar cells (PSCs) have realized efficiencies as high as 26.4% under full sun, much closer to those of silicon and thin film solar cells, with a power conversion efficiency (PCE) above 40% under indoor illumination, the use of lead and stability issues hinder their entry into the indoor PV market.9–14 The use of low-cost sustainable materials, along with less energy-intensive fabrication processes with the potential for recycling and the ability to control colour as well as transparency, provides added advantage to DSCs.15–25 Recently, DSCs proved to be the best photovoltaic technology for indoor/ambient light harvesting applications, realizing power conversion efficiencies above 30%.26–31 The primary batteries used in smart wireless electronics, including the Internet of Things (IoTs), are estimated to reach 27 billion by 2025.32 Replacing at least half of these primary batteries with sustainable and recyclable DSCs having a lower carbon footprint can tremendously bring down the carbon emissions.33Dye-sensitized solar cells used for ambient/indoor photovoltaics are different from the conventional DSCs that use ruthenium dyes, iodide/triiodide electrolyte and platinum counter electrodes.29,34 These re-engineered DSCs use co-sensitized organic dyes, the majority of them using triphenylamines (TPAs), having donor–π–acceptor (D–π–A) architecture with a higher absorption coefficient as well as broader light harvesting across the visible region.35–37 The molecular backbone of these sensitizers can be easily tailored to control the band gap and absorption properties. In the seminal contribution by Gratzel and co-workers in 2017, D35 was used as the co-sensitizer with XY1, leading to 28.9% power conversion efficiency (PCE).29 This was followed by using Y123 and XY1b co-sensitized dyes in direct contact architecture, leading to 32% efficient DSCs.26 Furthermore, the PCE was improved to 34% by using L1 along with XY1.28 Later, the use of MS5 having longer alkyl chains and XY1b enhanced the PCE to 34.5%.27 From the above observations, it is evident that even a slight change in the molecular architecture of sensitizers can significantly influence the indoor photovoltaic performance. It also needs to be noted that all the high-performing sensitizer combinations use dyes with triphenylamine as the donor core, and in all the above reports [Cu(tmby)2]2+/+ (tmby = bis-(4,4′,6,6′-tetramethyl-2,2′-bipyridine)) was used as the redox electrolyte.38
Recently, Suraj and co-workers introduced a new class of asymmetric dual-species Cu(II/I) electrolyte, [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+(dmp = 2,9-dimethyl-1,10-phenanthroline), which was used along with co-sensitized D35 and XY1b combination realizing 35.6% efficient DSCs.30 Taking into account these reported results and our previous experience with dendritic triphenylamine-based dyes,39–41 we have designed and synthesized a starburst propeller shaped triphenylamine sensitizer (RJ-C6) with hexyloxy chains grafted to the periphery along with ethynyl linkages which provide better conjugation and light harvesting properties covering the entire visible spectrum. The variation in optoelectronic properties of RJ-C6 was compared with that of two-parent dyes L0, having only triphenylamine and DN-F13, with an extended phenyl group having C4 alkyl chains (Fig. 1).42 The novel RJ-C6 dye, co-sensitized with XY1b, achieved an efficiency of 10.39 ± 0.06% under standard AM 1.5G, 100 mW cm−2 irradiation. The same combination realized an efficiency of 37.3 ± 0.22% under 1000 lux CFL illumination compared to 28.3 ± 0.44% using XY1b alone and achieved an outstanding efficiency of 40% (39.8 ± 0.15%) under 4000 lux, representing the highest reported PCE among DSCs to date. Notably, these devices consistently maintained an average efficiency above 35% across a broad range of ambient and indoor illumination levels, spanning from 100 lux to 6000 lux and consistent stability over 800 h. This robust performance makes them exceptionally well-suited for practical indoor photovoltaic applications as demonstrated by powering a temperature sensor/clock using two serially interconnected DSCs of <1 cm2 (0.64 cm2) active area.
2 Experimental
2.1 Synthesis
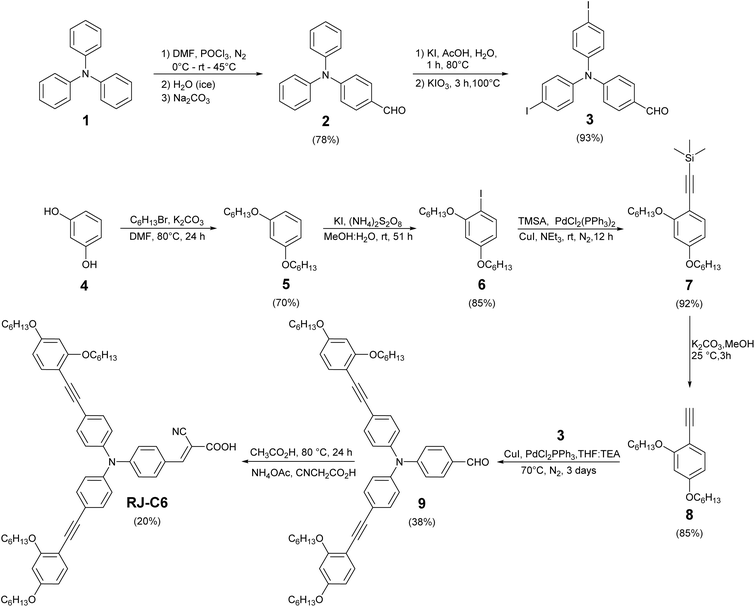
To improve the light-harvesting properties in the UV/vis spectral region, we introduced ethynyl linkages between the phenyl donor groups and the triphenylamine core (Fig. 1). This increases the π-conjugation of the RJ-C6 sensitizer and improves the molecular planarity, leading to enhanced dye packing on the semiconductor surface. Additionally, hexyloxy groups at ortho- and para-positions were introduced at the peripheral phenyl groups to suppress molecular aggregation of dyes as well as to reduce the recombination of injected electrons in titanium dioxide (TiO2) with oxidized Cu(II) species in the electrolyte. The novel RJ-C6 dye with custom made structural modification having D–π–A architecture was synthesized as detailed in Scheme 1. The donor part consists of a triphenylamine central core radially connected to an ortho, para-hexyloxy substituted resorcinol derivative through ethynyl linkages on either side. Cyanoacrylic acid is introduced as the acceptor and anchoring moiety, which binds the dyes to TiO2.Triphenylamine (1) was first subjected to the Vilsmeier–Haack reaction, and then it was iodinated to yield 4-(bis(4-iodophenyl)amino)benzaldehyde (3).43 Resorcinol (4) was alkylated with bromohexane,44 and then the alkylated intermediate (5) was subjected to iodination45 to yield 2,4-bis(hexyloxy)-1-iodobenzene (6), followed by Sonogashira coupling using trimethylsilylacetylene and deprotection to obtain the free acetylene moiety (8). The obtained 1-ethynyl-2,4-bis(hexyloxy)benzene (8) was coupled with (3) to yield the dye precursor (9).39 It was further subjected to Knoevenagel condensation to yield the desired dye, RJ-C6. In all the individual steps, the products were purified using silica gel column chromatography. The details of preparation of precursors and RJ-C6 dye along with characterization details are provided in Sections 1–3 and Fig. S1–S10 (ESI).‡
3 Results and discussion
3.1 Optical and electrochemical properties
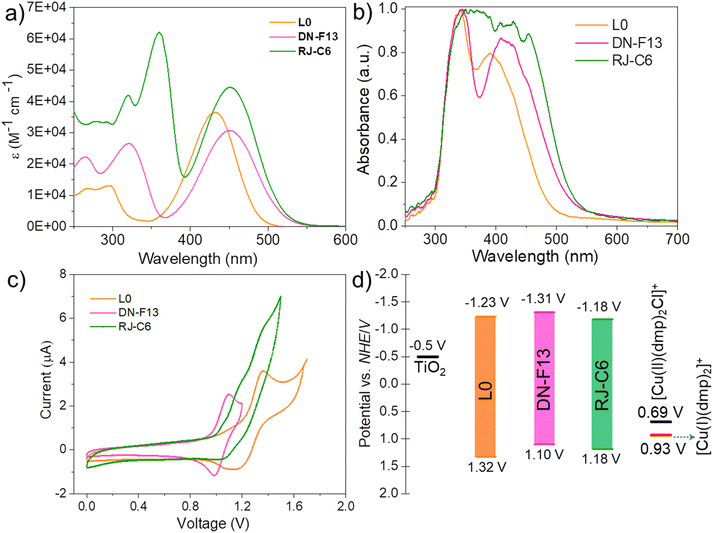
The sensitizer plays a critical role in dye-sensitized solar cells, as it determines the efficiency of light harvesting by converting incident light into electrons, which are then injected into the semiconductor.46 Thus, a comprehensive understanding of the optical and electrochemical properties is essential to establish a correlation between the structural/geometrical characteristics of the dye and the photovoltaic performance. The UV/vis absorption spectra of L0, DN-F13 and RJ-C6 in chloroform are shown in Fig. 2a, and their absorption peaks and molar extinction coefficients are summarized in Table 1. All three dyes displayed two prominent peaks in their respective UV/vis spectrum, i.e. one between 300 and 400 nm, while another between 400 and 500 nm. The higher energy UV region absorption bands can be assigned to localized aromatic π–π* transition. The intramolecular charge transfer (ICT) between the triphenylamine donor and the cyanoacrylic acid acceptor is the source for the lower energy absorption band in the visible region between 400 and 500 nm. L0 showed an absorption maximum of 432 nm, having a molar extinction coefficient of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1, while DN-F13 displayed a red-shifted absorption band at 451 nm compared to L0. On the other hand, moving to the more conjugated RJ-C6 dye, the absorption at 451 nm was close to that of DN-F13. Even though both DN-F13 and RJ-C6 displayed comparable absorption maxima, the extended π-conjugation and improved planarity introduced by the ethynyl linkages led to a higher absorption coefficient (44
500 M−1 cm−1, while DN-F13 displayed a red-shifted absorption band at 451 nm compared to L0. On the other hand, moving to the more conjugated RJ-C6 dye, the absorption at 451 nm was close to that of DN-F13. Even though both DN-F13 and RJ-C6 displayed comparable absorption maxima, the extended π-conjugation and improved planarity introduced by the ethynyl linkages led to a higher absorption coefficient (44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 M−1 cm−1) and higher light harvesting ability in the visible region for the RJ-C6 sensitizer compared to those of DN-F13 (30
480 M−1 cm−1) and higher light harvesting ability in the visible region for the RJ-C6 sensitizer compared to those of DN-F13 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1). Thus, introducing ethynyl groups into the RJ-C6 sensitizer contributed towards better conjugation and delocalization of charges throughout the entire π-conjugated backbone. The solid-state absorption spectra of all three dyes (L0, DN-F13 and RJ-C6) adsorbed on TiO2 are provided in Fig. 2b. All dyes display broader and red-shifted absorption after being anchored to TiO2. Also, there is a clear red shift in the absorption with increased conjugation going from L0 → DN-F13 → RJ-C6. The RJ-C6 sensitizer with acetylene bridges between the triphenylamine core and peripheral donor units with additional hexyloxy chains at the ortho and para positions of the peripheral phenyl groups has contributed to improved absorption. Additionally, enhanced planarity imparted by the acetylene bridge helped in having better close packing of dyes on the semiconductor, leading to higher dye loading using RJ-C6 (Table 2), contributing towards a broader photoresponse in the visible region overlapping with the spectra of artificial light, thereby improving the light-harvesting capability of our new custom-engineered RJ-C6 dye.
700 M−1 cm−1). Thus, introducing ethynyl groups into the RJ-C6 sensitizer contributed towards better conjugation and delocalization of charges throughout the entire π-conjugated backbone. The solid-state absorption spectra of all three dyes (L0, DN-F13 and RJ-C6) adsorbed on TiO2 are provided in Fig. 2b. All dyes display broader and red-shifted absorption after being anchored to TiO2. Also, there is a clear red shift in the absorption with increased conjugation going from L0 → DN-F13 → RJ-C6. The RJ-C6 sensitizer with acetylene bridges between the triphenylamine core and peripheral donor units with additional hexyloxy chains at the ortho and para positions of the peripheral phenyl groups has contributed to improved absorption. Additionally, enhanced planarity imparted by the acetylene bridge helped in having better close packing of dyes on the semiconductor, leading to higher dye loading using RJ-C6 (Table 2), contributing towards a broader photoresponse in the visible region overlapping with the spectra of artificial light, thereby improving the light-harvesting capability of our new custom-engineered RJ-C6 dye.
| Dyes | λ (max) (nm) | E HOMO (V vs. NHE) | E LUMO (V vs. NHE) | Band gap (Eo–o) (eV) | Molar absorption coefficient, ε (M−1 cm−1) |
|---|---|---|---|---|---|
| L0 | 432 | 1.32 | −1.23 | 2.55 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| DN-F13 | 451 | 1.10 | −1.31 | 2.41 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| RJ-C6 | 451 | 1.18 | −1.18 | 2.36 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
| Dyes | V oc (V) | J sc (mA cm−2) | FF | η (%) | Dye loading (mol cm−2 × 10−7) |
|---|---|---|---|---|---|
| L0 | 0.64 | 1.35 | 0.49 | 0.43 | 0.21 |
| DN-F13 | 0.81 | 3.44 | 0.63 | 1.78 | 0.45 |
| RJ-C6 | 1.024 | 6.19 | 0.69 | 4.34 | 0.89 |
Energetics of the sensitizers plays a significant role in dictating the efficiency of charge injection and regeneration in DSCs. Thus, a proper understanding of the ground and excited state energy levels in sensitizers is critical for evaluating the efficiency of the electron transfer process in these photoelectrochemical devices. The electrochemical characteristics of L0, DN-F13 and RJ-C6 were studied using cyclic voltammetry (CV) in chloroform using tetrabutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte, glassy carbon as the working electrode, platinum (Pt) as the counter electrode, and Ag/AgCl as the reference electrode. Ferrocene/ferrocenium (Fc/Fc+, E0 = 0.48 V vs. NHE) was used as the standard to calibrate the reference electrode. Fig. 2c provides the cyclic voltammograms of all three sensitizers, and the corresponding results are presented in Table 1.
The first oxidation potentials (Eox) of the dyes at their highest occupied molecular orbital (HOMO) levels (L0: 1.32 V, DN-F13: 1.10 V and RJ-C6: 1.18 V vs. NHE) are sufficiently more positive than the redox potential of the reduced copper(I) species, [Cu(I)(dmp)2]+ (0.93 V vs. NHE). This clearly suggests that all the oxidized dyes can be efficiently regenerated by the copper redox mediator. L0 dye with lower conjugation of the π-backbone exhibited a wider band gap of 2.86 eV compared to the more π-conjugated DN-F13 (2.41 eV) and RJ-C6 (2.36 eV) dyes. The introduction of acetylene bridges between the triphenylamine core and the peripheral phenyl groups in RJ-C6 dye has altered the energy levels of the frontier molecular orbitals, as seen from the positive shift in the HOMO energy level to 1.18 V as compared to 1.10 V in DN-F13. The theoretical studies further substantiate this shift in potential as the HOMO is mainly localised on the donor side, and it is discussed in the following section. The observed shift of 80 mV for RJ-C6 compared to DN-F13 could help with a better regeneration of its ground state by the reduced copper(I) species [Cu(I)(dmp)2]+ (0.93 V vs. NHE), which has a more positive redox potential. The LUMO energy levels of all the dyes are positioned at potentials with enough driving force to inject excited electrons into the conduction band of TiO2. The energy level diagram illustrating the positions of the HOMO–LUMO energy levels of L0, DN-F13 and RJ-C6 dyes, to the redox potential of the dual-species copper(II/I) electrolyte and TiO2 conduction band is given in Fig. 2d.
3.2 Theoretical studies
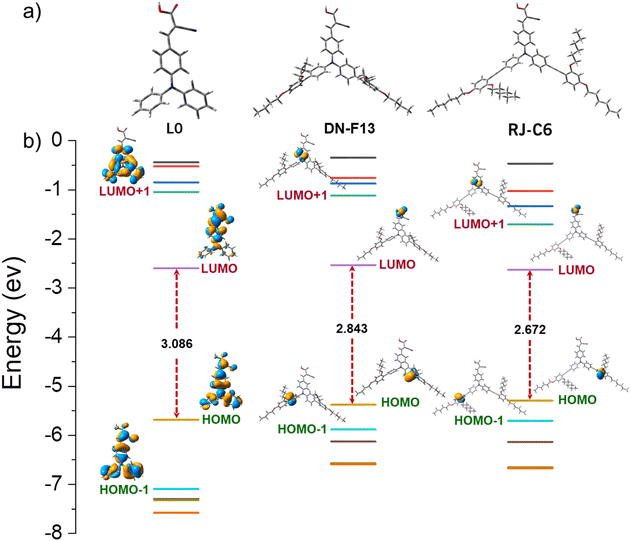
For a deeper understanding of structure–property relationships and the electron density distribution of L0, DN-F13 and RJ-C6 dyes, density functional theory (DFT) calculations were performed at the B3LYP exchange-correlation functional level of theory with the B3LYP/6-311G(d,p) basis set47,48 using the Gaussian G16 package, while GaussView 6.0 was used for the visualization of the structures.49,50 All the optimized structures were subjected to vibrational analysis to rule out any imaginary frequencies, and the corresponding energy-minimizing structures are provided in Fig. 3a. The details regarding the side view and length of dyes L0, DN-F13, RJ-C6, and XY1b are given in Fig. S11 and S12 (ESI).‡ The optimized structures of L0, DN-F13 and RJ-C6 indicate that the introduction of an ethynyl moiety in RJ-C6 reduced the dihedral angle and thus increased the planarity in the molecule. The dihedral angles between the adjacent phenyl rings (θ4 and θ5) are found to be (44.07° and 44.02°), whereas the ethynyl moiety forced the phenyl ring to remain in-plane with the dihedral angles spanning 0.68°–1.57° (Table S1, ESI‡). These reduced dihedral angles in RJ-C6 helped in better close packing of dyes and higher dye loading (Table 2). The more twisted phenyl rings of the DN-F13 dye can be attributed to the steric hindrance between adjacent aromatic moieties that eventually may reduce the intermolecular aggregation. Moreover, RJ-C6 is longer than L0 and DN-F13, which takes the alkyl chains outside, creating more recombination barriers, further proved by photovoltaic results.Furthermore, to simulate the theoretical UV/vis spectra of the dyes, the optimized geometries were utilized for single-point time-dependent DFT (TDDFT) calculations. The isodensity plots of frontier molecular orbitals and the energy levels of L0, DN-F13 and RJ-C6 are provided in Fig. 3b. It is evident from Fig. 3b that for RJ-C6 dye, the HOMO is localized towards the donor and the LUMO is localized on the acceptor unit facilitating charge separation and electron injection. Prominent HOMO and LUMO representation of energy levels for L0, DN-F13-Me and RJ-C6-Me are provided in Table S5, ESI.‡
3.3 Photovoltaic performance of L0, DN-F13 and RJ-C6 dyes with dual-species Cu(II/I) electrolyte under one sun illumination
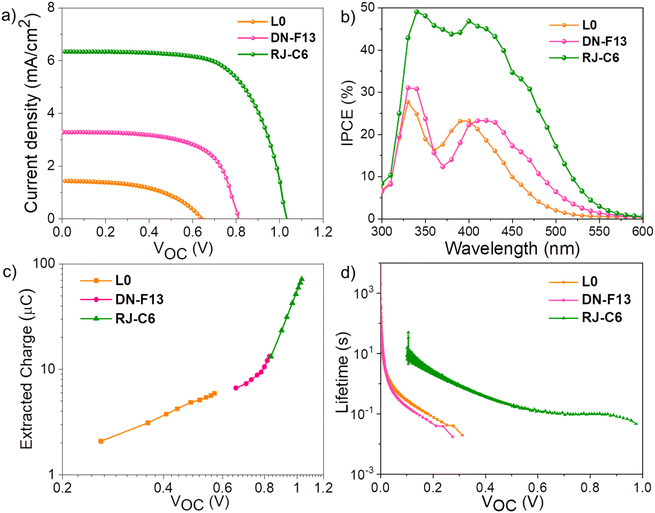
The introduction of copper-based electrolytes to replace conventional iodine and cobalt redox systems has revitalized photovoltaic research using dye-sensitized solar cells.51 These earth-abundant and environmentally friendly Cu(II/I) redox electrolytes offer inherent structural advantages that facilitate efficient dye regeneration with a required driving force as low as 100 mV.52–54 Leveraging the more positive redox potential and faster dye regeneration, Cu(II/I) electrolytes surpass other conventional redox mediators, achieving higher open-circuit potential and overall performance in DSCs. Despite these benefits, more positive redox potentials of copper redox electrolytes lead to increased recombination from injected electrons in TiO2 with oxidized Cu(II) species.55,56 Recently, we introduced a dual-species Cu(II/I) electrolyte using [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+, where structural modifications on the oxidized Cu(II) species provided better control of the redox potential, reducing the recombination driving force and improving the lifetime.30 It is important to note that copper redox mediators are ideally suited to work best with organic triphenylamine dyes, and even slight modifications to the structural backbone of the dyes and other peripheral changes can affect interactions with copper electrolyte, leading to variations in photovoltaic performance. In the present study, initially, we screened all the three dyes (L0, DN-F13 and RJ-C6) to understand how structural variations contribute to differences in photovoltaic performance using dmp-based dual-species [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+ electrolyte. Commercial dyes L0 and DN-F13, which are structurally similar to the RJ-C6 dye, were selected for comparison.42,57L0 features a single triphenylamine (TPA) core, while DN-F13 incorporates peripheral phenyl donors with C4 alkyl chains. RJ-C6 was custom-designed by introducing a rigidified acetylene bridge between the TPA core and donor phenyl units to enhance π-conjugation and replacing C4 alkyl chains with C6-hexyloxy units at ortho and para positions, which effectively passivate recombination.58–61Devices utilizing L0, DN-F13 and RJ-C6 dyes were fabricated following established protocols as adopted in our prior work, and the details are provided in the Experimental section.62 The well-aligned positive ground state (HOMO) level of the RJ-C6 dye (1.18 V vs. NHE, Fig. 2d and Table 1) makes the dual-species [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+ electrolyte an ideal redox electrolyte for assessing the photovoltaic performance. Fig. 4a provides the photocurrent–voltage (J–V) plots for the fabricated DSCs under standard one sun conditions (AM 1.5G/100 mW cm−2), and the corresponding PV parameters are provided in Table 2. The improvement in photovoltaic performance on shifting from the commercial dyes (L0 and DN-F13) to the newly designed RJ-C6 dye is evident with the enhancement in PCE. RJ-C6 dye delivered a PCE of 4.34% compared to 0.43% for L0 and 1.78% for DN-F13 dyes (Table 2). A comprehensive improvement of 26.4% in Voc (1.024 V), 79% in Jsc (6.19 mA cm−2) and 7.5% in fill factor (0.69) was observed for RJ-C6 dye compared to DN-F13 having a Voc of 0.81 V, a Jsc of 3.44 and a fill factor of 0.64. The improvement is far more prominent compared to the L0 dye achieving an increase of 60% in Voc, 35.8% in Jsc and 39.3% in FF. The incident photon-to-current conversion efficiency (IPCE) plots for the devices measured under the standard conditions with the three dyes (Fig. 4b) clearly show an improved light harvesting profile for RJ-C6 dye in the visible region compared to those of L0 and DN-F13, matching well with the solution state and solid-state absorption spectra of the corresponding dyes (Fig. 2a and b). This improved magnitude in light harvesting efficiency for RJ-C6 (nearly 50% in 350–450 nm region) and more red shift in absorption at longer wavelengths contributed to the improved Jsc for RJ-C6, as evident from Fig. 4b.
The strategy of introducing an alkynyl π-system to the molecular backbone helped RJ-C6 to realize better photon absorption, as evident from its UV/vis profile, as discussed in the previous section. The difference in the IPCE is associated with the variation in dye loading (Table 2), which is related to the molecular geometry (Fig. S13 and Table S5, ESI‡) of the dyes and their ability to close pack on the surface of the semiconductor photoanode. In addition to the better light harvesting behavior exhibited by RJ-C6 along with higher dye loading, we also need to consider the role of energetics in these dyes that act as a major contributor controlling the charge transfer reactions, in particular, electron injection and regeneration. Aside from the photovoltaic data obtained (Table 2), the Voc of RJ-C6 (1.024 V) showed improvements of 60% and 26% compared to L0 (0.64 V) and DN-F13 (0.81 V). This suggests that RJ-C6 dye is more efficient in preventing charge recombination by virtue of its planar nature, as evident from DFT studies (Table S1‡), contributing towards better close packing and improved dye loading. The presence of longer hexyloxy alkyl chains prevents the spatial closeness of Cu(II) with the injected electrons in TiO2 as well as a wider peripheral intramolecular distance of 33.41 Å for RJ-C6 compared to 24.66 Å for DN-F13 (Fig S12, ESI‡) also helps in creating a solid barrier against back electron transfer, making RJ-C6 a better candidate for using it with the Cu(II/I) electrolyte. Furthermore, we investigated changes in the conduction band using charge extraction (Fig. 4c), revealing the minimal influence of structural and geometrical variations in the dyes on the TiO2 conduction band and Fermi level shifting.63 Therefore, the improved Voc can primarily be attributed to the capability of the RJ-C6 dye in efficiently suppressing electron recombination.64
To assess the impact of recombination on the open-circuit potential, we carried out lifetime measurements using open-circuit voltage decay (OCVD), and the resulting trend is provided in Fig. 4d. As expected, the RJ-C6 dye, featuring longer hexyloxy chains, provided a significant barrier towards recombination, contributing to enhanced lifetime compared to L0 and DN-F13 dyes. Notably, L0 and DN-F13 showed similar lifetime trends despite DN-F13 having peripheral phenyl donors and C4 alkyl chains, which are believed to impede recombination by obstructing the approach of oxidized Cu(II) species, compared to a single TPA unit alone for L0. Despite their structural differences in peripheral substituents, the similarity in lifetime trends between L0 and DN-F13 highlights the critical role of dye packing and TiO2 passivation in controlling the recombination in these molecular light-harvesting electrochemical devices. This was previously discussed by Grätzel and Hagfeldt29 on co-sensitized dyes and copper electrolytes, and we have also observed similar influence of dye characteristics on the photovoltaic performance in DSCs.63
3.4 Co-sensitization approach to improve the photovoltaic performance under one sun illumination
As discussed above, the co-sensitization of organic dyes has proven to be one of the most effective strategies adopted recently for achieving efficient dye-sensitized solar cells for IPV applications.65 Careful consideration is essential while selecting co-sensitizers, as they influence dye packing on the photoanode, thereby controlling recombination and directly impacting the photovoltaic performance.66 The triphenylamine-based Donor–Acceptor–π–Acceptor (D–A–π–A) sensitizer XY1b is commonly chosen as the primary dye in most of the reported studies for attaining higher efficiencies, considering its ability to efficiently capture visible light over a wider window and effectiveness in suppressing recombination.26,27 Being a bulky and longer molecule with twisted geometrical configurations (Fig. S12d, ESI‡), particularly while attached to TiO2, provides easy and accessible channels for back electron transfer from TiO2 using XY1b. The co-sensitization approach using smaller dyes that occupy the spaces between the larger XY1b dye is therefore used to effectively block the charge recombination through the intermediate spaces between dyes, wherever TiO2 is exposed to the electrolyte. The co-sensitizing dye to be used to pair with XY1b should structurally form close-packed layers, thus passivating the TiO2 surface against recombination and should also exhibit complementary absorption characteristics, particularly in the near UV/vis region around 400 nm where XY1b has a lower absorption efficiency. Achieving this requires precise manipulation of the structural backbone of the sensitizer as well as adding or subtracting with peripheral functionalities to optimize the synergy between both the dyes. This concept is demonstrated by the photovoltaic results obtained by co-sensitizing XY1b dye with L0, DN-F13 and RJ-C6, which exhibit distinct structural and geometric differences. By using the same electrolyte across these dye combinations, we made a direct comparison to evaluate how structural variations in dyes contribute to differences in the photovoltaic performance. Initial optimization of the co-sensitization ratio was carried out with varying compositions of RJ-C6 and XY1b dyes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6, 1
1.6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.6
1, 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the corresponding J–V plots are provided in Fig. S13 and Table S5 (ESI).‡ The equimolar mixture (1
1), and the corresponding J–V plots are provided in Fig. S13 and Table S5 (ESI).‡ The equimolar mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of dye combinations (0.1 mM) delivered the highest PV performance; hence, the same is selected for all further studies. The photovoltaic results of co-sensitized DSCs obtained under standard one sun (AM 1.5G, 100 mW cm−2) illumination are provided in Table 3, and the corresponding current–voltage plots are given in Fig. 5a.
1) of dye combinations (0.1 mM) delivered the highest PV performance; hence, the same is selected for all further studies. The photovoltaic results of co-sensitized DSCs obtained under standard one sun (AM 1.5G, 100 mW cm−2) illumination are provided in Table 3, and the corresponding current–voltage plots are given in Fig. 5a.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, DN-F13
XY1b, DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b and RJ-C6
XY1b and RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized DSCs
XY1b co-sensitized DSCs
| Dyes | V oc (V) | J sc (mA cm−2) | FF | η (%) | Dye loading (mol cm−2 × 10−7) |
|---|---|---|---|---|---|
L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
0.99 ± 0.001 | 15.03 ± 0.06 | 0.67 ± 0.001 | 10.01 ± 0.06 | 0.79 |
DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
0.98 ± 0.001 | 15.03 ± 0.48 | 0.51 ± 0.020 | 7.53 ± 0.17 | 0.93 |
RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
1.003 ± 0.005 | 15.71 ± 0.18 | 0.66 ± 0.005 | 10.39 ± 0.06 | 1.43 |
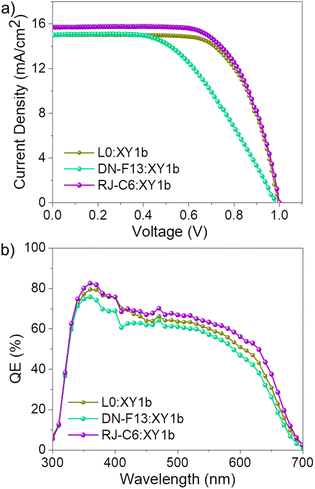 | ||
Fig. 5 (a) J–V curve under standard one sun illumination (AM 1.5G, 100 mW cm−2). (b) IPCE spectra of L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, DN-F13 XY1b, DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b and RJ-C6 XY1b and RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized dyes. XY1b co-sensitized dyes. | ||
The adopted co-sensitization strategy proved to be beneficial, as evident from the improved performance of all the dyes with co-sensitization (Table 3) compared to the PV performance of individual dyes (Table 2) and XY1b alone (Fig. S14 and Table S6, ESI‡) using similar device architecture and measuring conditions. The newly introduced RJ-C6 sensitizer co-sensitized with XY1b dye (RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b) in a 1
XY1b) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equimolar mixture of acetonitrile and t-butanol, using a 8 μm thick TiO2 layer, PEDOT counter electrode, UV epoxy spacer and [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+ (0.2 M Cu(I), 0.04 M Cu(II), 0.1 M LiTFSI and 0.6 M 1-methyl-benzimidazole (NMB) in acetonitrile) as electrolyte contributed to an efficiency of 10.39 ± 0.06% with a Jsc = 15.71 mA cm−2, Voc = 1.003 V and FF = 0.66. Both L0 and DN-F13 co-sensitized with XY1b delivered lower photovoltaic performances of 10.01 ± 0.056% (L0
1 equimolar mixture of acetonitrile and t-butanol, using a 8 μm thick TiO2 layer, PEDOT counter electrode, UV epoxy spacer and [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+ (0.2 M Cu(I), 0.04 M Cu(II), 0.1 M LiTFSI and 0.6 M 1-methyl-benzimidazole (NMB) in acetonitrile) as electrolyte contributed to an efficiency of 10.39 ± 0.06% with a Jsc = 15.71 mA cm−2, Voc = 1.003 V and FF = 0.66. Both L0 and DN-F13 co-sensitized with XY1b delivered lower photovoltaic performances of 10.01 ± 0.056% (L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b) and 7.53 ± 0.17% (DN-F13
XY1b) and 7.53 ± 0.17% (DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b). The improved current density for RJ-C6
XY1b). The improved current density for RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b is associated with a higher dye loading (Table 3) and a better external quantum efficiency profile (Fig. 5b).
XY1b is associated with a higher dye loading (Table 3) and a better external quantum efficiency profile (Fig. 5b).
Interestingly, it is noticed that the trend in the increasing PV performance for the co-sensitized dyes (RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b > L0
XY1b > L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b > DN-F13
XY1b > DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, Table 3) is different from the trend in the photovoltaic performance observed using individual dyes (RJ-C6 > DN-F13 > L0, Table 2). This clearly points out the importance of controlling the structural and geometrical aspects of individual dyes in a co-sensitized cocktail mixture, which influences the PV performance. The variation in photovoltaic parameters and device performance was further probed using various electrical and optical perturbation tools to understand the interlaying relationship between the molecular structural and geometrical aspects of individual dyes in a co-sensitized mixture, which influences the PV performance.
XY1b, Table 3) is different from the trend in the photovoltaic performance observed using individual dyes (RJ-C6 > DN-F13 > L0, Table 2). This clearly points out the importance of controlling the structural and geometrical aspects of individual dyes in a co-sensitized cocktail mixture, which influences the PV performance. The variation in photovoltaic parameters and device performance was further probed using various electrical and optical perturbation tools to understand the interlaying relationship between the molecular structural and geometrical aspects of individual dyes in a co-sensitized mixture, which influences the PV performance.
The charge extraction measurements reveal that there is a negative shift in the TiO2 conduction band using DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b mixture compared to the other two dye combinations (Fig. 6a) which could be related to the variation in dye coverage. As expected, L0, being a small dye, easily fits into the space between the larger XY1b dye, whereas DN-F13, with a twisted geometry having bulkier alkoxy chains, restricts the close packing of the dyes. Thus, the non-optimal dye coverage and a negative shift in the conduction band contribute to more recombination for the co-sensitized devices. This contributed to a lower lifetime, as seen from intensity-modulated photovoltage measurements (IMVS) (Fig. 6b). This lowers the lifetime, resulting in low open-circuit potential and fill factor for DN-F13
XY1b mixture compared to the other two dye combinations (Fig. 6a) which could be related to the variation in dye coverage. As expected, L0, being a small dye, easily fits into the space between the larger XY1b dye, whereas DN-F13, with a twisted geometry having bulkier alkoxy chains, restricts the close packing of the dyes. Thus, the non-optimal dye coverage and a negative shift in the conduction band contribute to more recombination for the co-sensitized devices. This contributed to a lower lifetime, as seen from intensity-modulated photovoltage measurements (IMVS) (Fig. 6b). This lowers the lifetime, resulting in low open-circuit potential and fill factor for DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b devices.
XY1b devices.
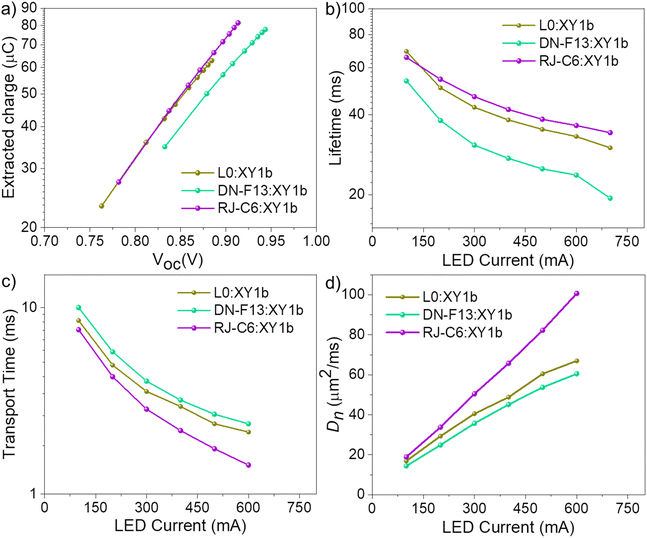 | ||
Fig. 6 (a) Extracted charge, (b) electron lifetime (τn), (c) transport time (τd) and (d) diffusion coefficient (Dn) of L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, DN-F13 XY1b, DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b and RJ-C6 XY1b and RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized DSCs. XY1b co-sensitized DSCs. | ||
The RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized devices delivered improved lifetime (Fig. 6b), faster transport time (Fig. 6c) and higher diffusion coefficient (Fig. 6d) among all the three co-sensitized devices, contributing towards better current, voltage and fill factor. Now, carefully examining the structural variation of RJ-C6 with DN-F13, we can identify that making the dye backbone more planar by introducing an acetylene bridge helped to achieve not only improved absorption but also better close packing and dye loading, leading to improved current, as evident from the J–V data as well as from interfacial, optical and electrical perturbation results. Furthermore, introduction of the acetylene bridge also contributed to increasing the length of the donor arms for RJ-C6 (Fig. S12, ESI‡). With the introduction of hexyloxy chains compared to the C4 alkyl chains in DN-F13, a pin-hole free barrier was made on TiO2 which along with synergetic interactions with XY1b efficiently blocked the oxidized Cu(II) complex in reaching the semiconductor surface, thereby arresting recombination and achieving improved lifetime. This points out the fact that in a cocktail combination either the co-sensitizer should be a small molecule like L0, which can easily get in between the primary dye or else the sensitizer needs to be custom-modified with the ability to make it a best fit in the gap, enabling it to have favourable interactions with the other dye. Under one sun illumination, more or less, the efficiencies are the same using both strategies, whereas indoor photovoltaic performance varies quite a lot, which is discussed in the following section. In the present work, since our primary interest was indoor photovoltaics, device architecture and additives, including their concentration, were selected based on our previous experience, which is best performing for indoor illumination. Thus, we expect further improvement in one sun results with more systematic optimizations and using other copper redox electrolytes like [Cu(II/I)(tmby)2]2+/+, which are less mass transport limited under high intensity one sun illumination. Fig. S15 (ESI‡) provides the charge collection efficiency (ηcc) vs. LED current plot, and it is evident that RJ-C6
XY1b co-sensitized devices delivered improved lifetime (Fig. 6b), faster transport time (Fig. 6c) and higher diffusion coefficient (Fig. 6d) among all the three co-sensitized devices, contributing towards better current, voltage and fill factor. Now, carefully examining the structural variation of RJ-C6 with DN-F13, we can identify that making the dye backbone more planar by introducing an acetylene bridge helped to achieve not only improved absorption but also better close packing and dye loading, leading to improved current, as evident from the J–V data as well as from interfacial, optical and electrical perturbation results. Furthermore, introduction of the acetylene bridge also contributed to increasing the length of the donor arms for RJ-C6 (Fig. S12, ESI‡). With the introduction of hexyloxy chains compared to the C4 alkyl chains in DN-F13, a pin-hole free barrier was made on TiO2 which along with synergetic interactions with XY1b efficiently blocked the oxidized Cu(II) complex in reaching the semiconductor surface, thereby arresting recombination and achieving improved lifetime. This points out the fact that in a cocktail combination either the co-sensitizer should be a small molecule like L0, which can easily get in between the primary dye or else the sensitizer needs to be custom-modified with the ability to make it a best fit in the gap, enabling it to have favourable interactions with the other dye. Under one sun illumination, more or less, the efficiencies are the same using both strategies, whereas indoor photovoltaic performance varies quite a lot, which is discussed in the following section. In the present work, since our primary interest was indoor photovoltaics, device architecture and additives, including their concentration, were selected based on our previous experience, which is best performing for indoor illumination. Thus, we expect further improvement in one sun results with more systematic optimizations and using other copper redox electrolytes like [Cu(II/I)(tmby)2]2+/+, which are less mass transport limited under high intensity one sun illumination. Fig. S15 (ESI‡) provides the charge collection efficiency (ηcc) vs. LED current plot, and it is evident that RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b with ηcc approaching 95% has outperformed the other two commercial dye combinations, thereby explaining its improved PCE of 10.39 ± 0.064%. Along with the J–V data, the interfacial studies for RJ-C6
XY1b with ηcc approaching 95% has outperformed the other two commercial dye combinations, thereby explaining its improved PCE of 10.39 ± 0.064%. Along with the J–V data, the interfacial studies for RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b combinations in various ratios are provided in the ESI (Fig. S16–S19).‡ Furthermore, electrochemical impedance spectroscopy (EIS) was carried out for all three co-sensitized devices under dark conditions (voltage ∼ Voc), revealing higher electron recombination resistance (second semi-circle) for RJ-C6
XY1b combinations in various ratios are provided in the ESI (Fig. S16–S19).‡ Furthermore, electrochemical impedance spectroscopy (EIS) was carried out for all three co-sensitized devices under dark conditions (voltage ∼ Voc), revealing higher electron recombination resistance (second semi-circle) for RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b device followed by L0
XY1b device followed by L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b and DN-F13
XY1b and DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, respectively (Fig. S20 (ESI)).‡ These results are in good agreement with the observed Voc trend for the devices, further corroborating the lifetime measurement results.
XY1b, respectively (Fig. S20 (ESI)).‡ These results are in good agreement with the observed Voc trend for the devices, further corroborating the lifetime measurement results.
3.5 Indoor photovoltaics using cocktail starburst triphenylamine dyes and dual-species copper(II/I) electrolyte
Under low-intensity indoor/ambient illumination, recombination is the predominant process limiting the performance of the DSCs.53,67,68 The effectiveness of blocking the back electron transfer and recombination of injected electrons from the conduction band and sub-band gap states, as well as FTO, plays a crucial role in realizing higher efficiency in DSCs under indoor illumination69–71 This can be achieved through various methods involving precise control of the semiconductor, dye, electrolyte, and device architecture. In the present study, we introduced a newly designed starburst propeller shaped triphenylamine sensitizer (RJ-C6) with enhanced π-conjugation and planarity that collectively contribute to improved dye loading, enhanced light harvesting, and efficient suppression of recombination, thereby enhancing the photovoltaic performance, particularly under low-intensity indoor and artificial light conditions.The indoor photovoltaic performance measured for all three co-sensitized dye combinations (L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, DN-F13
XY1b, DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b and RJ-C6
XY1b and RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b) using dual-species copper(II/I) electrolyte ([Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+) under standard 1000 lux warm white CFL illumination (Pin: 283 μW cm−2) is provided in Fig. 7a and Table 4.
XY1b) using dual-species copper(II/I) electrolyte ([Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+) under standard 1000 lux warm white CFL illumination (Pin: 283 μW cm−2) is provided in Fig. 7a and Table 4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b, DN-F13
XY1b, DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b and RJ-C6
XY1b and RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized DSCs (*4000 lux)
XY1b co-sensitized DSCs (*4000 lux)
| Dyes | V oc (V) | J sc (μA cm−2) | FF | η (%) | P out (μW cm−2) |
|---|---|---|---|---|---|
L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
0.79 ± 0.004 | 146.9 ± 0.19 | 0.83 ± 0.002 | 34.3 ± 0.02 | 96.9 |
DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
0.82 ± 0.002 | 123.2 ± 1.19 | 0.77 ± 0.003 | 27.1 ± 0.18 | 76.7 |
RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
0.87 ± 0.001 | 149.7 ± 0.87 | 0.81 ± 0.001 | 37.3 ± 0.22 | 105.6 |
*RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b XY1b |
0.91 ± 0.002 | 628.0 ± 6.53 | 0.79 ± 0.003 | 39.8 ± 0.15 | 448.9 |
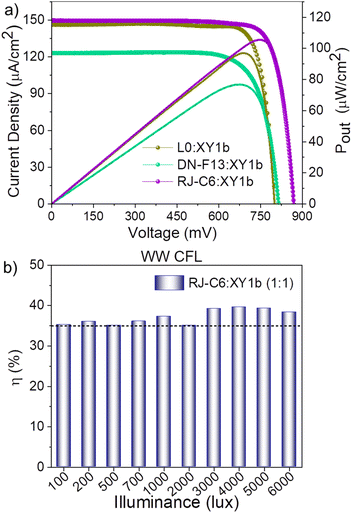
The dual-species Cu(II/I) electrolyte is known for its ability to efficiently address recombination with a more negatively shifted Cu(II) redox potential, providing a lower recombination driving force and achieving an improved lifetime when used with organic sensitizers. Recently, Sruthi et al. achieved an efficiency of 35.6% using the dual-species copper(II/I) electrolyte coupled with the D35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized dye combination.30 In the present study, replacing D35 with the RJ-C6 sensitizer enhanced the indoor photovoltaic performance to 37.3% (Pout: 105.6 μW cm−2) under standard 1000 lux warm white CFL illumination. This improved performance is primarily attributed to the enhanced light harvesting profile exhibited by the RJ-C6
XY1b co-sensitized dye combination.30 In the present study, replacing D35 with the RJ-C6 sensitizer enhanced the indoor photovoltaic performance to 37.3% (Pout: 105.6 μW cm−2) under standard 1000 lux warm white CFL illumination. This improved performance is primarily attributed to the enhanced light harvesting profile exhibited by the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b mixture, contributing to a higher current density of 149 μA cm−2 compared to 135 μA cm−2 observed for D35
XY1b mixture, contributing to a higher current density of 149 μA cm−2 compared to 135 μA cm−2 observed for D35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b. The improved current density results from extended π-conjugation and better close packing of the planar RJ-C6 sensitizer, as evident from the absorption and IPCE results. Consequently, the RJ-C6
XY1b. The improved current density results from extended π-conjugation and better close packing of the planar RJ-C6 sensitizer, as evident from the absorption and IPCE results. Consequently, the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized DSCs demonstrate complementary absorption across the entire visible region, aligning well with the warm white CFL spectra, as shown in Fig. S21 (ESI‡), ultimately leading to enhanced current and efficiency.
XY1b co-sensitized DSCs demonstrate complementary absorption across the entire visible region, aligning well with the warm white CFL spectra, as shown in Fig. S21 (ESI‡), ultimately leading to enhanced current and efficiency.
Under the same illumination conditions and using the dual-species Cu(II/I) electrolyte, L0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized DSCs achieved a PCE of 34.3% (Pout: 96.9 μW cm−2) with a Jsc of 146.9 μA cm−2, Voc of 0.79 V, and FF of 0.83, while DN-F13
XY1b co-sensitized DSCs achieved a PCE of 34.3% (Pout: 96.9 μW cm−2) with a Jsc of 146.9 μA cm−2, Voc of 0.79 V, and FF of 0.83, while DN-F13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b-based DSCs exhibited a lower efficiency of 27.1% (Pout: 76.7 μW cm−2) with a Jsc of 123.3 μA cm−2, Voc of 0.82 V, and FF of 0.77. Both efficiencies are lower than those of the RJ-C6
XY1b-based DSCs exhibited a lower efficiency of 27.1% (Pout: 76.7 μW cm−2) with a Jsc of 123.3 μA cm−2, Voc of 0.82 V, and FF of 0.77. Both efficiencies are lower than those of the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b combination (PCE: 37.3%, Pout: 105.6 μW cm−2). This trend aligns with our observations under one sun illumination (Fig. 5a and Table 2) using the same co-sensitized systems. XY1b alone, under the same measurement conditions (1000 lux CFL illumination), delivered an efficiency of 28.3% and Pout of 80.03 μW cm−2 (Fig. S14 and Table S6, ESI).‡ The RJ-C6 sensitizer, with its extended π-backbone featuring ethynyl linkages, exhibits superior light-harvesting behavior, especially in the UV/vis region around 400 nm, where XY1b absorption is limited. Additionally, the triple bond imparts better planarity to the molecular structure, facilitating efficient dye packing on the semiconductor and achieving improved dye loading compared to L0 and DN-F13 dyes (Table 3), resulting in a higher current density (149 μA cm−2vs. 123 μA cm−2). Furthermore, hexyloxy chains, as opposed to butyl chains in DN-F13, provide a more effective barrier against recombination, thereby enhancing the voltage from 820 to 870 mV (6%). This illustrates the critical impact of even slight structural modifications of sensitizers on the performance of co-sensitized indoor DSCs.
XY1b combination (PCE: 37.3%, Pout: 105.6 μW cm−2). This trend aligns with our observations under one sun illumination (Fig. 5a and Table 2) using the same co-sensitized systems. XY1b alone, under the same measurement conditions (1000 lux CFL illumination), delivered an efficiency of 28.3% and Pout of 80.03 μW cm−2 (Fig. S14 and Table S6, ESI).‡ The RJ-C6 sensitizer, with its extended π-backbone featuring ethynyl linkages, exhibits superior light-harvesting behavior, especially in the UV/vis region around 400 nm, where XY1b absorption is limited. Additionally, the triple bond imparts better planarity to the molecular structure, facilitating efficient dye packing on the semiconductor and achieving improved dye loading compared to L0 and DN-F13 dyes (Table 3), resulting in a higher current density (149 μA cm−2vs. 123 μA cm−2). Furthermore, hexyloxy chains, as opposed to butyl chains in DN-F13, provide a more effective barrier against recombination, thereby enhancing the voltage from 820 to 870 mV (6%). This illustrates the critical impact of even slight structural modifications of sensitizers on the performance of co-sensitized indoor DSCs.
The stability of copper electrolytes over a more extended period in a working DSC is influenced by the formation of multiple Cu(II) species in the electrolyte that retards the counter-electrode reactions. These species are formed with Cu(II) and pyridine bases like tert-butylpyridine (t-TBP) and N-methyl benzimidazole (NMB), which are used as additives in the electrolyte.72,73 We have successfully demonstrated that using structurally different dual-species copper(II/I) electrolytes can efficiently suppress these reactions, further improving the charge regeneration at the counter electrode and stability. Using the same dual-species Cu(II/I) electrolyte, the stability of RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized DSCs was further evaluated using an accelerated indoor stability testing chamber,33 which demonstrated promising stability with no degradation even after 800 hours (Fig. S22, ESI‡). Furthermore, in situ post-mortem analysis74 using IPCE and EIS of the stable RJ-C6
XY1b co-sensitized DSCs was further evaluated using an accelerated indoor stability testing chamber,33 which demonstrated promising stability with no degradation even after 800 hours (Fig. S22, ESI‡). Furthermore, in situ post-mortem analysis74 using IPCE and EIS of the stable RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b devices was carried out (Fig. S23, ESI‡) after long-term accelerated stability tests. As is evident from the IPCE curve, the RJ-C6
XY1b devices was carried out (Fig. S23, ESI‡) after long-term accelerated stability tests. As is evident from the IPCE curve, the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b device exhibited a nearly identical IPCE profile even after 800 h of accelerated stability tests (Fig. S23(a), ESI‡), proving the stable light absorption and charge collection capability of the RJ-C6
XY1b device exhibited a nearly identical IPCE profile even after 800 h of accelerated stability tests (Fig. S23(a), ESI‡), proving the stable light absorption and charge collection capability of the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b system. Furthermore, EIS analysis revealed a slightly lower second semicircle, corresponding to reduced recombination resistance, which might have contributed to the marginal drop in the device performance (a reduced PCE of 36.1% from 37.3% under 1000 lux WW CFL illumination) after 800 h of accelerated testing. An ex situ post-mortem analysis was also carried out by opening the device and re-assembling the same using a hole-free, spacer-free (HF-SF) device architecture23 and further performing the EIS analysis. As observed from the EIS data (Fig. S23(b), ESI‡), the HF-SF-based re-fabricated RJ-C6
XY1b system. Furthermore, EIS analysis revealed a slightly lower second semicircle, corresponding to reduced recombination resistance, which might have contributed to the marginal drop in the device performance (a reduced PCE of 36.1% from 37.3% under 1000 lux WW CFL illumination) after 800 h of accelerated testing. An ex situ post-mortem analysis was also carried out by opening the device and re-assembling the same using a hole-free, spacer-free (HF-SF) device architecture23 and further performing the EIS analysis. As observed from the EIS data (Fig. S23(b), ESI‡), the HF-SF-based re-fabricated RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b device exhibited a response with a reduced third semi-circle (electrolyte diffusion) owing to the minimal inter-electrode spacing while maintaining the other characteristics. These results further confirm the long-term stability of our novel RJ-C6-based co-sensitized indoor solar cells.
XY1b device exhibited a response with a reduced third semi-circle (electrolyte diffusion) owing to the minimal inter-electrode spacing while maintaining the other characteristics. These results further confirm the long-term stability of our novel RJ-C6-based co-sensitized indoor solar cells.
A comprehensive analysis of indoor photovoltaic performance under various warm white CFL illumination intensities ranging from 100 to 6000 lux (100, 200, 500, 700, 1000, 2000, 3000, 4000, 5000, and 6000 lux) was conducted using RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized devices with the dual-species Cu(II/I) electrolyte. The J–V plots obtained are provided in Fig. S24 (ESI),‡ and the data are presented in Table S7 (ESI).‡Fig. 7b illustrates the variation in indoor photovoltaic performance as a function of illumination intensity (100–6000 lux). It is evident from the obtained photovoltaic results that DSCs fabricated using the RJ-C6
XY1b co-sensitized devices with the dual-species Cu(II/I) electrolyte. The J–V plots obtained are provided in Fig. S24 (ESI),‡ and the data are presented in Table S7 (ESI).‡Fig. 7b illustrates the variation in indoor photovoltaic performance as a function of illumination intensity (100–6000 lux). It is evident from the obtained photovoltaic results that DSCs fabricated using the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b combination and dual-species copper(II/I) electrolyte delivered consistent PCE above 35% across the entire range of indoor illumination (100 to 6000 lux), highlighting their potential to be used to power a wide range of low-power wireless electronics. Under 4000 lux illumination, the above-mentioned dye-electrolyte combination achieved a record PCE of 40% (39.8 ± 0.15%, Pout: 451.30 μW cm−2), representing the highest indoor photovoltaic performance in DSC to date. A comparison of the present results with those reported in the literature is provided in Tables S8 and S9 (ESI).‡ The capability of these devices was further demonstrated by powering a clock and temperature sensor (ACETEQ DC-2) using two of these devices connected in series, with a total active area of 0.68 cm2, operating autonomously under 1000 lux CFL illumination (Fig. S25 and Video S1, ESI‡).
XY1b combination and dual-species copper(II/I) electrolyte delivered consistent PCE above 35% across the entire range of indoor illumination (100 to 6000 lux), highlighting their potential to be used to power a wide range of low-power wireless electronics. Under 4000 lux illumination, the above-mentioned dye-electrolyte combination achieved a record PCE of 40% (39.8 ± 0.15%, Pout: 451.30 μW cm−2), representing the highest indoor photovoltaic performance in DSC to date. A comparison of the present results with those reported in the literature is provided in Tables S8 and S9 (ESI).‡ The capability of these devices was further demonstrated by powering a clock and temperature sensor (ACETEQ DC-2) using two of these devices connected in series, with a total active area of 0.68 cm2, operating autonomously under 1000 lux CFL illumination (Fig. S25 and Video S1, ESI‡).
4 Conclusions
In this work, we introduced a newly designed starburst triphenylamine sensitizer (RJ-C6) for indoor photovoltaic DSC applications. Compared to the parent dye DN-F13, the RJ-C6 sensitizer features a more rigid and conjugated π-backbone. Combining RJ-C6 with XY1b enhanced the light harvesting behaviour in the visible region, overlapping well with indoor light spectra. Using the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b co-sensitized starburst cocktail dye in combination with dual-species [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+ electrolyte, we achieved a PCE of 10.40% under standard AM 1.5G one sun illumination. The improved performance is attributed to better close packing of RJ-C6 dyes, leading to enhanced dye loading and improved light harvesting in the visible region attributed to the structural modifications along with improved lifetime. Under standard 1000 lux CFL illumination, the RJ-C6
XY1b co-sensitized starburst cocktail dye in combination with dual-species [Cu(II)(dmp)2Cl]+/[Cu(I)(dmp)2]+ electrolyte, we achieved a PCE of 10.40% under standard AM 1.5G one sun illumination. The improved performance is attributed to better close packing of RJ-C6 dyes, leading to enhanced dye loading and improved light harvesting in the visible region attributed to the structural modifications along with improved lifetime. Under standard 1000 lux CFL illumination, the RJ-C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XY1b combination demonstrated an impressive PCE of 37.3%. Notably, under 4000 lux illumination, a record PCE of 40% was achieved. These DSCs maintained consistent efficiency above 35% across a wide range of indoor illumination from 100 to 6000 lux, making them suitable for various indoor environments. Replacing primary batteries with such efficient DSCs can significantly reduce carbon emissions associated with millions of discarded batteries ending up in landfills. With lower production costs, environmentally friendly materials, and reduced carbon footprint through recycling, DSCs emerge as leaders in indoor photovoltaics.
XY1b combination demonstrated an impressive PCE of 37.3%. Notably, under 4000 lux illumination, a record PCE of 40% was achieved. These DSCs maintained consistent efficiency above 35% across a wide range of indoor illumination from 100 to 6000 lux, making them suitable for various indoor environments. Replacing primary batteries with such efficient DSCs can significantly reduce carbon emissions associated with millions of discarded batteries ending up in landfills. With lower production costs, environmentally friendly materials, and reduced carbon footprint through recycling, DSCs emerge as leaders in indoor photovoltaics.
Data availability
The data supporting this article have been included as part of the ESI.‡Author contributions
S. S. conceived the work, designed the experiments, supervised the study, wrote the manuscript and carried out revisions with inputs from the co-authors; R. J. P. synthesized and characterized RJ-C6 dye with support from J. J.; R. J. P. carried out all photophysical and electrochemical characterization; R. K. M. carried out the DFT calculations; A. S. G. and R. J. P. fabricated, assembled and characterized the devices; S. S. obtained the funding and coordinated the work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. S. acknowledges financial support from the DST-Solar Challenge Award (DST/ETC/CASE/RES/2023/05(C)/(G)) and the CSIR-FTT project (FTT 060511). R. J. P. acknowledges Ms. Sruthi M. M. for providing the dual-species copper electrolyte and Ms. Sreelekshmy M. R. for the initial device optimizations. R. J. P. thanks the CSIR and A. S. G. thanks the DST-Solar Challenge Award for research fellowships.References
- S. Rahman, A. Haleem, M. Siddiq, M. K. Hussain, S. Qamar, S. Hameed and M. Waris, RSC Adv., 2023, 13, 19508–19529 RSC.
- I. Mathews, S. N. Kantareddy, T. Buonassisi and I. M. Peters, Joule, 2019, 3, 1415–1426 CrossRef CAS.
- G. Gokul, S. C. Pradhan and S. Soman, Dye-Sensitized Solar Cells as Potential Candidate for Indoor/Diffused Light Harvesting Applications: From BIPV to Self-powered IoTs, in Advances in Solar Energy Research, ed. H. Tyagi, A. K. Agarwal, P. R. Chakraborty and S. Powar, Springer, Singapore, 1st edn, 2019 Search PubMed.
- A. B. Muñoz-García, I. Benesperi, G. Boschloo, J. J. Concepcion, J. H. Delcamp, E. A. Gibson, G. J. Meyer, M. Pavone, H. Pettersson, A. Hagfeldt and M. Freitag, Chem. Soc. Rev., 2021, 50, 12450–12550 RSC.
- Y. Ren, D. Zhang, J. Suo, Y. Cao, F. T. Eickemeyer, N. Vlachopoulos, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nature, 2023, 613, 60–65 CrossRef CAS.
- M. Kokkonen, P. Talebi, J. Zhou, S. Asgari, S. A. Soomro, F. Elsehrawy, J. Halme, S. Ahmad, A. Hagfeldt and S. G. Hashmi, J. Mater. Chem. A, 2021, 9, 10527–10545 RSC.
- Q. Huaulmé, V. M. Mwalukuku, D. Joly, J. Liotier, Y. Kervella, P. Maldivi, S. Narbey, F. Oswald, A. J. Riquelme, J. A. Anta and R. Demadrille, Nat. Energy, 2020, 5, 468–477 CrossRef.
- A. Chakraborty, G. Lucarelli, J. Xu, Z. Skafi, S. Castro-Hermosa, A. B. Kaveramma, R. G. Balakrishna and T. M. Brown, Nano Energy, 2024, 128, 109932 CrossRef CAS.
- J. Park, J. Kim, H. S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. Il Seok, Nature, 2023, 616, 724–730 CrossRef CAS PubMed.
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS.
- Y. Zhao, Z. Qu, S. Yu, T. Shen, H. Deng, X. Chu, X. Peng, Y. Yuan, X. Zhang and J. You, Science, 2022, 534, 531–534 CrossRef.
- H. Chen, C. Liu, J. Xu, A. Maxwell, W. Zhou, Y. Yang, Q. Zhou, S. Teale, Y. Liu, M. I. Saidaminov, M. Li, N. Rolston and S. Hoogland, Science, 2024, 384, 189–193 CrossRef CAS.
- C. K. Vipin, S. Chandra, K. N. N. Unni and S. Soman, Sol. Energy, 2024, 276, 112705 CrossRef CAS.
- Z. Guo, A. K. Jena and T. Miyasaka, ACS Energy Lett., 2023, 8, 90–95 CrossRef CAS.
- Y. Liu, S. Zhu, W. Li, Y. Su, H. Zhou, R. Chen, W. Chen, W. Zhang, X. Niu, X. Chen and Z. An, Phys. Chem. Chem. Phys., 2022, 24, 22580–22588 RSC.
- H. N. Tsao, C. Yi, T. Moehl, J. H. Yum, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, ChemSusChem, 2011, 4, 591–594 CrossRef CAS.
- W. Naim, V. Novelli, I. Nikolinakos, N. Barbero, I. Dzeba, F. Grifoni, Y. Ren, T. Alnasser, A. Velardo, S. Haacke, S. M. Zakeeruddin, M. Graetzel and C. Barolo, JACS Au, 2021, 1, 409–426 CrossRef CAS PubMed.
- T. W. Hamann, R. A. Jensen, A. B. F. Martinson, V. Ryswyk, J. T. Hupp and T. Hamann, Energy Environ. Sci., 2008, 1, 66–78 RSC.
- A. B. F. Martinson, T. W. Hamann, M. J. Pellin and J. T. Hupp, Chem.–Eur. J., 2008, 14, 4458–4467 CrossRef CAS.
- N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338–2345 CrossRef CAS PubMed.
- A. Singh, A. K. Singh, R. Dixit, K. Vanka, K. Krishnamoorthy and J. Nithyanandhan, ACS Omega, 2024, 9, 16429–16442 CrossRef CAS PubMed.
- Y. Numata, S. P. Singh, A. Islam, M. Iwamura, A. Imai, K. Nozaki and L. Han, Adv. Funct. Mater., 2013, 23, 1817–1823 CrossRef CAS.
- A. S. George, S. C. Pradhan, K. N. N. Unni and S. Soman, RSC Sustainability, 2024, 2, 2839–2843 RSC.
- A. K. Patra, A. Dutta and A. Bhaumik, J. Phys. Chem. C, 2014, 118, 16703–16709 CrossRef CAS.
- K. Park, S. Jae, R. Gomes and A. Bhaumik, Chem. Eng. J., 2015, 260, 393–398 CrossRef CAS.
- Y. Cao, Y. Liu, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Joule, 2018, 2, 1108–1117 CrossRef CAS.
- D. Zhang, M. Stojanovic, Y. Ren, Y. Cao, F. T. Eickemeyer, E. Socie, N. Vlachopoulos, J. E. Moser, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nat. Commun., 2021, 12, 2–11 CrossRef.
- H. Michaels, M. Rinderle, R. Freitag, I. Benesperi, T. Edvinsson, R. Socher, A. Gagliardi and M. Freitag, Chem. Sci., 2020, 11, 2895–2906 RSC.
- M. Freitag, J. Teuscher, Y. Saygili, X. Zhang, F. Giordano, P. Liska, J. Hua, S. M. Zakeeruddin, J. E. Moser, M. Grätzel and A. Hagfeldt, Nat. Photonics, 2017, 11, 372–378 CrossRef CAS.
- S. M. Meethal, S. C. Pradhan, J. Velore, S. Varughese, R. S. Pillai, F. Sauvage, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2023, 12, 1081–1093 RSC.
- R. Haridas, J. Velore, C. Pradhan, A. Vindhyasarumi, K. Yoosaf, S. Soman, K. N. N. Unni and A. Ajayaghosh, Mater. Adv., 2021, 2, 7773–7787 RSC.
- S. Das and E. Mao, Sustainable Energy, Grids Networks, 2020, 24, 100408 CrossRef.
- A. Jagadeesh, G. Veerappan, P. S. Devi, K. N. N. Unni and S. Soman, J. Mater. Chem. A, 2023, 11, 14748–14759 RSC.
- S. Ahmad, E. Guillén, L. Kavan, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2013, 6, 3439–3466 RSC.
- Y. K. Eom, S. H. Kang, I. T. Choi, Y. Yoo, J. Kim and H. K. Kim, J. Mater. Chem. A, 2017, 5, 2297–2308 RSC.
- C. Aumaitre, C. Rodriguez-seco and O. Bardagot, J. Mater. Chem. A, 2018, 6, 10074–10084 RSC.
- A. Farokhi, H. Shahroosvand, F. Zisti, M. Pilkington and M. K. Nazeeruddin, J. Mater. Chem. A, 2023, 11, 25136–25215 RSC.
- Y. Saygili, M. Söderberg, N. Pellet, F. Giordano, Y. Cao, A. B. Munoz-García, S. M. Zakeeruddin, N. Vlachopoulos, M. Pavone, G. Boschloo, L. Kavan, J. E. Moser, M. Grätzel, A. Hagfeldt and M. Freitag, J. Am. Chem. Soc., 2016, 138, 15087–15096 CrossRef CAS.
- M. V. Vinayak, T. M. Lakshmykanth, M. Yoosuf, S. Soman and K. R. Gopidas, Sol. Energy, 2016, 124, 227–241 CrossRef CAS.
- M. Yoosuf, S. C. Pradhan, M. M. Sruthi, S. Soman and K. R. Gopidas, Sol. Energy, 2021, 216, 151–163 CrossRef CAS.
- M. V. Vinayak, M. Yoosuf, S. C. Pradhan, T. M. Lakshmykanth, S. Soman and K. R. Gopidas, Sustainable Energy Fuels, 2018, 2, 303–314 RSC.
- T. Kitamura, M. Ikeda, K. Shigaki, T. Inoue, N. A. Anderson, X. Ai, T. Lian and S. Yanagida, Chem. Mater., 2004, 16, 1806–1812 CrossRef CAS.
- R. Yuan, L. Zhang, L. Chen, H. Zhang, P. Dou, X. Ren, W. Chen, H. Zhou, Y. Wan and H. Wu, Tetrahedron Lett., 2019, 60, 1803–1807 CrossRef CAS.
- H. Zhou, Z. Wang, C. Gao, J. You and G. Gao, Chem. Commun., 2013, 49, 1832–1834 RSC.
- N. C. Ganguly, S. K. Barik and S. Dutta, Synthesis, 2010, 9, 1467–1472 CrossRef.
- A. Sen, M. H. Putra, A. K. Biswas, A. K. Behera and A. Groβ, Dyes Pigm., 2023, 213, 111087 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785–789 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView, Version 6.1, Semichem Inc., Shawnee Mission, KS, 2016 Search PubMed.
- C. E. Housecroft and E. C. Constable, Chem. Soc. Rev., 2015, 44, 8386–8398 RSC.
- C. E. Housecroft and E. C. Constable, Chem. Sci., 2022, 13, 1225–1262 RSC.
- J. Velore, S. C. Pradhan, T. W. Hamann, A. Hagfeldt, K. N. N. Unni and S. Soman, ACS Appl. Energy Mater., 2022, 5, 2647–2654 CrossRef CAS.
- M. Freitag, F. Giordano, W. Yang, M. Pazoki, Y. Hao, B. Zietz, M. Grätzel, A. Hagfeldt and G. Boschloo, J. Phys. Chem. C, 2016, 120, 9595–9603 CrossRef CAS.
- M. Fairoos, M. Kavungathodi, P. Wagner, S. Mori and A. J. Mozer, J. Phys. Chem. C, 2023, 127, 7618–7627 Search PubMed.
- M. Narita, M. F. M. Kavungathodi, M. Dheendayal, P. Wagner, S. Mori and A. J. Mozer, J. Am. Chem. Soc., 2024, 146, 12310–12314 CrossRef CAS.
- M. Jilakian and T. H. Ghaddar, ACS Appl. Energy Mater., 2022, 5, 257–265 CrossRef CAS.
- Q. Feng, G. Zhou and Z. S. Wang, J. Power Sources, 2013, 239, 16–23 CrossRef CAS.
- Y. Zhang, Y. Zhang, Z. Wang, M. Liang, D. Jia, Q. Wu and S. Xue, J. Power Sources, 2014, 253, 167–176 CrossRef CAS.
- J. E. Kroeze, N. Hirata, S. Koops, M. K. Nazeeruddin, L. Schmidt-Mende, M. Grätzel and J. R. Durrant, J. Am. Chem. Soc., 2006, 128, 16376–16383 CrossRef CAS PubMed.
- Q. Y. Yu, J. Y. Liao, S. M. Zhou, Y. Shen, J. M. Liu, D. Bin Kuang and C. Y. Su, J. Phys. Chem. C, 2011, 115, 22002–22008 CrossRef CAS.
- S. C. Pradhan, J. Velore, A. Hagfeldt and S. Soman, J. Mater. Chem. C, 2022, 10, 3929–3936 RSC.
- S. C. Pradhan, J. Velore, S. M. Meethal and S. Soman, Energies, 2023, 16, 6913 CrossRef CAS.
- N. C. D. Nath, H. J. Lee, W. Y. Choi and J. J. Lee, Electrochim. Acta, 2013, 109, 39–45 CrossRef CAS.
- J. M. Cole, G. Pepe, O. K. Al Bahri and C. B. Cooper, Chem. Rev., 2019, 119, 7279–7327 CrossRef CAS PubMed.
- M. Filipič, M. Berginc, F. Smole and M. Topič, Curr. Appl. Phys., 2012, 12, 238–246 CrossRef.
- E. M. Barea, J. Ortiz, F. J. Payá, F. Fernández-Lázaro, F. Fabregat-Santiago, A. Sastre-Santos and J. Bisquert, Energy Environ. Sci., 2010, 3, 1985–1994 RSC.
- S. C. Pradhan, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2018, 6, 22204–22214 RSC.
- Q. Wang, Z. Zhang, S. M. Zakeeruddin and M. Gräetzel, J. Phys. Chem. C, 2008, 112, 7084–7092 CrossRef CAS.
- M. M. Sruthi, S. C. Pradhan, A. S. George, P. R. Nitha, R. K. Mishra, J. John and S. Soman, Photochem. Photobiol., 2024, 100, 1127–1139 CrossRef PubMed.
- L. Sivasankaran, S. C. Pradhan, R. K. Mishra, S. Soman and A. Ajayaghosh, Sol. Energy, 2022, 236, 182–194 CrossRef CAS.
- D. Joshy, S. B. Narendranath, Y. A. Ismail and P. Periyat, Nanoscale Adv., 2022, 73, 5202–5232 RSC.
- J. Y. Kim, J. Y. Kim, D. K. Lee, B. Kim, H. Kim and M. J. Ko, J. Phys. Chem. C, 2012, 116, 22759–22766 CrossRef CAS.
- D. A. Chalkias, D. D. Loizos and G. C. Papanicolaou, Sol. Energy, 2020, 207, 841–850 CrossRef CAS.
Footnotes |
| † The present article is dedicated to Professor Anders Hagfeldt on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Materials and methods, Fig. S1–S25, Tables S1–S9, and Video S1. See DOI: https://doi.org/10.1039/d4ta05513f |
| This journal is © The Royal Society of Chemistry 2024 |