Efficient and stable near-infrared Y2Mg2Al2Si2O12:Cr3+ phosphor: analysis of the luminescence source by a site elimination strategy†
Yixin
Sun
a,
Mengmeng
Shang
 *a,
Yining
Wang
a,
Yiying
Zhu
a,
Xiaole
Xing
a,
Peipei
Dang
b and
Jun
Lin
*a,
Yining
Wang
a,
Yiying
Zhu
a,
Xiaole
Xing
a,
Peipei
Dang
b and
Jun
Lin
 *b
*b
aKey Laboratory for Liquid–Solid Structural Evolution and Processing of Materials Ministry of Education, School of Material Science and Engineering, Shandong University, 17923 Jingshi Road, Jinan 250061, P. R. China. E-mail: mmshang@sdu.edu.cn
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: jlin@ciac.ac.cn
First published on 18th January 2024
Abstract
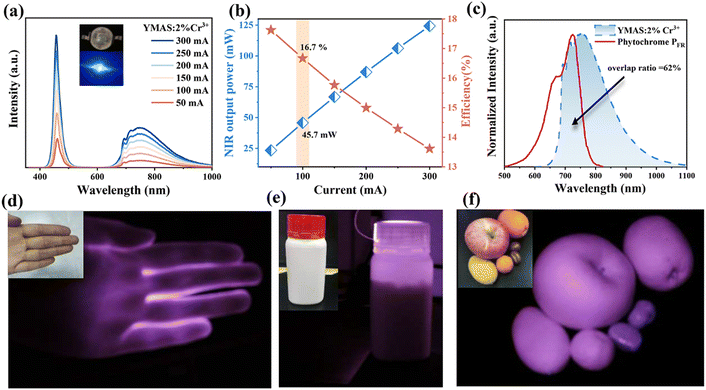
Near-infrared (NIR) phosphor-converted light-emitting diodes (pc-LEDs) are the ideal light source for next-generation NIR spectroscopy, and developing novel NIR phosphors with high performance is crucial to achieve a wide range of NIR pc-LED applications. In this work, NIR luminescent material Y2Mg2Al2Si2O12 (YMAS):2%Cr3+ with good quantum efficiency (79%) and excellent thermal stability (88%@100 °C) is prepared. Under 430 nm light excitation, YMAS:2%Cr3+ emits 620–1100 nm NIR light with a full-width at half maximum (FWHM) of 153 nm. Fluorescence decay curves, photoluminescence (PL) spectra, 7 K PL spectra, the Rietveld refinement, 27Al solid-state nuclear magnetic resonance (ss-NMR) spectra and time-resolved emission spectroscopy (TRS) confirm that Cr3+ has two different luminescence centers in the YMAS lattice. Benefiting from the site elimination strategy, the double luminescence centers are proven to be derived from Cr3+ ions occupying the two sites [Al1O6] and [Al2O4]. The NIR packaging device prepared using YMAS:2%Cr3+ has outstanding NIR output power and photovoltaic conversion efficiency as high as 45.7 mW@100 mA and 16.7%@100 mA, respectively. Owing to the excellent performance of the YMAS:2%Cr3+ sample, it presents excellent practical performance in plant growth, bioimaging, non-destructive testing and night vision.
1. Introduction
As an integral part of the electromagnetic spectrum, broadband NIR light plays an unprecedentedly important role in biological detection, laboratory instrumentation, chemical industry, optoelectronic devices, and medical diagnostics.1–6 In particular, NIR light in the 700–1100 nm region is known as the first biological window, which can be used in bio-imaging and non-destructive testing due to its considerable penetration depth in biological tissues besides plant growth and night vision application.7–9 Traditional NIR light sources such as incandescent lamps and halogen lamps have a series of problems like large size, low output, and high energy consumption, which hinder the further development of portable and integrated NIR technology. And the GaAs(Al/In)-based NIR LEDs are more competitive with the advantages of high efficiency and long lifetime. But their narrow FWHM (less than 50 nm) and high price limit their application. NIR pc-LEDs offer extraordinary advantages over other conventional light sources in terms of low power consumption, small size for easy integration, eco-friendliness, durability and long lifetime.10–12 NIR pc-LEDs are fabricated to coat NIR phosphors on the LED chip or the package wall. The NIR phosphor as a critical component of pc-LEDs affects the overall luminescence performance and emission spectra of the devices. However, for a long time, research studies on solid luminescent materials have mainly focused on visible and ultraviolet light-emitting materials. There is no corresponding mature NIR phosphor product on the market, and further research and development are urgently needed.The core of the NIR luminescent material is the activator, which determines the intrinsic electron-transition mechanism of luminescent materials. Cr3+ ions are one of the most favored NIR emission activators because of their tunable broadband and efficient NIR emissions, such as CaLu2Mg2Si3O12:Cr3+,13 K2SrGe8O18:Cr3+,14 Ca3Sc2Si3O12:Cr3+,15 and ScBO3:Cr3+.16 Among them, garnet-type compounds are rich in a variety of cationic sites, which are suitable for Cr3+ doping, and Cr3+ doped garnet structure NIR phosphors have attracted much attention due to their variable composition, adjustable spectrum, high quantum efficiency and good thermal stability, as listed in Table 1.
| Phosphor | λ em (nm) | FWHM (nm) | IQE (%) | I 150°C (%) | Output power (100 mA) | Efficiency (100 mA) | Ref. |
|---|---|---|---|---|---|---|---|
| LiInSi2O6:Cr3+ | 840 | 143 | 75 | 77 | 51.6 mW | 17.2% | 17 |
| ScBO3:Cr3+ | 800 | 120 | 65 | 51 | 26 mW | 7% | 16 |
| NaScSi2O6:Cr3+ | 860 | 156 | 22.2 | 69 | 203 mW@300 mA | 5.7%@300 mA | 18 |
| SrGa12O19:Cr3+ | 750 | 100 | 82.4 | — | — | — | 19 |
| Sr2ScSbO6:Cr3+ | 890 | 130 | 82 | 66 | — | — | 20 |
| Ca3Sc2Si3O12:Cr3+ | 770 | 110 | 92.3 | 97.4 | 97.8 mW@520 mA | 64.7%@520 mA | 15 |
| CaLu2Mg2Si3O12:Cr3+ | 765 | 120 | ∼41 | 82.3 | 34.75 mW | 11.6% | 13 |
| Y2Mg2Ga2Si2O12:Cr3+ | 790 | 180 | 44.3 | 70@100 °C | 14 mW | 5% | 21 |
| Ca2LuScAl2Si2O12:Cr3+ | 752 | 142 | 73.7 | 76 | 24.59 mW | 8.22% | 22 |
| Y2Mg2Al2Si2O12:Cr3+ | 750 | 153 | 79 | 89@100 °C | 45.7 mW | 16.7% | This work |
In this work, a novel Y2Mg2Al2Si2O12 (YMAS):Cr3+ NIR phosphor with garnet structure is reported for the first time. There are three suitable sites for Cr3+ occupation in the YMAS lattice, i.e. [AlO6] octahedron, [MgO6] octahedron and [AlO4] tetrahedron. The optimized YMAS:2%Cr3+ phosphor has a broadband NIR emission of 620–1100 nm (the peak at 750 nm and FWHM of 153 nm). A site elimination strategy is proposed to eliminate the [MgO6] octahedral sites by introducing [Al3+–Al3+] ion pairs in place of [Mg2+–Si4+] without drastic structural changes, which verifies the dual luminescence centers (Cr3+ in the [AlO6] octahedron and [AlO4] tetrahedron) of Cr3+. And the detailed PL mechanism is discussed in this paper. Moreover, YMAS:2%Cr3+ has excellent internal quantum efficiency (79%) and thermal stability (≈88%@100 °C). The NIR packaged device prepared from YMAS:2%Cr3+ and 460 nm chip has NIR output power and photovoltaic conversion efficiency of up to 45.7 mW@100 mA and 16.7%@100 mA, respectively. Due to the outstanding performance of the YMAS:2%Cr3+ sample, it is a potential candidate for practical applications in the field of plant growth, bioimaging, non-destructive testing and night vision.
2. Experimental section
2.1. Materials and synthesis
Powder samples with compositions of Y2Mg2Al2Si2O12 (YMAS):xCr3+ (x = 0–30%), Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ (y = 0–1) and Y2MgAl4SiO12 (YMA4S):zCr3+ (z = 0–20%) were synthesized by a high-temperature solid state reaction. The raw materials Y2O3 (99.99%, Sinopharm Chemical Reagent Co.), MgO (98.5%, Sinopharm Chemical Reagent Co.), Al2O3 (99.99%, Sinopharm Chemical Reagent Co.), SiO2 (99%, Sinopharm Chemical Reagent Co.), and Cr2O3 (99.95%, Aladdin) were weighed according to the stoichiometric amounts and then ground in an agate mortar for 30 min. Subsequently, the powder mixture was transferred into an alumina crucible and sintered at 1400 °C for 5–6 h in air. Finally, the as-prepared phosphors were cooled down to room temperature (RT) and ground into fine powders for subsequent testing.2.2. NIR pc-LED fabrication
The as-prepared NIR phosphors were first mixed with epoxy resins (the mass ratio of the phosphor to the resin is fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then coated on the 460 nm GaN blue chips with 1 W power. The mixtures were cured at 120 °C for 1 h to form the final LED devices.
1) and then coated on the 460 nm GaN blue chips with 1 W power. The mixtures were cured at 120 °C for 1 h to form the final LED devices.
2.3. Characterization
The absorption spectra were recorded using a UV-vis-NIR spectrophotometer equipped with an integrating sphere (Cary 5000). The composition and phase purity of products were studied by powder X-ray diffraction (XRD) measurements using a D8 Focus diffractometer (Bruker) with Cu Kα radiation (λ = 0.15405 nm). The general structure analysis system program was used to conduct the structure refinements. The morphology and elemental composition were determined using a field emission scanning electron microscope (FESEM, S4800, Hitachi) equipped with an energy-dispersive spectrometer (EDS). The diffuse reflectance (DR) spectra were recorded using a UV-2600 LabSolutions UV-Vis spectrophotometer. The PL, PLE spectra, decay curves, TRS, 7 K spectra and temperature-dependent PL spectra (25–200 °C) were recorded using an Edinburgh fluorescence spectrometer (FLS1000) equipped with a continuous 450 W Xenon lamp as the steady-state excitation source, a pulsed high energy xenon flash lamp (μF2) as the transient excitation source, and a heating stage (TAP-02) as the temperature-controlled stage. The 27Al solid-state nuclear magnetic resonance (ss-NMR) spectra were recorded on a Bruker Avance Neo 400WB. The photoluminescence quantum yield (QY) analysis was performed on an absolute PL quantum yield measurement system (C9920-02, Hamamatsu Photonics K.K., Japan). The optical properties of the NIR pc-LED devices were measured on a HAAS 2000 photoelectric measuring system from EVER FINE. The inductively coupled plasma mass spectrometry (ICP-MS) was performed on an Agilent 5110 (OES).3. Results and discussion
3.1. Phase and crystal structure
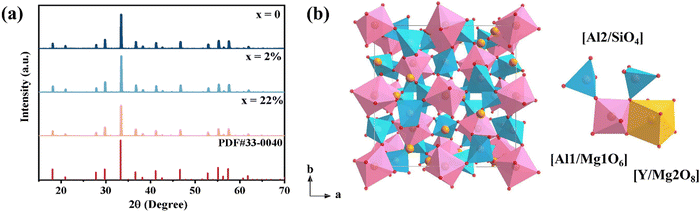
The X-ray diffraction (XRD) patterns of prepared YMAS:xCr3+ (x = 0, 2% and 22%) are presented in Fig. 1a. The diffraction peaks of all the samples are in concordance with the standard data of Y3Al5O12 (PDF #33-0040), indicating that garnet-type YMAS is successfully synthesized and the doping of Cr3+ ions does not obviously change the structure of the host. Fig. 1b shows the crystal structure of YMAS. Similar to Y3Al5O12,23 it can be viewed as a dense connection network composed of dodecahedrons, octahedrons and tetrahedrons. In YMAS, the octahedral cationic sites are occupied by Al1 and Mg1 atoms forming [Al1/Mg1O6] octahedrons, [Al2/SiO4] tetrahedrons are constructed by Al2 and Si atoms to form tetrahedral sites, and the dodecahedral cationic sites are composed of [Y/Mg2O8] dodecahedrons. Through oxygen atoms, the [Al1/Mg1O6] octahedron is connected to [Al2/SiO4] with a shared corner and connected to [Y/Mg2O8] with a shared edge. The activator ion Cr3+ (0.615 Å, CN = 6; 0.46 Å, CN = 4) tends to occupy octahedron and tetrahedron sites (compared to the dodecahedrons) due to the fact that it is more stable in an environment of coordination where the crystal field stabilization energy is stronger.24–28 Combining the principle of similar ion radius and valence state shows that there are three types of cation sites in YMAS suitable for Cr3+ ions to occupy and form luminescent centers, which are Al1 (0.535 Å, CN = 6), Al2 (0.39 Å, CN = 4) and Mg1 (0.72 Å, CN = 6), respectively. This issue of site occupancy will be further discussed in the section on the PL properties and site elimination sections.The morphology characterization of the scanning electron microscopy (SEM) and the elemental distribution of the energy-dispersive spectrometer (EDS) are illustrated in Fig. S1 (ESI†). The YMAS:Cr3+ product consists of non-uniform, micron-sized, and irregularly shaped particles. The elements Y, Mg, Al, Si, O and Cr are uniformly distributed throughout the specimen, indicating the successful doping of Cr3+ ions.
3.2. Photoluminescence properties
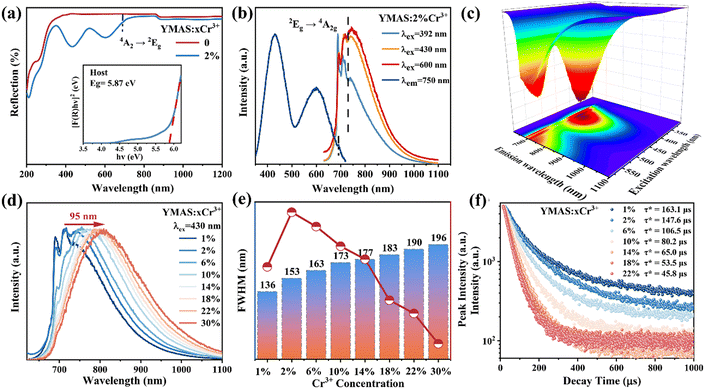
The diffuse reflection (DR) spectra of YMAS:xCr3+ (x = 0, 2%) are shown in Fig. 2a. There is almost no observable absorption in the visible region for YMAS, which means that a wide band gap can be expected. The optical band gap (Eg) can be calculated using the Kubelka–Munk formula29,30 (eqn (S1) and (S2), ESI†), and the calculated Eg of YMAS is 5.87 eV (inset of Fig. 2a). The DR spectrum of YMAS:2%Cr3+ presents two strong absorption bands with peaks at 430 nm (blue region) and 600 nm (red region), and it also has a weak absorption at 690 nm, which is attributed to the 4A2g → 2Eg transition of Cr3+ ions. In addition, the characteristic absorption band of Cr4+ ions at 850–1200 nm from the 3A2 → 3T2 (3F) transition is not observed,31,32 which means that only Cr3+ exists in the samples (it should be noted that the breakover at 850–1200 nm is caused by instrument parameters).Fig. 2b shows the photoluminescence excitation (PLE) and PL spectra of the YMAS:2%Cr3+ sample. The PLE spectrum has two broad bands in the blue (430 nm) and red (600 nm) spectral regions, which is consistent with the DR spectrum. Upon 430 nm light excitation, a broad NIR emission band (620–1100 nm, FWHM = 153 nm) is observed for YMAS:2%Cr3+. In addition, it can be found that the shape of the emission spectra changes with different excitation wavelengths (392, 430, and 600 nm). All the emission spectra are composed of R line (2Eg → 4A2g) and broadband emission, but the ratio of R line to broadband is significantly different. Apparently, the 392 nm light excitation favors R-line emission for the same doping concentration. The change in the emission spectra with excitation wavelength means that there may be multiple luminescent centers in the YMAS:Cr3+ sample. For more intuitive observation, the PL mapping spectrum of YMAS:2%Cr3+ is measured, as shown in Fig. 2c. Two sharp peak emissions at 690 nm and 710 nm and a broadband emission dominated by 800 nm are observed, which also proves multiple luminescent centers in the spectrum.
The normalized PL spectra (Fig. 2d) of YMAS:xCr3+(x = 1–30%) doped with different Cr3+ concentrations show that there is a significant difference between the low and high Cr3+ concentration doped samples in the position and shape of the emission peaks. When the concentration (x) is lower than 10%, the PL spectra contain both sharp and broadband emission; when the concentration (x) is higher than 14%, only a broadband NIR emission peak is observed. In addition, there is a greater redshift of 95 nm (715 nm → 810 nm) with increasing doping concentration. And the FWHM is significantly expanded from 136 nm (x = 1%) to 196 nm (x = 30%), as shown in Fig. 2e. Such significant changes in the PL spectra are mainly due to the multi-site occupation of Cr3+, and the details will be discussed in the low-temperature spectra. According to the experimental results, the optimal concentration is determined to be 2%, the IQE of which is as high as 79% (Fig. S2, ESI†). It is worth mentioning that when the doping concentration exceeds 14%, the emission intensity of the sample decreases sharply; at the same time, the luminescence of the sharp line is almost undetectable. Obviously, there is energy transfer (ET) from the strong crystal field luminescence center to the weak crystal field luminescence center.33
Fig. 2f shows the luminescence decay curves of YMAS:xCr3+ (x = 1% ∼ 22%) samples monitored at the corresponding emission wavelengths (715–810 nm, Table S1, ESI†), which can be calculated by the double exponential decay model, as follows:34
| It = I0 + A1e(−t/τ1) + A2e(−t/τ2) | (1) |
| τ* = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (2) |
In particular, the measurement of the spectra at extremely low temperatures can not only greatly reduce the lattice vibration, but also represent the intrinsic emission of the phosphor, which helps to accurately allocate energy levels.35–37 The PL spectra of YMAS:2%Cr3+ at 7 K are shown in Fig. 3a (λex = 600 nm) and Fig. S3a (λex = 430 nm, ESI†). Fig. 3a shows the NIR emission in the range of 650–1000 nm, consisting of three sharp peaks and a weak broadband emission, which is different from the PL spectrum at RT. The three sharp emission peaks are located at 691 nm, 709 nm and 734 nm, respectively, and the peak at 691 nm is dominant. Whereas the broadband emission near 800 nm is weak. It should be noted that the sharp and broadband emission peaks may come from two different luminescence centers. For this purpose, 691 nm, 709 nm, 734 nm and 850 nm light are selected for monitoring the wavelength and the PLE spectra at 7 K are obtained (Fig. 3b). The peak positions of excitation spectra detected by the three sharp peaks are similar and there is no significant difference. However, the peak position of the excitation spectrum at the detection wavelength of 850 nm has a significant redshift. The excitation peaks at different positions further prove that the two types of emission peaks come from two different luminescent centers.
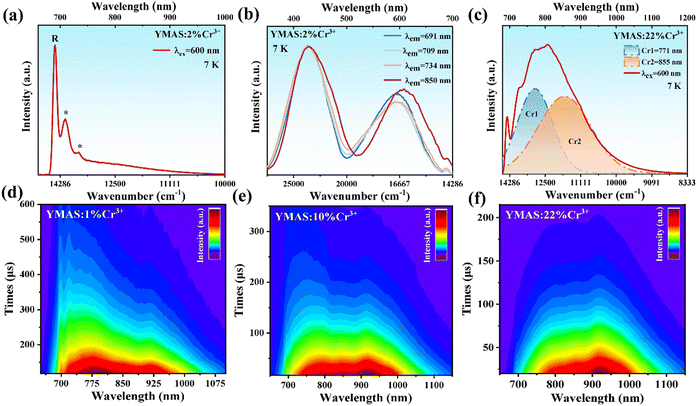 | ||
| Fig. 3 (a) The PL spectrum, (b) the PLE spectra of YMAS:2%Cr3+, and (c) the PL spectrum of YMAS:22%Cr3+ at 7 K. The TRS images of (d) YMAS:1%Cr3+, (e) YMAS:10%Cr3+, and (f) YMAS:22%Cr3+. | ||
Combined with the crystal structure, there are three different cation sites [Al1O6], [Mg1O6] and [Al2O4] in the YMAS host, which is suitable for Cr3+ occupation. The peaks at 690 nm are zero-phonon R line emission (2Eg → 4A2g transition),38 which represents Cr3+ in the unperturbed six-coordinate octahedral crystal field environment, usually indicating a strong crystal field. The lines at 709 nm and 734 nm denoted by (*) in Fig. 3a are Stokes phonon sidebands.39 Based on the principle of the crystal field, the short ion radius usually results in a strong crystal field environment. Therefore, the sharp peak emission should come from Cr3+ in the occupied [Al1O6] octahedron (denoted as Cr1). Additionally, there are two possibilities for broadband emission of Cr3+. One is that Cr3+ ions locate at the octahedral position with the 4T2 → 4A2 spin-allowed transition (Fig. S4a, ESI†).40 The second is that Cr3+ occupies the tetrahedral position with the 4T1 → 2T1 spin-allowed transition (Fig. S4b, ESI†).26,33,41,42 Therefore, both [Mg1O6] and [Al2O4] are likely to be occupied by Cr3+ to form the broadband emission near 850 nm (denoted as Cr2).
In addition, the emission spectrum dominated by the sharp peak emission (7 K, Fig. 3a) gradually transforms into the broadband emission (RT, Fig. 2b) with increasing temperature, which is mainly attributed to thermal activation. At 7 K, the 2E (spin-forbidden transition, sharp peak emission) state is the lowest excited state of Cr3+, while the 4T2 (spin-allowed transition, broadband emission) state is the higher state, and the PL spectrum is dominated by spikes. As the temperature increases, the probability of electrons in Cr3+ ions transitioning from the 2E state to the 4T2 state increases, resulting in more electrons being thermally occupied at the 4T2 state,43 which in turn causes the intensity of broadband emission to gradually increase. Therefore, broadband emission dominates the PL spectrum at RT.
In order to explore the reasons for emission peak redshift and the increase of FWHM, the intrinsic emission spectrum at high doping concentration is further measured. The emission spectra of YMAS:22%Cr3+ at 7 K are measured and shown in Fig. 3c (λex = 600 nm) and Fig. S3b (ESI†) (λex = 430 nm, ESI†). Different from the emission spectrum dominated by spikes at low doping concentrations, the PL spectrum of YMAS:22% Cr3+ is dominated by the broadband emission, and can also be fitted by Gaussian to synthesize two broadband (Fig. 3c). With the increase of substitution amount, the distortion of [Al1O6] octahedron caused by larger Cr3+ replacing smaller Al3+ increases, and the structural disorder is enhanced, which leads to the decrease of crystal field strength. Therefore, the gradual transition of sharp peak emission (Cr1) into broadband emission at high concentration is one of the reasons causing the red shift of emission peak and the increase of FWHM. Another reason is that the proportion of Cr2 with long wavelength and broadband emission is gradually increasing.
The two Cr3+ emission centers and their interactions are further supported by TRS analysis.39Fig. 3d shows the TRS image of YMAS:1%Cr3+ at 0–600 μs time interval under 430 nm pulse Xe lamp excitation. Cr1 ions are in charge of the sharp and broadband emission, covering 650–840 nm. Cr2 only contributes to the broadband emission, covering the NIR range of 840–1100 nm. In Fig. 3d, it can be observed that the lifetime of Cr1 is longer than Cr2. However, with the increase of concentration (x), the lifetime of Cr1 is shorter than Cr2 (Fig. 3f), which means that Cr1 is more easily quenched by concentration.
Here, the assignment of long-wavelength broadband emission (Cr2) is still uncertain: Cr3+ occupying [Mg1O6] or [Al2O4]? Therefore, a novel design method of a site elimination strategy is proposed. According to the radius matching principle, [Al1O6]–[Al2O4] are introduced to replace [Mg1O6]–[SiO4] without drastic changes in the structure. When [Mg1O6] is completely eliminated, the chemical formula is Y2MgAl4SiO12 (YMA4S). The remaining Mg ions and Y ions form the dodecahedron in the YMA4S structure since large ions reside in a large polyhedral volume position in the garnet structure.44 If Cr3+ occupies [Al1O6] and [Mg1O6] sites in the original YMAS host, after the elimination of the [Mg1O6] octahedron, the emission spectrum would be composed of only one luminescent center. If the emission spectrum still has two luminescent centers as before substitution, Cr3+ would occupy the [Al1O6] and [Al2O4] polyhedron.
Furthermore, the phase stability of YMAS (Y2Mg2−yAl2+2ySi2−yO12, y = 0) and YMA4S (Y2Mg2−yAl2+2ySi2−yO12, y = 1) is confirmed by the tolerance factor (Dτ) which is used to predict and analyze the phase stability of the (A)3{B}2[C]3O12 garnet structure. For real garnet-based compounds, the Dτ value should fluctuate around 1, in the range of 0.748–1.333. The calculation formula of Dτ is as follows:44
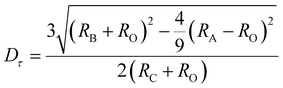 | (3) |
3.3. Site elimination strategy
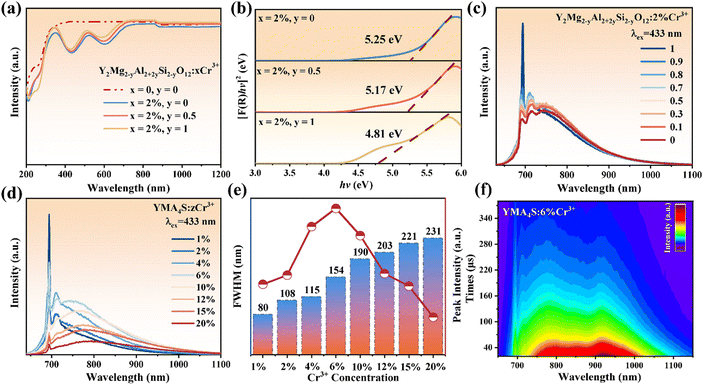
The XRD patterns of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ (y = 0.1, 0.5 and 1) powder samples are shown in Fig. S5 (ESI†). With the increase of y value, the standard card of the matched diffraction peak is still PDF #33-0040, indicating that the Y2Mg2−yAl2+2ySi2−yO12 solid solutions were successfully synthesized and did not cause dramatic changes in the crystal structure.The DR spectra of Y2Mg2−yAl2+2ySi2−yO12:xCr3+(x = 0, 2%; y = 0, 0.5 and 1) are shown in Fig. 4a. According to the formula in eqn (S1) and (S2) (ESI†), the Eg of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ is 5.25 eV (y = 0), 5.17 eV (y = 0.5) and 4.81 eV (y = 1), respectively, as listed in Fig. 4b. The co-substitution of [Al3+–Al3+] pairs gradually reduce the optical band gap, which means that the introduction of [Al3+–Al3+] pairs induce the rearrangement of electrons in the host. This is because of the different electronegativity between [Al3+–Al3+] and [Mg2+–Si4+] ion pairs.45
Fig. 4c shows the PL spectra of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ (y = 0–1). With the gradual increase of the substitution amount, it can be observed that the emission peak of the R line at 690 nm increases sharply under the excitation of 433 nm light. This means that the introduction of [Al3+–Al3+] co-substituted [Mg2+–Si4+] increases the order of the lattice, and results in the enhancement of the [Al1O6] octahedral crystal field strength where it is occupied by Cr1. In the process of gradually eliminating the [Mg1O6] octahedron, the emission spectrum is still broadband emission, and the broadband intensity except the R line does not change much, indicating that the introduction of [Al3+–Al3+] with different electronegativity has little effect on the broad emission band. Even though the [Mg1O6] octahedron is completely eliminated (y = 1, YMA4S), the broadband emission remains virtually unchanged. The intensity of the broadband emission seems to be less affected by the crystal field strength, which indicates that YMA4S:2%Cr3+ still has two luminescent centers, and also confirms that Cr2 is located in the tetrahedral (Al2O4) position rather than the octahedron (Mg1O6).
To further measure the composition of YMA4S, the content of the YMA4S:2%Cr3+ sample is measured by inductively coupled plasma mass spectrometry (ICP-MS) and the results are given in Table S2 (ESI†). According to the measurement results, the chemical formula is Y2.03Mg0.91Al3.4SiO12:1.5%Cr3+. However, it is necessary to dilute a higher multiple before testing, which will lead to some test errors.
Fig. S6 (ESI†) shows the Rietveld refinement results for typical Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ (y = 0 and 1) samples. Cr3+ ions are constrained to the Al1 and Al2 positions in the Rietveld refinement process. In Tables S3 and S4 (ESI†), the atom positions, occupations, and thermal vibration parameters were improved by convergence and accorded well with the reflection conditions: Rwp = 8.96%, Rp = 7.52% for y = 0 and Rwp = 9.53%, Rp = 8.72% for y = 1. The YMAS:2%Cr3+ is confirmed to be a trigonal Ia-3d structure for the unit cell parameters of a = b = c = 11.967 Å, V = 1713.69 Å3, α = β = γ = 90°. And the lattice parameters for YMA4S:2%Cr3+ are calculated to be a = b = c = 11.953 Å, V = 1707.78 Å3. The decrease of the refined volume suggests that [Al1O6]–[Al2O4] successfully replaces [Mg1O6]–[SiO4] after the site elimination strategy, resulting in a decrease in cell parameters. Reliable refinement results confirm that Cr ions occupy both [Al1O6] and [Al2O4] sites.
The 27Al solid-state nuclear magnetic resonance (ss-NMR) spectra of YMAS:xCr3+ (x = 0 and 22%) samples shown in Fig. S7 (ESI†) provide direct evidence of Cr3+ ions entering the local structure of the [Al2O4] tetrahedron and [Al1O6] octahedron. The 27Al ss-NMR spectrum of the Cr3+ undoped YMAS sample shows two bands from −30 to +25 ppm and 50 to 100 ppm, corresponding to the [Al1O6] and [Al2O4] sites in the garnet structure.46 After Cr3+ doping, the 27Al ss-NMR peaks of the YMAS:22%Cr3+ sample are broadened, indicating that the simultaneous entry of Cr3+ ions into the [Al2O4] and [Al1O6] polyhedron destroys the local symmetry.
To further verify that Cr3+ occupies two sites of [Al1O6] and [Al2O4], a series of samples of YMA4S (y = 1):zCr3+ (z = 1–20%) are prepared. The corresponding PL spectra show pronounced redshift and broadening of the emission bands (Fig. 4d and e), which is consistent with YMAS:xCr3+. Besides, the concentration quenching of YMA4S (y = 1):zCr3+ is observed until z = 6%, which is higher than that of YMAS (y = 0):xCr3+ (x = 2%). The reason is that the substitution of [Al1O6]–[Al2O4] for [Mg1O6]–[SiO4] produces more sites suitable for Cr3+ occupation, which extends the concentration quenching value from 2% (YMAS, y = 0) to 6% (YMA4S, y = 1). The TRS plot of YMA4S (y = 1):6%Cr3+ (Fig. 4f) is very similar to that of YMAS (y = 0):10%Cr3+ (Fig. 3e). Two luminescence centers are still observed in YMA4S (y = 1):6%Cr3+ without the [Mg1O6] octahedron, and lifetime values of Cr1 is still longer than Cr2 in YMA4S (y = 1), which is also consistent with YMAS (y = 0). Fig. S8a and b (ESI†) are the luminescence decay curve of two luminescence centers in the YMA4S:xCr3+ (x = 2–15%) samples. In Fig. S8c (ESI†), the different decay rates of Cr1 and Cr2 can be clearly observed. The Cr2 lifetime is short but the attenuation rate is slow, and for Cr1 is the opposite. This means that Cr1 is more easily quenched by concentration. Until then, ample experimental evidence proves that Cr2 should be the emission of Cr3+ occupying the [Al2O4] polyhedron.
3.4. High-temperature thermal stability
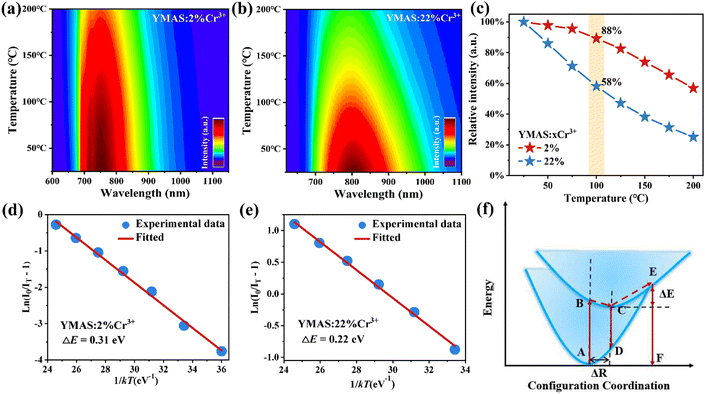
Fig. 5a and b show the PL spectra of YMAS:2%Cr3+ and YMAS:22%Cr3+ excited with 430 nm light at different temperatures, respectively. The emission intensity of both phosphors decreases with increasing temperature, but the decrease for the YMAS:22%Cr3+ phosphor is significantly greater than that for the YMAS:2%Cr3+ phosphor. Fig. 5c presents the change in the relative intensity of the emission peaks of YMAS:xCr3+ (x = 2%, 22%) as a function of temperature. The integral intensities of these samples remain 88%@100 °C (x = 2%) and 58%@100 °C (x = 22%) of that at RT, respectively, indicating excellent thermal stability of YMAS:2%Cr3+. In order to further explain the TQ behavior, the activation energy (ΔE) of YMAS:xCr3+ (x = 2%, 22%) is the energy (ΔE) of YMAS:xCr3+ (x = 2%, 22%) and is calculated by the Arrhenius formula:47,48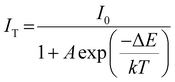 | (4) |
In the YMAS host, the larger Eg (5.87 eV, as shown in Fig. 2a) inhibits the possibility that the excited electrons are further pumped into the conduction band of the host lattice by thermal energy and then undergo non-radiative recombination with the photooxidation luminescence center or are trapped by some traps in the host.49 However, the thermal stability of the YMAS:22%Cr3+ sample is not excellent. Thus, thermal ionization is not the dominant reason for the good thermal stability of YMAS:2%Cr3+. So why does YMAS:2%Cr3+ have better thermal stability, much higher than that of YMAS:22%Cr3+? There are two main reasons: (1) the electron–phonon coupling generates cross-relaxation, and electron returns from the excited state to the ground state through nonradiative transitions rather than radiative transitions, in which the energy barrier required to cause nonradiative transitions is ΔE, as shown in Fig. 5f. Whereas ΔE = 0.31 eV (YMAS:2%Cr3+) is larger than ΔE = 0.22 eV (YMAS:22%Cr3+). It is difficult for the electrons in YMAS:2%Cr3+ to cross the energy barrier, and the amount of energy dissipated in the form of thermal energy through nonradiative leaps is reduced. (2) In heavily doped materials, the distance between Cr3+ ions is shorter. The increase in temperature enhances the lattice vibration, which promotes the non-radiative relaxation and increases the possibility of the energy transfer process between Cr3+ ions, making it easier for photons to enter the killing centers. Therefore, the thermal stability of lightly doped YMAS:2%Cr3+ is better.
In addition, Fig. S9a–e (ESI†) shows the PL spectra of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ (y = 0, 0.1, 0.5, 0.9 and 1) excited with 430 nm light at different temperatures, respectively. Their corresponding emission intensities show the same decreasing trend with increasing temperature. The integral intensities of all samples can be maintained more than 80%@100 °C room temperature, as shown in Fig. S9f (ESI†). Among them, YMA4S:2%Cr3+ exhibits the most excellent thermal stability (92%@100 °C).
3.5. Applications
To further evaluate the application potential of YMAS:Cr3+ phosphors, pc-LED was fabricated using YMAS:2%Cr3+ and a commercial 460 nm blue chip (insert in in Fig. 6a). Fig. 6a shows the PL spectral increment curve of the pc-LED device. As the driving current increases from 50 to 300 mA, the spectral intensity continues to increase without saturation. Fig. 6b presents the photoelectric characteristics test results of NIR pc-LED, which indicates a gradual increase in output power at the cost of reduced photoelectric efficiency. Under a forward current of 100 mA, the output power and photoelectric efficiency of the NIR pc-LED prepared by the YMAS:2%Cr3+ phosphor are 45.7 mW and 16.7%, respectively. By comparing the reported Cr3+-doped garnet-type luminescent materials (Table 1), the photoelectric performance of NIR pc-LED prepared by YMAS:2%Cr3+ is more desirable for application.Fig. 6c compares the emission spectrum of YMAS:2%Cr3+ and the absorption spectrum of plant phytochrome PFR.50–52 The spectral similarity index is calculated to be 62% by quantitative analysis, which indicates that the material could effectively promote plant growth and mainly play a role in regulating phytochrome PFR. In addition, its practical performance in bioimaging, non-destructive testing and night vision is also explored. NIR light from an LED can penetrate the palms and fingers, and can see the bones clearly (Fig. 6d). Under the illumination of the prepared pc-LED device, the drug allowance can be clearly observed in the dark environment with the help of a NIR camera, which means that it also has a good application prospect in non-destructive testing (Fig. 6e). Finally, in the dark environment, with the help of NIR light, different kinds of fruits and foods can be clearly distinguished, indicating that it also has a good application prospect in night vision (Fig. 6f).
4. Conclusions
In this work, a series of YMAS:Cr3+ NIR-emitting phosphors were successfully developed and synthesized. The broadband NIR emission accompanied by sharp peak emission is obtained due to the occupation of two different luminescent centers of Cr3+ in the YMAS lattice. Benefiting from the site elimination strategy, the double luminescent centers are proven to be derived from the two sites of [Al1O6] (Cr1) and [Al2O4] (Cr2). It provides a new design idea for studying the occupation of different lattice sites of doped ions. Moreover, YMAS:2%Cr3+ has an excellent quantum efficiency (79%) and thermal stability (≈ 88%@100 °C). The NIR output power and photoelectric conversion efficiency of the NIR packaging material prepared using YMAS:2%Cr3+ are as high as 45.7 mW@100 mA and 16.7%@100 mA, respectively. Owing to the excellent performance of the YMAS:2%Cr3+ sample, the as-fabricated NIR device exhibits potential application performance in plant growth, bioimaging, non-destructive testing and night vision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC No. 12374376 and 12304461), the Natural Science Foundation of Shandong Province (ZR2021ZD10), and the Project of the Qilu Young Scholar Program of Shandong University.References
- Y. Liang, Q. Cao, Y. Zhou, W. Zhou, J. Zhang, L. Yu, S. Lian and Z. Qiu, Chem. Eng. J., 2023, 454, 140235 CrossRef CAS.
- S. Mura, C. Cappai, G. F. Greppi, S. Barzaghi, A. Stellari and T. M. P. Cattaneo, Comput. Electron. Agric., 2019, 159, 92 CrossRef.
- J. Wang, X. Wang, C. Zhang, X. Zhang, T. Zhou and R.-J. Xie, J. Mater. Chem. C, 2023, 11, 9030–9036 RSC.
- Z. Chang, W. Deng, X. Ren, X. Liu, G. Luo, Y. Tan, X. Zhang and J. Jie, ACS Appl. Mater. Interfaces, 2023, 15, 32037 CrossRef CAS PubMed.
- J. Meng, Y. Cui and Y. Wang, J. Mater. Chem. B, 2022, 10, 8596 RSC.
- H. Jiang, L. Liu, K. Yu, X. Yin, S. Zheng, L. Song, J. Shi and Y. Zhang, J. Rare Earths, 2022, 40, 1389 CrossRef CAS.
- R. Li, G. Wei, Z. Wang, Y. Wang, J. Li, S. He, L. Li, H. Suo, W. Ding and P. Li, Laser Photonics Rev., 2023, 17, 2200589 CrossRef CAS.
- Y. Wang, M. Shang, Y. Sun, M. Deng, X. Xing, P. Dang and J. Lin, J. Mater. Chem. C, 2023, 11, 9291–9299 RSC.
- M. M. Nagy, S. Wang and M. A. Farag, Trends Food Sci. Technol., 2022, 123, 290 CrossRef CAS.
- H. Suo, Y. Wang, X. Zhao, X. Zhang, L. Li, K. Guan, W. Ding, P. Li, Z. Wang and F. Wang, Laser Photonics Rev., 2022, 16, 2200012 CrossRef CAS.
- G. B. Nair, H. C. Swart and S. J. Dhoble, Prog. Mater. Sci., 2020, 109, 100622 CrossRef CAS.
- Y. Shi, Z. Wang, J. Peng, Y. Wang, S. He, J. Li, R. Li, G. Wei, Y. Yang and P. Li, Mater. Today Adv., 2022, 16, 100305 CrossRef CAS.
- R. Li, Y. Liu, C. Yuan, G. Leniec, L. Miao, P. Sun, Z. Liu, Z. Luo, R. Dong and J. Jiang, Adv. Opt. Mater., 2021, 9, 2100388 CrossRef CAS.
- Y. Sun, M. Shang, Y. Wang, Y. Zhu, X. Xing, P. Dang and J. Lin, Ceram. Int., 2023, 49, 32619 CrossRef CAS.
- Z. Jia, C. Yuan, Y. Liu, X.-J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS PubMed.
- Q. Shao, H. Ding, L. Yao, J. Xu, C. Liang and J. Jiang, RSC Adv., 2018, 8, 12035 RSC.
- X. Xu, Q. Shao, L. Yao, Y. Dong and J. Jiang, Chem. Eng. J., 2020, 383, 123108 CrossRef CAS.
- Y. Yan, M. Shang, S. Huang, Y. Wang, Y. Sun, P. Dang and J. Lin, ACS Appl. Mater. Interfaces, 2022, 14, 8179 CrossRef CAS PubMed.
- S. Liu, Y. Zheng, D. Peng, J. Zhao, Z. Song and Q. Liu, Adv. Funct. Mater., 2023, 33, 2209275 CrossRef CAS.
- M. Zhao, S. Liu, H. Cai, F. Zhao, Z. Song and Q. Liu, Sci. China Mater., 2022, 65, 748–756 CrossRef CAS.
- S. Wu, H. Liang, Y. Li, W. Zou, Z. Liao, W. Wang and X. Ye, Dalton Trans., 2023, 52, 6569 RSC.
- Y. Jin, Z. Zhou, R. Ran, S. Tan, Y. Liu, J. Zheng, G. Xiang, L. Ma and X. Wang, Adv. Opt. Mater., 2022, 10, 2202049 CrossRef CAS.
- T. Xu, L. Yuan, Y. Chen, Y. Zhao, L. Ding, J. Liu, W. Xiang and X. Liang, Opt. Mater., 2019, 91, 30 CrossRef CAS.
- C. Wang, X. Wang, Y. Zhou, S. Zhang, C. Li, D. Hu, L. Xu and H. Jiao, ACS Appl. Electron. Mater., 2019, 1, 1046 CrossRef CAS.
- X. Zou, H. Zhang, W. Li, M. Zheng, M. S. Molokeev, Z. Xia, Y. Zheng, Q. Li, Y. Liu, X. Zhang and B. Lei, Adv. Opt. Mater., 2022, 10, 2200882 CrossRef CAS.
- V. Rajendran, M.-H. Fang, G. N. D. Guzman, T. Lesniewski, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, Y.-S. Lin, K.-M. Lu, C.-M. Lin, H. Chang, S.-F. Hu and R.-S. Liu, ACS Energy Lett., 2018, 3, 2679 CrossRef CAS.
- L. Jiang, X. Jiang, L. Zhang, Q. Liu, X. Mi, Z. Yu, G. Lv and Y. Su, Inorg. Chem., 2023, 62, 4220 CrossRef CAS PubMed.
- L. Wang, Y. Liu, H. Yu, C. Yang, L. Mei, H. Ding and R. Mi, J. Alloys Compd., 2023, 961, 170745 CrossRef CAS.
- B. Bai, P. Dang, D. Huang, H. Lian and J. Lin, Inorg. Chem., 2020, 59, 13481 CrossRef CAS PubMed.
- T. Gao, W. Zhuang, R. Liu, Y. Liu, X. Chen and Y. Xue, J. Alloys Compd., 2020, 848, 156557 CrossRef CAS.
- Y. Wang, G. Liu and Z. Xia, Laser Photonics Rev., 2023, 2300717 Search PubMed.
- F. Zhao, Z. Song and Q. Liu, Laser Photonics Rev., 2022, 16, 2200380 CrossRef CAS.
- Y. Zhou, C. Li and Y. Wang, Adv. Opt. Mater., 2022, 10, 2102246 CrossRef CAS.
- C. Yang, C. Fan, F. Hussain, W. Sheng, K. Song, J. Wu, Q. Huang, W. Su, J. Xu, S. Sun and D. Wang, J. Rare Earths, 2023, 41, 489 CrossRef CAS.
- V. Rajendran, M.-H. Fang, W.-T. Huang, N. Majewska, T. Lesniewski, S. Mahlik, G. Leniec, S. M. Kaczmarek, W. K. Pang, V. K. Peterson, K.-M. Lu, H. Chang and R.-S. Liu, J. Am. Chem. Soc., 2021, 143, 19058 CrossRef CAS PubMed.
- H. Lin, G. Bai, T. Yu, M.-K. Tsang, Q. Zhang and J. Hao, Adv. Opt. Mater., 2017, 5, 1700227 CrossRef.
- H. Zeng, T. Zhou, L. Wang and R.-J. Xie, Chem. Mater., 2019, 31, 5245 CrossRef CAS.
- Q. Zhang, X. Wei, J. Zhou, B. Milićević, L. Lin, J. Huo, J. Li, H. Ni and Z. Xia, Adv. Opt. Mater., 2023, 11, 2300310 CrossRef CAS.
- L. Yao, Q. Shao, M. Shi, T. Shang, Y. Dong, C. Liang, J. He and J. Jiang, Adv. Opt. Mater., 2022, 10, 2102229 CrossRef CAS.
- L. Jiang, X. Jiang, J. Xie, H. Sun, L. Zhang, X. Liu, Z. Bai, G. Lv and Y. Su, J. Alloys Compd., 2022, 920, 165912 CrossRef CAS.
- Z. Song and Q. Liu, Adv. Theory Simul., 2022, 5, 2200466 CrossRef CAS.
- T. Wang, Y. Wang, W. Chen and Z. Xia, Laser Photonics Rev., 2023, 2300784 Search PubMed.
- M.-H. Fang, G. N. A. De Guzman, Z. Bao, N. Majewska, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, C.-W. Yang, K.-M. Lu, H.-S. Sheu, S.-F. Hu and R.-S. Liu, J. Mater. Chem. C, 2020, 8, 11013 RSC.
- Z. Song, D. Zhou and Q. Liu, Acta Crystallogr., Sect. C: Struct. Chem., 2019, 75, 2053–2296 Search PubMed.
- T. Lang, M. Cai, S. Fang, T. Han, S. He, Q. Wang, G. Ge, J. Wang, C. Guo, L. Peng, S. Cao, B. Liu, V. I. Korepanov, A. N. Yakovlev and J. Qiu, Adv. Opt. Mater., 2022, 10, 2101633 CrossRef CAS.
- J. Feng, H. Liu, Z. Ma, J. Feng, L. Chen, J. Li, Y. Cai, Q. Zeng, D. Wen and Y. Guo, Chem. Eng. J., 2022, 449, 137892 CrossRef CAS.
- H. Liu, F. Zhao, H. Cai, Z. Song and Q. Liu, J. Mater. Chem. C, 2022, 10, 9232 RSC.
- Q. Wang, S. Wang, R. Pang, T. Tan, T. Tan, H. Wen, S. Zhang, H. You, C. Li and H. Zhang, Mater. Res. Bull., 2023, 163, 112222 CrossRef CAS.
- G. Liu and Z. Xia, J. Phys. Chem. Lett., 2022, 13, 5001 CrossRef CAS PubMed.
- S. J. Gai, C. Zhou, L. Peng, M. H. Wu, P. X. Gao, L. J. Su, M. S. Molokeev, Z. Zhou and M. Xia, Mater. Today Chem., 2022, 26, 101107 CrossRef CAS.
- X. Zou, X. Wang, H. Zhang, Y. Kang, X. Yang, X. Zhang, M. S. Molokeev and B. Lei, Chem. Eng. J., 2022, 428, 132003 CrossRef CAS.
- Y. Zhang, Z. Zeng, G. Li, P. Gao, Y. Li, M. Xia and Z. Zhou, Spectrochim. Acta, Part A, 2023, 302, 123122 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Main parameters of processing and refinement results of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+ (y = 0 and 1). The calculated average lifetime of YMAS:xCr3+ (x = 1–22%) and YMA4S:xCr3+ (x = 2–15%). The content of each element in YMA4S:2%Cr3+. The SEM image and quantum yield diagram of YMAS:2%Cr3+. And the PL spectra of YMAS:xCr3+ (x = 2% and 22%) at 7 K. Tanabe-Sugano diagram for Cr3+ ion in octahedral coordination and tetrahedral coordination. XRD patterns of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+(y = 0.1, 0.5 and 1). Rietveld refinement of YMAS:2%Cr3+ and YMA4S:2%Cr3+. 27Al ss-NMR spectra of YMAS:xCr3+ (x = 0 and 22%). Temperature-dependent PL spectra and integral intensity of Y2Mg2−yAl2+2ySi2−yO12:2%Cr3+(y = 1, 0.1, 0.5, 0.9 and 1). The Kubelka–Munk formula. See DOI: https://doi.org/10.1039/d3tc04245f |
| This journal is © The Royal Society of Chemistry 2024 |