Accelerating photocatalytic hydrogen production by anchoring Pt single atoms on few-layer g-C3N4 nanosheets with Pt–N coordination
Qi
Zhang
ab,
Miao
Yue
ab,
Peng
Chen
ab,
Qingmiao
Ren
ab,
Weihu
Kong
a,
Chenxia
Jia
a,
Qianyu
Lu
ab,
Jizhou
Wu
 abc,
Yuqing
Li
abc,
Wenliang
Liu
abc,
Peng
Li
abc,
Yuqing
Li
abc,
Wenliang
Liu
abc,
Peng
Li
 a,
Yongming
Fu
a,
Yongming
Fu
 *ab and
Jie
Ma
*abc
*ab and
Jie
Ma
*abc
aSchool of Physics and Electronic Engineering & Institute of Laser Spectroscopy, State Key Laboratory of Quantum Optics and Quantum Optics Devices, Shanxi University, Taiyuan 030006, China. E-mail: fuyongming@sxu.edu.cn; mj@sxu.edu.cn
bXinzhou Institute of Innovation Ecosystem, Shanxi University, Xinzhou 034000, China
cCollaborative Innovation Center of Extreme Optics, Shanxi University, Taiyuan 030006, China
First published on 6th February 2024
Abstract
Graphitic carbon nitride (g-C3N4) has gained considerable attention as a promising photocatalyst for hydrogen production through water splitting. However, its catalytic efficiency remains severely limited due to the rapid recombination of charge carriers and poor charge-transfer properties. Here, g-C3N4 is subjected to modification through the introduction of well-isolated Pt single atoms using a low-temperature incipient wetness impregnation method. The Pt single atoms exhibit a maximum weight ratio of 1.26%, resulting in a giant enhancement of the photocatalytic H2 evolution rate (336.8 μmol h−1), approximately two orders of magnitude higher than that of pristine g-C3N4 (1.8 μmol h−1) during a 22-h-long test with an apparent quantum yield (AQY) of 13.5% at 405 nm. The improved performance and excellent stability in photocatalytic H2 evolution can be attributed to the formation of Pt–N bonds between Pt single atoms and g-C3N4, which creates a new energy level of the N 2p–Pt 5d hybrid orbital for remarkably inhibiting the recombination of photogenerated electron–hole pairs and reducing interfacial charge-transfer resistance.
1. Introduction
Photocatalytic water splitting is anticipated to be an eco-friendly and enduring method for generating hydrogen (H2) devoid of detrimental byproducts or emissions.1–3 Extensive research has been conducted to identify the most suitable semiconductor-based photocatalysts that possess visible-light responsivity, stability, and reusability. In particular, carbon nitride allotropes have received extensive attention in photocatalysis due to their extended conjugation for fast separation and transfer of the charges.4–6 Some studies indicate that graphitic carbon nitride (g-C3N4) has emerged as a highly promising candidate for photocatalytic water splitting due to its distinctive electronic structure, exceptional photoelectric characteristics, remarkable stability, and cost-effectiveness.7–11 However, the efficiency of g-C3N4 in H2 production is hindered by the limited carrier mobility and the rapid recombination of electron–hole pairs generated during the photocatalytic process.12–14 To enhance the efficiency of photocatalytic H2 production, a significant approach is the incorporation of cocatalysts, which can facilitate charge transfer and provide reactive sites.Platinum (Pt) has been widely acknowledged as one of the most efficient cocatalysts for photocatalytic H2 production due to its low overpotential and favorable Gibbs free energy in H2 adsorption.15–17 Traditional large-scale particulate Pt cocatalysts exhibit limited low atomic utilization and a scarcity of active sites, significantly constraining the utilization efficiency of Pt atoms. Consequently, it becomes imperative to incorporate Pt species in the form of clusters or even isolated single atoms, as this approach offers a viable strategy to optimize atomic efficiency.18 With the reduction in the volume of the particulate cocatalyst, there is a progressive enhancement in both the specific surface area and atomic utilization. When the volume is decreased to atomic clusters comprising merely a few dozen atoms, a pronounced interaction between the support and the Pt atoms emerges, exerting a substantial effect on the photocatalytic process.19–21 Ultimately, upon reducing the cocatalysts to a state of single atom dispersion, the ideal atom utilization attains 100% and the selectivity achieves its utmost level.22–25 The presence of homogenous pores in layer-structured g-C3N4 offers stable coordination sites for anchor atoms, while the N atoms within the structure facilitate bonding between pyridine N and active atoms. Thus, g-C3N4 can serve as an appropriate support for the deposition of Pt single atoms, enabling efficient photocatalytic H2 production.26 In recent years, numerous investigations have documented diverse approaches for synthesizing single-atom Pt catalysts on g-C3N4, including ion exchange,27 reflux reaction,28 photoreduction,29–33 and high-temperature pyrolysis.34–38 Nevertheless, these methods face constraints when it comes to attaining high loading levels of single-atom Pt catalysts. In this respect, preparing Pt single atom anchored g-C3N4 with high loading and secure trapping is necessary to develop highly efficient photocatalysts.
In this study, the synthesis of Pt single atom anchored g-C3N4 nanosheets with varying Pt contents (Ptx/g-C3N4) has been successfully achieved through a low-temperature incipient wetness impregnation method followed by high-temperature pyrolysis. The incipient wetness impregnation method is frequently employed to enhance the loading capacity of single atoms,39–41 and lowering the impregnation temperature can additionally suppress the occurrence of agglomeration during the impregnation process. By employing the sequence of low-temperature incipient wetness impregnation, lyophilization, and high-temperature pyrolysis, the dispersion of high-density single atoms can be effectively improved. Characterization analyses have revealed that the isolated Pt single atoms are uniformly dispersed on the surface of g-C3N4, and the formation of Pt–N bonds notably enhances the charge separation and transfer capabilities. The photocatalytic rate of H2 evolution for Ptx/g-C3N4 demonstrates a notable increase compared to both pristine g-C3N4 and g-C3N4 modified with Pt nanoparticles.
2. Experimental
2.1. Synthesis of bulk g-C3N4 powder
Bulk g-C3N4 powder was synthesized through the thermal polymerization of urea. Specifically, 10 g of urea powder was placed in a closed alumina crucible within a muffle furnace (KSL-1100X-S, MTI, China) under a N2 atmosphere. The furnace was gradually heated to 550 °C at a rate of 1 °C min−1 and maintained at this temperature for a duration of 2 h. Subsequently, the resulting yellow g-C3N4 powder was collected and allowed to cool to room temperature before further use.2.2. Synthesis of few-layer g-C3N4 nanosheets
Few-layer g-C3N4 nanosheets were obtained through the process of ultrasonic-assisted liquid exfoliation. 100 mg of bulk g-C3N4 powder were dispersed in 100 mL of isopropanol and subjected to exfoliation using an ultrasonic crusher with a power of 1 kW for 12 h at room temperature. The resulting dispersion was then centrifuged using a high-speed centrifuge (HC-3018, Zonkia, China) at a speed of 8000 rpm for 10 min. The upper liquid was dried in a vacuum at 50 °C for 6 h to obtain pale yellow few-layer g-C3N4 nanosheets.2.3. Synthesis of Ptx/g-C3N4
The g-C3N4 nanosheets were loaded with Pt single atoms through low-temperature incipient wetness impregnation. A stable suspension was prepared by dispersing 100 mg of the as-prepared few-layer g-C3N4 nanosheets in 100 mL of deionized water and subjecting to ultrasonic treatment at a power of 200 W for 30 min. Subsequently, 0.5, 1, and 1.5 mL of H2PtCl6 solutions with a Pt concentration of 1 g L−1 were added drop by drop to the suspension. The resulting mixture was then transferred to a refrigerator and allowed to stand for 12 h at 0 °C to facilitate abundant adsorption, followed by rapid cooling to −60 °C. After undergoing freeze-drying in a freeze dryer (SCIENTZ-10N, Ningbo Scientz, China), the as-dried powders were transferred to a muffle furnace under a N2 atmosphere and subjected to a heating process at 450 °C for 2 h to facilitate the generation of Pt single atoms on the surface of g-C3N4. Finally, the resulting products were dissolved in deionized water, filtered with a 0.22 μm PTFE filter membrane, and dried using a freeze dryer to remove any impurities. The as-prepared samples were designated as Pt0.5/g-C3N4, Pt1/g-C3N4, and Pt1.5/g-C3N4, respectively. To establish a control, 10 mL of H2PtCl6 solution with a Pt concentration of 1 g L−1 was introduced into 100 mL of g-C3N4 solution and stirred for 30 min under xenon lamp irradiation to form Pt nanoparticles on few-layer g-C3N4.2.4. Characterization
The Pt content of the samples was quantified using inductively coupled plasma-optical emission spectroscopy (ICP–OES, Agilent 5510, USA). The surface morphology was characterized with a scanning electron microscope (SEM, Regulus8230, Hitachi) and a transmission electron microscope (TEM, JEM-F200, JEOL) at an acceleration voltage of 200 kV. The identification of crystalline phases, chemical functional groups, and surface chemical states was performed using X-ray diffraction (XRD, Rigaku Miniflex 600, Japan), Fourier transform infrared spectroscopy (FTIR, Thermo Scientific Nicolet iS20, USA), and X-ray photoelectron spectrometry (XPS, Thermo Scientific K-Alpha, USA), respectively. The surface area analyzer (Quantachrome Nova 4000e, USA) was utilized to obtain the Brunauer–Emmett–Teller (BET) specific surface area and pore size distribution. Diffuse reflectance spectroscopy (DRS, Hitachi UH5700, Japan) was employed to acquire the UV-vis absorption spectroscopy data. High angle annular dark-field scanning transmission electron microscopy (HAADF–STEM) images were captured using an aberration corrected JEOL JEM-ARM200F operating at 200 keV. X-ray absorption spectroscopy (XAS) measurements of the Pt element were conducted at the XAS beam line of the Shanghai Synchrotron Radiation Facility (SSRF) utilizing an Si(111) double-crystal monochromator, where the experimental data were acquired in the fluorescence mode using an ionization chamber. The steady and time-resolved photoluminescence (PL) spectra were measured using a photoluminescence spectrometer (Edinburgh FLS1000, UK).2.5. Photoelectrochemical measurements
Electrochemical measurements were performed using an electrochemical workstation (CHI 660E, China) with a standard three-electrode system. The Ag/AgCl electrode and Pt foil were used as the reference electrode and counter electrode in 0.5 M Na2SO4 aqueous solution, respectively. Fluorine–tin oxide (FTO) glass coated with the photocatalyst was used as the working electrode. To prepare the working electrode, 5 mg of the photocatalyst was dispersed in 1 mL of ethanol and 20 μL of Nafion anisole solution (5%) under ultrasonic treatment for 30 min to form a homogeneous slurry. Then, 50 μL of the obtained slurry was dropped on the conductive surface (10 mm × 10 mm) of FTO (10 mm × 30 mm × 1.1 mm) using a pipette and dried at 60 °C for 2 h. The transient photocurrent curves were measured in the i–t mode by periodically switching a 300 W Xe-lamp (PLS-SXE300, Perfectlight, China) at a 20 s interval under a continuous bias of 0.2 V. The electrochemical impedance spectroscopy (EIS) was conducted at an open circuit voltage with frequency ranging from 0.01 to 100 kHz and an amplitude of 5 mV.2.6. Photocatalytic H2 evolution
Photocatalytic water splitting reactions were implemented in a photocatalytic trace-gas generation and analysis system (Labsolar-6A, Perfectlight, China), involving a sealed top-irradiation quartz vessel connected to a closed gas circulation system. In a typical experiment, 50 mg of photocatalysts were added to 90 mL of deionized water and 10 mL of triethanolamine (TEOA) was added as a sacrificial agent. Prior to the photocatalytic test, the air in the reactor is evacuated using a vacuum pump. The evolved H2 gas was analyzed every 30 min using gas chromatography (GC-2060, FULI, China) with N2 serving as carrier gas. During the photocatalytic test, a simulated sunlight source was provided by the 300 W Xe-lamp. The reaction temperature was maintained at 2 °C using a cooling water circulator. The apparent quantum yield (AQY) of Pt1.5/g-C3N4 was determined using the same equipment under irradiation passing through different bandpass filters (365, 405, 420 and 500 nm, respectively) for 4 h. The AQY value was calculated using eqn (1), | (1) |
3. Results and discussion
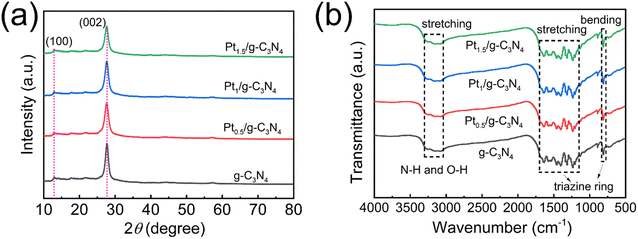
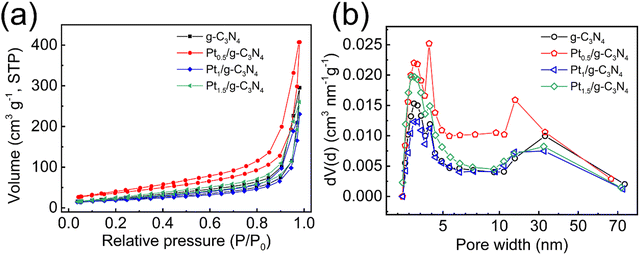
g-C3N4 samples loaded with varying Pt contents have been successfully synthesized using the low-temperature incipient wetness impregnation method, and the ICP–OES analysis reveals that the actual Pt loading percentages of Pt0.5/g-C3N4, Pt1/g-C3N4, Pt1.5/g-C3N4 and PtNP/g-C3N4 are determined to be 0.38%, 0.90%, 1.26%, and 3.95%, respectively. The observed loss in Pt content can potentially be attributed to incomplete adsorption of Pt species on the surface of g-C3N4 during low-temperature treatment. The XRD patterns of all the samples exhibit two characteristic diffraction peaks at 12.9° and 27.7° (Fig. 1a), which correspond to the (100) and (002) planes of hexagonal g-C3N4. No discernible peaks or peak-shifts associated with Pt species can be observed for the Ptx/g-C3N4 samples, suggesting that the presence of Pt does not significantly alter the crystal structure of g-C3N4. The FTIR spectrum of few-layer g-C3N4 demonstrates distinct peaks at approximately 3100–3500, 1200–1600, and 810 cm−1, which correspond to the vibrational modes of N–H/O–H bonds, aromatic C–N heterocyclic units, and triazine units, respectively (Fig. 1b).34 The FTIR spectra of the Ptx/g-C3N4 samples exhibit similar peaks to that of pristine g-C3N4, suggesting that the introduction of Pt species does not alter the chemical composition of g-C3N4.The BET analyses are depicted in Fig. 2. The N2 adsorption–desorption isotherms of the samples exhibit a type-IV behavior (Fig. 2a), indicating the presence of mesopores. The BET surface areas of g-C3N4 and Pt0.5/g-C3N4 are 71.097 and 132.904 m2 g−1, respectively. Fig. 2b displays a quite wide distribution of pore size, confirming the existence of both micropores and mesopores. Interestingly, the pore volumes of pristine g-C3N4, Pt0.5/g-C3N4, Pt1/g-C3N4, and Pt1.5/g-C3N4 are 0.322, 0.539, 0.268, and 0.302 cm3 g−1, while the mean pore diameters of these samples are 2.571, 1.897, 2.197, and 1.963 nm, respectively, implying that the adsorption behavior is primarily influenced by micropores and the presence of Pt atoms can enhance the formation of micropores to provide more active sites. The significant increase in the surface area and pore volume of Pt0.5/g-C3N4 can be attributed to the fact that the uniform dispersion of isolated Pt atoms can enlarge the interlayer distance of g-C3N4 structures.27,42,43 As the Pt content exceeds 0.5%, the specific surface area and pore volume exhibit a declining trend, but Ptx/g-C3N4 still demonstrates the ability to offer an increased number of micropores as active sites for the photocatalytic reaction.
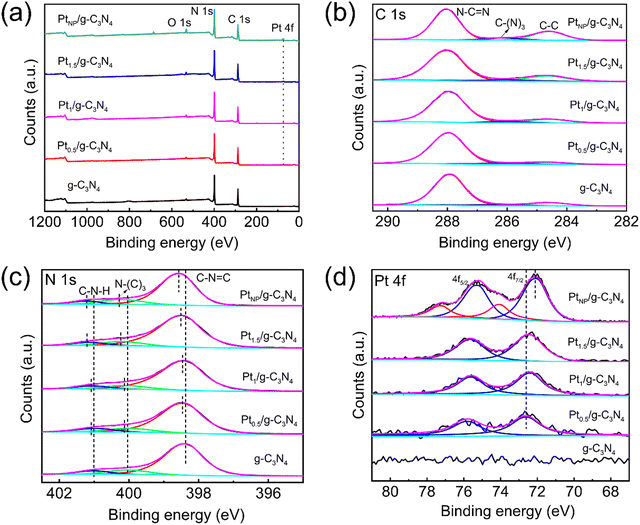
Fig. 3 presents the XPS analysis conducted on the samples to examine their chemical composition and oxidation states. The survey spectra indicate the presence of C and N in the g-C3N4 sample and Pt in the Ptx/g-C3N4 and PtNP/g-C3N4 samples (Fig. 3a). The high-resolution C 1s spectra are well fitted to three distinct peaks at 287.98, 286.02, and 284.6 eV, corresponding to the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–(N)3, and C
N, C–(N)3, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively (Fig. 3b).44 In the N 1s spectrum of g-C3N4, there are three characteristic peaks including 398.36 eV for C–N
C bonds, respectively (Fig. 3b).44 In the N 1s spectrum of g-C3N4, there are three characteristic peaks including 398.36 eV for C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, 400.04 eV for N–(C)3, and 401.00 eV for C–N–H, respectively (Fig. 3c).44 Compared to g-C3N4, the three peaks of N 1s shift towards higher energy with increasing Pt loading.40 For Pt1.5/g-C3N4, the shift values assigned to C–N
C, 400.04 eV for N–(C)3, and 401.00 eV for C–N–H, respectively (Fig. 3c).44 Compared to g-C3N4, the three peaks of N 1s shift towards higher energy with increasing Pt loading.40 For Pt1.5/g-C3N4, the shift values assigned to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–(C)3, and C–N–H groups are 0.09, 0.14, and 0.13 eV, respectively. The binding energy of N 1s in PtNP/g-C3N4 exhibits a similar shift.45 In Fig. 3d, the Pt 4f spectrum of PtNP/g-C3N4 is divided into two sets of double peaks, with the high-energy and low-energy peaks corresponding to the high-oxidation state of Pt clusters and metallic Pt nanoparticles, respectively.28,46,47 The Pt 4f7/2 and 4f5/2 orbitals related to Pt nanoparticles are located at 72.05 and 75.25 eV, while those of Ptx/g-C3N4 are located at 72.45 and 75.65 eV, respectively.46,48 The Pt 4f orbitals of Ptx/g-C3N4 roughly corresponding to Pt2+ species positively shift by 0.4 eV compared to Pt nanoparticles, which is ascribed to the weakened shielding effect due to the lack of 5d electrons of the outer orbital.47,49 Therefore, the alteration in the electron density of the N atom is ascribed to the modification of the Pt atom, thereby confirming the robust electronic interaction between Pt and N atoms (Pt–N bond) at higher density of Pt single atoms.
C, N–(C)3, and C–N–H groups are 0.09, 0.14, and 0.13 eV, respectively. The binding energy of N 1s in PtNP/g-C3N4 exhibits a similar shift.45 In Fig. 3d, the Pt 4f spectrum of PtNP/g-C3N4 is divided into two sets of double peaks, with the high-energy and low-energy peaks corresponding to the high-oxidation state of Pt clusters and metallic Pt nanoparticles, respectively.28,46,47 The Pt 4f7/2 and 4f5/2 orbitals related to Pt nanoparticles are located at 72.05 and 75.25 eV, while those of Ptx/g-C3N4 are located at 72.45 and 75.65 eV, respectively.46,48 The Pt 4f orbitals of Ptx/g-C3N4 roughly corresponding to Pt2+ species positively shift by 0.4 eV compared to Pt nanoparticles, which is ascribed to the weakened shielding effect due to the lack of 5d electrons of the outer orbital.47,49 Therefore, the alteration in the electron density of the N atom is ascribed to the modification of the Pt atom, thereby confirming the robust electronic interaction between Pt and N atoms (Pt–N bond) at higher density of Pt single atoms.
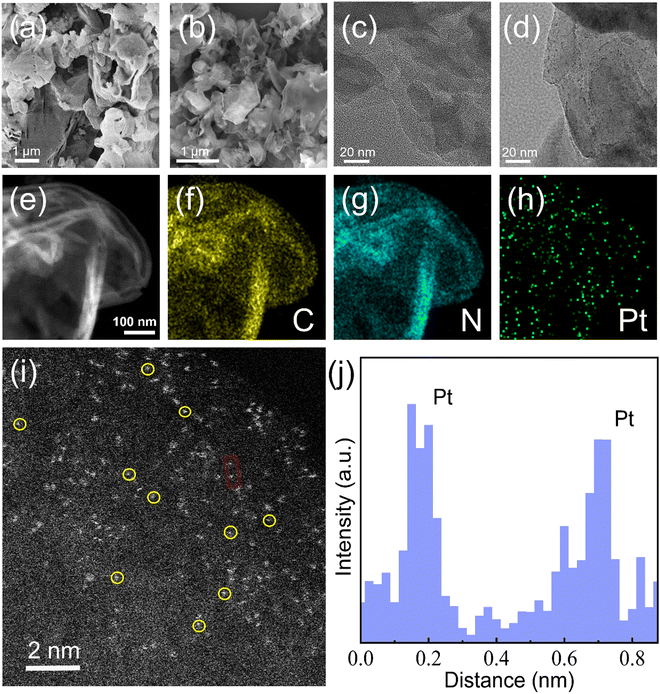
The distribution and configuration of Pt atoms at high loading density in Pt1.5/g-C3N4 are accurately determined through the characterization of an HAADF–STEM image, as illustrated in Fig. 4. Fig. 4a and b are the SEM images of Pt1.5/g-C3N4 and PtNP/g-C3N4, indicating that the nanosheets remain ultra-thin after the introduction of Pt species. The corresponding high-resolution TEM images are shown in Fig. 4c and d, respectively. The Pt nanoparticles can only be observed in PtNP/g-C3N4. Fig. 4e provides a low-resolution STEM depiction of the crumpled few-layer structure of Pt1.5/g-C3N4. The elemental mapping profiles presented in Fig. 4f–h demonstrate the homogeneous distribution of C, N, and Pt elements, confirming the presence of trace Pt atoms dispersed throughout g-C3N4. Furthermore, the HAADF–STEM image in Fig. 4i reveals densely dispersed bright spots corresponding to Pt atoms, which exist in an isolated single-atom state on the surface of g-C3N4. To quantify the dispersion of Pt single atoms, the distance between adjacent Pt single atoms is determined to be approximately 0.5 nm (Fig. 4j), suggesting a high level of dispersion and individualization of Pt atoms.
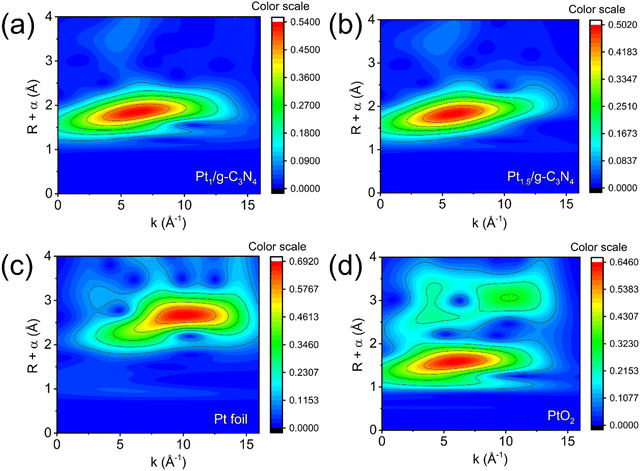
The detailed atomic-level structure and coordination information on Pt species is provided by synchrotron-based X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) at the Pt L3-edge. Pt1/g-C3N4 and Pt1.5/g-C3N4 exhibit identical XANES spectra with the absorption edge intensity falling between that of Pt foil and PtO2 (Fig. 5a), confirming that the Pt single atoms in Ptx/g-C3N4 are highly reduced to an oxidation state between Pt0 and Pt4+.50 The correlation between the peak intensity of L3-edge absorption and the unoccupied density of states of Pt 5d orbitals is evident.51 Thus, the Pt atoms in Ptx/g-C3N4 possess a greater abundance of unoccupied 5d-electron states, which aligns with the observed electron transfer indicated by the Pt 4f XPS spectra. To further analyze the local coordination environment of the Pt single atoms, Fourier-transform EXAFS analysis is performed (Fig. 5b). The peaks observed in the spectra correspond to distinct coordination shells surrounding the Pt atoms. The prominent peaks of Pt foil and PtO2 are clearly observed at 2.55 and 1.60 Å, respectively. However, these peaks are absent for Pt1/g-C3N4 and Pt1.5/g-C3N4, confirming the presence of Pt single atoms, which is consistent with the HAADF–STEM observation. Previous studies have shown that Pt single atoms can be stabilized through covalent bonds with adjacent N and C atoms.27,47,50,52 Therefore, the dominant peak at approximately 1.9 Å can be assigned to the Pt–N/C bonds. In this case, the experimental EXAFS spectra of Pt1/g-C3N4 and Pt1.5/g-C3N4 exhibit a satisfactory fit while employing the Pt–N/C pathway. Although the exact coordination of Pt atoms with either N or C presents difficulties, it is more probable to observe Pt–N coordination by examining the significant energy shift of N 1s spectra of Ptx/g-C3N4. Based on the EXAFS fitting outcomes (Fig. 5c and d), both the Pt1/g-C3N4 and Pt1.5/g-C3N4 demonstrate comparable coordination numbers (Pt1–N: 6.3 ± 0.4; Pt1.5–N: 6.4 ± 0.4) and Pt–N bond lengths (∼2.41 Å). It should also be noted that when the Pt atom is situated within the central position of a sixfold cavity in g-C3N4, it is expected to exhibit a Pt–N bond length of approximately 2.38 Å.40 The EXAFS wavelet transforms provide a method to obtain both the radial distance resolution (R space) and k space resolution simultaneously. Fig. 6a and b show that the maximum intensities of Pt1/g-C3N4 and Pt1.5/g-C3N4 centered at about 6.55 and 6.30 Å−1 (k space) are resolved at 1.90 Å (R space), respectively, which are both assigned to the Pt–N coordination structure.45,53 Note that the intensities corresponding to Pt–Pt (10.05 Å−1, Fig. 6c) and Pt–O (6.05 Å−1, Fig. 6d) coordination for the Pt foil and PtO2 are not observed for Pt1/g-C3N4 and Pt1.5/g-C3N4. The coordination analyses provide confirmation of the highly reduced state of the Pt single atoms and their strong interaction with N atoms in an isolated state.
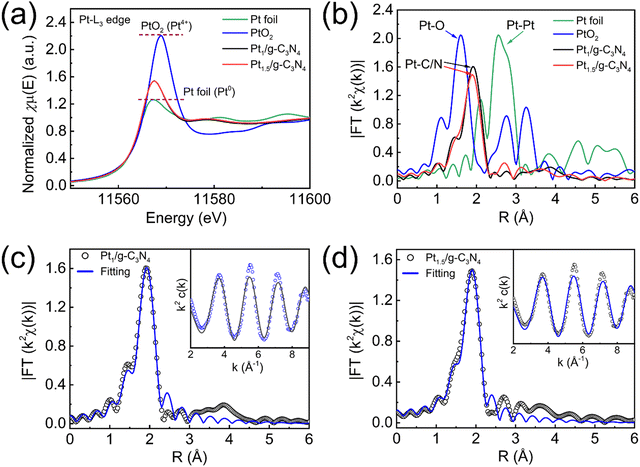 | ||
| Fig. 5 XAFS of Pt1/g-C3N4 and Pt1.5/g-C3N4. (a) L3-edge XANES spectra. (b) k2-weighted FT-EXAFS spectra. (c) Fitted FT-EXAFS spectra of Pt1/g-C3N4. (d) Fitted FT-EXAFS spectra of Pt1.5/g-C3N4. | ||
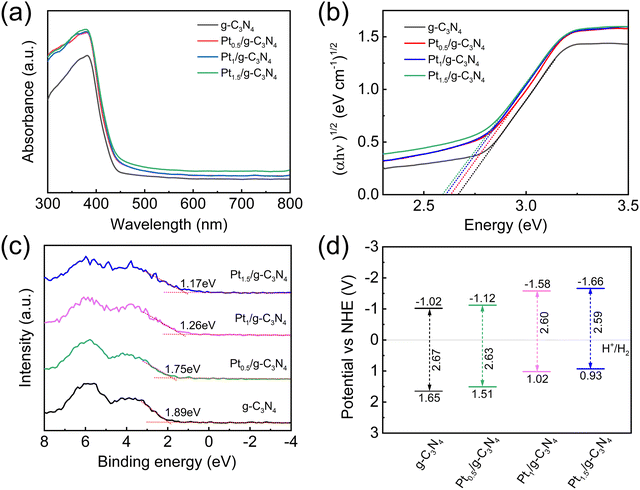
The band structures of the samples are depicted in Fig. 7. The DRS results indicate an increasing absorption intensity in the UV region and a slight red-shift in the absorption edge at approximately 430 nm with increasing Pt content (Fig. 7a), which can be ascribed to the presence of a higher concentration of Pt–N bonds.34 By extrapolating the intercept of the x-axis in the linear region of Tauc plots, the band gaps (Eg) of g-C3N4 and Ptx/g-C3N4 are calculated to be 2.67, 2.63, 2.60, and 2.59 eV, respectively (Fig. 7b).54 The corresponding valence band potentials (EVB) are calculated using valence band XPS (VB-XPS), yielding values of 1.89, 1.75, 1.20, and 1.17 eV, respectively (Fig. 7c). The EVB and the standard hydrogen electrode (EVB,NHE) exhibit a correlation expressed as EVB,NHE = φ + EVB,XPS − 4.44, where φ represents the work function of the XPS analyzer (4.2 eV).55 Thus, the EVB,NHE values of the respective samples are determined to be 1.65, 1.51, 1.02, and 0.93 eV. The band structures of g-C3N4 and Ptx/g-C3N4 are shown in Fig. 7d, where the conduction band potential (ECB) is calculated using the empirical formula: ECB = EVB − Eg. It is noteworthy that the ECB of Ptx/g-C3N4 tends to be more negative than that of g-C3N4, suggesting that Ptx/g-C3N4 has a stronger reduction in protons compared to g-C3N4 in thermodynamics.
The PL spectra reveal that all of the samples show a similar intrinsic fluorescence emission peak within the range of 400–550 nm (Fig. 8a).30 The PL intensity of Ptx/g-C3N4 is significantly diminished as a result of the incorporation of Pt single atoms, indicating a pronounced suppression of recombination of photogenerated charge carriers.22,46,56 The time-resolved PL-decay spectrum fitted by third-order exponential terms provides direct evidence of the duration of the photoexcited charge carriers (Fig. 8b), showing that the presence of Pt single atoms leads to a shorter lifetime (6.00, 5.80, and 5.90 ns for Pt0.5/g-C3N4, Pt1/g-C3N4, and Pt1.5/g-C3N4, respectively) compared to g-C3N4 (8.09 ns). The rapid migration of charge carriers between Pt single atoms and g-C3N4 enhances exciton dissociation, thereby effectively inhibiting the recombination of electron–hole pairs.49 To assess the charge-separation ability, the photocurrent and EIS properties are examined. In Fig. 8c, the photoelectrode of Ptx/g-C3N4 shows a higher photocurrent density in comparison to g-C3N4, indicating superior charge-transfer properties and more efficient photogenerated charge separation in Ptx/g-C3N4. Furthermore, the EIS Nyquist plot of Ptx/g-C3N4 displays a smaller semicircle compared to g-C3N4 (Fig. 8d), suggesting enhanced charge-transfer ability at the photocatalyst interface and improved electronic utilization. These results indicate that the incorporation of well-dispersed Pt single atoms can effectively augment optical absorption, diminish the band gap and valence band, impede photogenerated carrier recombination, and facilitate interfacial charge transfer. Moreover, the improving effect becomes stronger with the increasing content of Pt single atoms. These outcomes hold significant implications for enhancing the efficiency of the photocatalytic H2 evolution reaction.
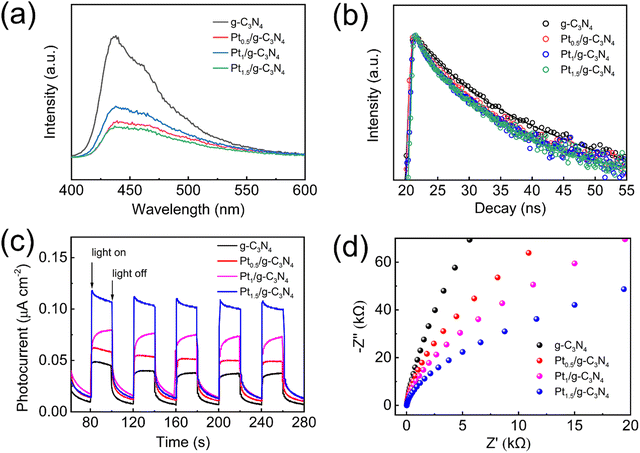 | ||
| Fig. 8 (a) PL spectra. (b) Time-resolved PL spectra. (c) Transient photocurrent responses. (d) EIS Nyquist plots. | ||
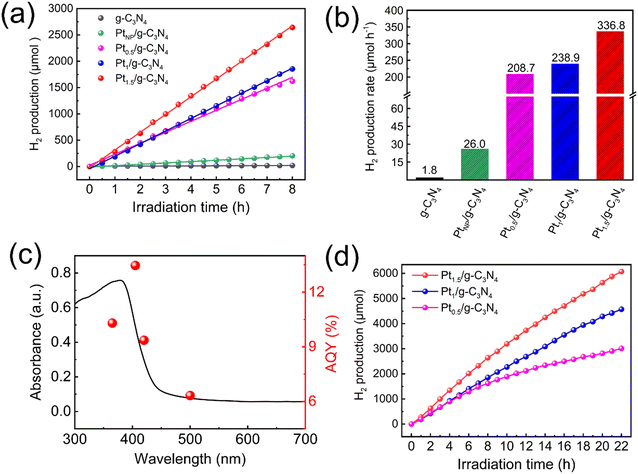
The advantage of loading Pt single atoms in photocatalytic H2 evolution is evaluated by varying the Pt content and aggregation. As shown in Fig. 9a, the initial H2 production rate of pristine g-C3N4 is relatively low (1.8 μmol h−1), which experiences an increase to 26.0 μmol h−1 upon loading Pt nanoparticles. Once g-C3N4 is modified with Pt single atoms at a loading content of merely 0.5%, a substantial enhancement of the photocatalytic H2 production rate is achieved (208.7 μmol h−1). The H2 production rate of Pt0.5/g-C3N4 is ∼116 times that of g-C3N4 and ∼8 times that of PtNP/g-C3N4, despite the amount of Pt source being only 1/20th of PtNP/g-C3N4. With an increase in Pt concentration, Pt1/g-C3N4 and Pt1.5/g-C3N4 exhibit elevated H2 production rates of 238.9 and 336.8 μmol h−1, respectively (Fig. 9b). These results indicate a positive correlation between the H2 production rate and the loading content of Pt single atoms within a reasonable range. The AQY of Pt1.5/g-C3N4 under irradiation at different wavenumbers is shown in Fig. 9c. Against irradiation wavenumbers of 365, 405, 420, and 500 nm, the AQYs are 10.30%, 13.46%, 9.35%, and 6.32%, respectively. The high AQY value and the good match between AQY and the absorption spectrum indicate that most of the captured photons are involved in the photocatalytic reaction.45,57 Compared with other Pt single-atom modified g-C3N4 photocatalysts prepared by different methods, Pt1.5/g-C3N4 reported in this work exhibits a relatively high loading content of Pt single atoms and excellent photocatalytic H2 evolution performances, as shown in Table 1.28,40,49,58,59
| Samples | Pt loading | Methods | Light sources | H2 production rates (μmol h−1) | AQYs | Ref. |
|---|---|---|---|---|---|---|
| Pt–CN | 0.74% | Immersion | 30 W LED (λ > 520) | 34.2 | 0.84% λ = 520 nm | 28 |
| Pt/CN | 0.16% | Immersion | 300 W Xe lamp (λ > 420) | 318 | — | 40 |
| Pt/CN | 0.11% | Photo-chemical reduction | 300 W Xe lamp (AM1.5) | 40.1 | — | 49 |
| Pt-CN | 0.38% | Immersion | 300 W Xe lamp (AM1.5) | 2.9 | — | 58 |
| PtHD-CNNT | 0.32% | Pyrolysis | 300 W Xe lamp (λ > 420) | 106.1 | 13.1% λ = 420 nm | 59 |
| Pt1.5/g-C3N4 | 1.26% | IWI | 300 W Xe lamp | 336.8 | 13.5% λ = 405 nm | This work |
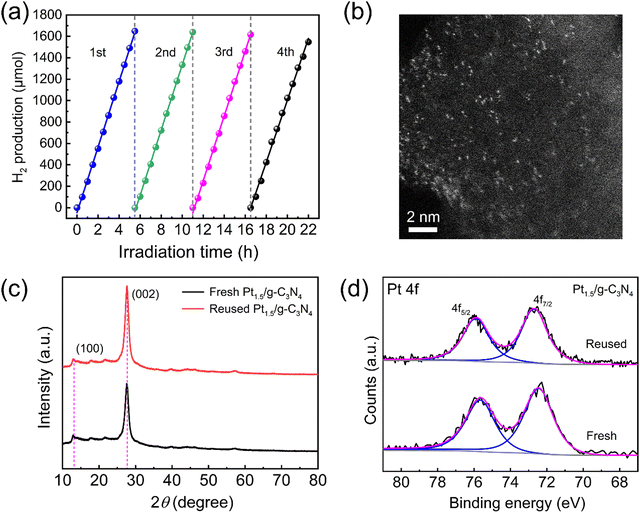
To study the stability of photocatalytic H2 production, an extended duration of the H2 evolution test is conducted. Fig. 9d shows that the H2 production rate of Pt0.5/g-C3N4 experiences a noticeable decline after 10 h, which may be attributed to the rupture of the Pt–N bond resulting from inadequate Pt filling between 3-s-triazines. Interestingly, the H2 production rates of Pt1/g-C3N4 and Pt1.5/g-C3N4 remain relatively constant for 22 h, indicating that the sufficient dispersion of Pt single atoms facilitates an optimal and robust interaction with g-C3N4. To further investigate the reusability, the photocatalytic test of Pt1.5/g-C3N4 is repeated four times, and the used catalyst after the reaction is characterized again, as depicted in Fig. 10. Fig. 10a shows the H2 production rate of Pt1.5/g-C3N4 during four cycles. After four cycles, the H2 production capability is 5.8% lower than the initial value, exhibiting excellent photocatalytic stability. The HAADF–STEM image of Pt1.5/g-C3N4 after photocatalysis is shown in Fig. 10b, indicating that the Pt single atoms maintain a high dispersion in an isolated state without apparent aggregation. Comparing the XRD patterns (Fig. 10c) and Pt 4f XPS spectra (Fig. 10d) before and after 4-cycle-photocatalysis, both the structure of g-C3N4 and the valence state of Pt single atoms are not significantly changed. These results demonstrate that Ptx/g-C3N4 exhibits excellent photocatalytic stability after a long photocatalytic reaction even when loaded with a high density of Pt single atoms.
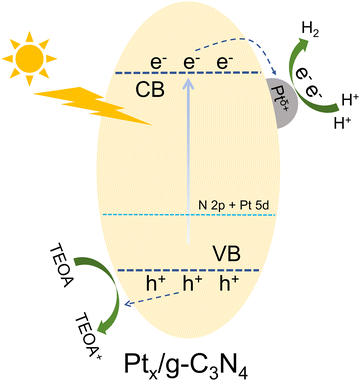
The enhanced mechanism of photocatalytic H2 production of Ptx/g-C3N4 is illustrated in Fig. 11. Low-temperature treatment reduces the average pore size of the small pores in g-C3N4, which may promote the adsorption of H+. Furthermore, the strong interaction between Pt 5d and N 2p for the formation of the Pt–N bond plays a key role in the kinetics of the H2 evolution reaction by effectively accelerating charge transfer and stabilizing active sites for photocatalysis.34,40,42,60 Pt single atoms incorporated in the center of the sixfold cavity and forming robust Pt–N6 coordination introduce new energy levels within the band structure of g-C3N4, leading to improved separation of photo-induced electron–hole pairs. Under light irradiation, the photogenerated electrons migrate to Pt single atoms for participating in the H2 evolution reaction. The stable and efficient production of H2 is attributed to the optimal dispersion of Pt atoms and the improved charge carrier dynamics facilitated by the interaction between Pt and g-C3N4.
4. Conclusions
In summary, Ptx/g-C3N4 photocatalysts with different Pt contents are synthesized using a low-temperature incipient wetness impregnation method. Pt atoms loaded on g-C3N4 in the form of the isolated single atoms even at a high density of 1.5 wt% are demonstrated by HAADF–STEM and EXAFS results. The well-dispersed Pt single atoms provide active sites for H+ adsorption. The Pt single atoms incorporated in the sixfold cavity between the three adjacent triazines form strong interaction between Pt and N6, which generates new doping energy levels and boosts higher transportation and separation efficiency of photo-generated charge carriers for improving photocatalytic H2 evolution. The photocatalytic H2 production rates of Ptx/g-C3N4 are roughly two orders of magnitude better than that of the pristine g-C3N4, and the stability increases with increasing Pt loading content. This research reflects a significant advancement in photocatalyst design and opens new avenues for the development of efficient hydrogen production systems.Author contributions
Qi Zhang: resources, investigation; Miao Yue: investigation, writing – original draft; Peng Chen: investigation, formal analysis; Qingmiao Ren: investigation; Weihu Kong: investigation; Chenxia Jia: visualization, formal analysis; Qianyu Lu: resources, writing – reviewing and editing; Jizhou Wu: formal analysis, writing – original draft; Yuqing Li: methodology; Wenliang Liu: writing – reviewing and editing; Peng Li: visualization; Yongming Fu: conceptualization, investigation, writing – original draft; supervision; and Jie Ma: writing – reviewing and editing, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFA0304203), the National Natural Science Foundation of China (62020106014, 62175140, 61901249, 92165106, 12104276), PCSIRT (IRT—17R70), 111 project (D18001), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi (OIT), the Applied Basic Research Project of Shanxi Province, China (201901D211191, 201901D211188), and the collaborative grant by the Russian Foundation for Basic Research and NSF of China (62011530047, 20-53-53025 in the RFBR classification).Notes and references
- C. Bie, L. Wang and J. Yu, Challenges for photocatalytic overall water splitting, Chem, 2022, 8, 1567–1574 CAS.
- T. Hisatomi, J. Kubota and K. Domen, Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- B.-J. Ng, L. K. Putri, X. Y. Kong, Y. W. Teh, P. Pasbakhsh and S.-P. Chai, Z-Scheme Photocatalytic Systems for Solar Water Splitting, Adv. Sci., 2020, 7, 1903171 CrossRef CAS PubMed.
- J. Zhang, G. Ye, C. Zhang, Z. M. Pan, S. B. Wang, G. G. Zhang and X. C. Wang, Heptazine-Based Ordered-Distorted Copolymers with Enhanced Visible-Light Absorption for Photocatalytic Hydrogen Production, ChemSusChem, 2022, 15, e202201616 CrossRef CAS PubMed.
- M. H. Liu, G. G. Zhang, X. C. Liang, Z. M. Pan, D. D. Zheng, S. B. Wang, Z. Y. Yu, Y. D. Hou and X. C. Wang, Rh/Cr2O3 and CoOx Cocatalysts for Efficient Photocatalytic Water Splitting by Poly (Triazine Imide) Crystals, Angew. Chem., Int. Ed., 2023, 62, e202304694 CrossRef CAS PubMed.
- Q. Wang, G. Zhang, W. Xing, Z. Pan, D. Zheng, S. Wang, Y. Hou and X. Wang, Bottom-up Synthesis of Single-Crystalline Poly (Triazine Imide) Nanosheets for Photocatalytic Overall Water Splitting, Angew. Chem., Int. Ed., 2023, 62, e202307930 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- Z. A. Lan, G. G. Zhang and X. C. Wang, A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting, Appl. Catal., B, 2016, 192, 116–125 CrossRef CAS.
- X. B. Li, J. Xiong, X. M. Gao, J. T. Huang, Z. J. Feng, Z. Chen and Y. F. Zhu, Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction, J. Alloys Compd., 2019, 802, 196–209 CrossRef CAS.
- L. H. Lin, Z. Y. Yu and X. C. Wang, Crystalline Carbon Nitride Semiconductors for Photocatalytic Water Splitting, Angew. Chem., Int. Ed., 2019, 58, 6164–6175 CrossRef CAS PubMed.
- S. B. Yang, Y. J. Gong, J. S. Zhang, L. Zhan, L. L. Ma, Z. Y. Fang, R. Vajtai, X. C. Wang and P. M. Ajayan, Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- Z. P. Yu, Y. F. Li, A. Torres-Pinto, A. P. LaGrow, V. M. Diaconescu, L. Simonelli, M. J. Sampaio, O. Bondarchuk, I. Amorim, A. Araujo, A. M. T. Silva, C. G. Silva, J. L. Faria and L. F. Liu, Single-atom Ir and Ru anchored on graphitic carbon nitride for efficient and stable electrocatalytic/photocatalytic hydrogen evolution, Appl. Catal., B, 2022, 310, 121318 CrossRef CAS.
- C. J. Wang, J. Xie, N. Chen, W. F. Chen, P. H. Bai and H. Wang, Non-Noble-Metal Catalyst of Cu/g-C3N4 for Efficient Photocatalytic Hydrogen Evolution, ACS Appl. Energy Mater., 2021, 4, 13796–13802 CrossRef CAS.
- W. Li, X. S. Chu, F. Wang, Y. Y. Dang, X. Y. Liu, X. C. Wang and C. Y. Wang, Enhanced cocatalyst-support interaction and promoted electron transfer of 3D porous g-C3N4/GO-M (Au, Pd, Pt) composite catalysts for hydrogen evolution, Appl. Catal., B, 2021, 288, 120034 CrossRef CAS.
- Z. Wang, Y. Luo, T. Hisatomi, J. J. M. Vequizo, S. Suzuki, S. S. Chen, M. Nakabayashi, L. H. Lin, Z. H. Pan, N. Kariya, A. Yamakata, N. Shibata, T. Takata, K. Teshima and K. Domen, Sequential cocatalyst decoration on BaTaO2N towards highly-active Z-scheme water splitting, Nat. Commun., 2021, 12, 1005 CrossRef CAS PubMed.
- C. M. Wolff, P. D. Frischmann, M. Schulze, B. J. Bohn, R. Wein, P. Livadas, M. T. Carlson, F. Jäckel, J. Feldmann, F. Würthner and J. K. Stolarczyk, All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods, Nat. Energy, 2018, 3, 862–869 CrossRef CAS.
- J. D. Xiao, Q. C. Shang, Y. J. Xiong, Q. Zhang, Y. Luo, S. H. Yu and H. L. Jiang, Boosting Photocatalytic Hydrogen Production of a Metal-Organic Framework Decorated with Platinum Nanoparticles: The Platinum Location Matters, Angew. Chem., Int. Ed., 2016, 55, 9389–9393 CrossRef CAS PubMed.
- Y. Attia and M. Samer, Metal clusters: New era of hydrogen production, Renew. Sustainable Energy Rev., 2017, 79, 878–892 CrossRef CAS.
- Y. Chen, L. Soler, C. Cazorla, J. Oliveras, N. G. Bastus, V. F. Puntes and J. Llorca, Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution, Nat. Commun., 2023, 14, 6165 CrossRef CAS PubMed.
- H. Zhai, Z. Liu, J. Tong, Y. Zhang, B. Zhou, P. Tan, R. Sa and J. Pan, Tuning charge transfer between size-controlled Pt cluster and N vacancy engineered ultrathin g-C3N4 for efficient photocatalytic hydrogen evolution, Ceram. Int., 2023, 49, 36857–36865 CrossRef CAS.
- F.-G. Zhang, M. Cheng, Y.-J. Yuan, Q.-Y. Liu, Q. Cheng and J. Guan, Boosted charge transfer in Pt cluster anchored TiO2 microspheres with rich oxygen vacancies for solar driven H2 production from lignocellulosic biomass, Inorg. Chem. Front., 2023, 10, 7369–7380 RSC.
- X. Z. Fang, Q. C. Shang, Y. Wang, L. Jiao, T. Yao, Y. F. Li, Q. Zhang, Y. Luo and H. L. Jiang, Single Pt Atoms Confined into a Metal-Organic Framework for Efficient Photocatalysis, Adv. Mater., 2018, 30, 1705112 CrossRef PubMed.
- B. T. Qiao, A. Q. Wang, X. F. Yang, L. F. Allard, Z. Jiang, Y. T. Cui, J. Y. Liu, J. Li and T. Zhang, Single-atom catalysis of CO oxidation using Pt1/FeOx, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- A. Q. Wang, J. Li and T. Zhang, Heterogeneous single-atom catalysis, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef CAS.
- Q. Zuo, T. T. Liu, C. S. Chen, Y. Ji, X. Q. Gong, Y. Y. Mai and Y. F. Zhou, Ultrathin Metal-Organic Framework Nanosheets with Ultrahigh Loading of Single Pt Atoms for Efficient Visible-Light-Driven Photocatalytic H2 Evolution, Angew. Chem., Int. Ed., 2019, 58, 10198–10203 CrossRef CAS PubMed.
- G. F. S. R. Rocha, M. A. R. da Silva, A. Rogolino, G. A. A. Diab, L. F. G. Noleto, M. Antonietti and I. F. Teixeira, Carbon nitride based materials: more than just a support for single-atom catalysis, Chem. Soc. Rev., 2023, 52, 4878–4932 RSC.
- Z. Zeng, Y. Su, X. Quan, W. Choi, G. Zhang, N. Liu, B. Kim, S. Chen, H. Yu and S. Zhang, Single-atom platinum confined by the interlayer nanospace of carbon nitride for efficient photocatalytic hydrogen evolution, Nano Energy, 2020, 69, 104409 CrossRef CAS.
- Y. Xue, Y. Lei, X. Liu, Y. Li, W. Deng, F. Wang and S. Min, Highly active dye-sensitized photocatalytic H2 evolution catalyzed by a single-atom Pt cocatalyst anchored onto g-C3N4 nanosheets under long-wavelength visible light irradiation, New J. Chem., 2018, 42, 14083–14086 RSC.
- C. Wu, S. Xue, Z. Qin, M. Nazari, G. Yang, S. Yue, T. Tong, H. Ghasemi, F. C. R. Hernandez, S. Xue, D. Zhang, H. Wang, Z. M. Wang, S. Pu and J. Bao, Making g-C3N4 ultra-thin nanosheets active for photocatalytic overall water splitting, Appl. Catal., B, 2021, 282, 119557 CrossRef CAS.
- L. Zhang, R. Long, Y. Zhang, D. Duan, Y. Xiong, Y. Zhang and Y. Bi, Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts, Angew. Chem., Int. Ed., 2020, 59, 6224–6229 CrossRef CAS PubMed.
- P. Zhou, F. Lv, N. Li, Y. Zhang, Z. Mu, Y. Tang, J. Lai, Y. Chao, M. Luo, F. Lin, J. Zhou, D. Su and S. Guo, Strengthening reactive metal-support interaction to stabilize high-density Pt single atoms on electron-deficient g-C3N4 for boosting photocatalytic H2 production, Nano Energy, 2019, 56, 127–137 CrossRef CAS.
- L. Wang, R. Tang, A. Kheradmand, Y. Jiang, H. Wang, W. Yang, Z. Chen, X. Zhong, S. P. Ringer, X. Liao, W. Liang and J. Huang, Enhanced solar-driven benzaldehyde oxidation with simultaneous hydrogen production on Pt single-atom catalyst, Appl. Catal., B, 2021, 284, 119759 CrossRef CAS.
- G. Huang, Q. Niu, J. Zhang, H. Huang, Q. Chen, J. Bi and L. Wu, Platinum single-atoms anchored covalent triazine framework for efficient photoreduction of CO2 to CH4, Chem. Eng. J., 2022, 427, 131018 CrossRef CAS.
- Y. Hu, Y. Qu, Y. Zhou, Z. Wang, H. Wang, B. Yang, Z. Yu and Y. Wu, Single Pt atom-anchored C3N4: A bridging Pt–N bond boosted electron transfer for highly efficient photocatalytic H2 generation, Chem. Eng. J., 2021, 412, 128749 CrossRef CAS.
- D. Vasilchenko, A. Zhurenok, A. Saraev, E. Gerasimov, S. Cherepanova, S. Tkachev, P. Plusnin and E. Kozlova, Highly efficient hydrogen production under visible light over g-C3N4-based photocatalysts with low platinum content, Chem. Eng. J., 2022, 445, 136721 CrossRef CAS.
- H. Yang, N. Lu, J. Zhang, R. Wang, S. Tian, M. Wang, Z. Wang, K. Tao and F. Ma, S. Peng. Ultra-low single-atom Pt on g-C3N4 for electrochemical hydrogen peroxide production, Carbon Energy, 2023, 5, e337 CrossRef CAS.
- Y. Yao, Z. Huang, P. Xie, L. Wu, L. Ma, T. Li, Z. Pang, M. Jiao, Z. Liang, J. Gao, Y. He, D. J. Kline, M. R. Zachariah, C. Wang, J. Lu, T. Wu, T. Li, C. Wang, R. Shahbazian-Yassar and L. Hu, High temperature shockwave stabilized single atoms, Nat. Nanotechnol., 2019, 14, 851–857 CrossRef CAS PubMed.
- X. Liu, S. Wang, W. Yu, J. Zhang, S. Fang, J. Zhang, J. Qiu, F. Kong and X. Duan, Single platinum atoms anchored on holy carbon nitride for efficient photodegradation of sulfonylurea herbicide, Chem. Eng. J., 2022, 446, 137426 CrossRef CAS.
- Z. Chen, S. Mitchell, E. Vorobyeva, R. K. Leary, R. Hauert, T. Furnival, Q. M. Ramasse, J. M. Thomas, P. A. Midgley, D. Dontsova, M. Antonietti, S. Pogodin, N. Lopez and J. Perez-Ramirez, Stabilization of Single Metal Atoms on Graphitic Carbon Nitride, Adv. Funct. Mater., 2017, 27, 1605785 CrossRef.
- X. Li, W. Bi, L. Zhang, S. Tao, W. Chu, Q. Zhang, Y. Luo, C. Wu and Y. Xie, Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution, Adv. Mater., 2016, 28, 2427–2431 CrossRef CAS PubMed.
- M. Ou, S. Wan, Q. Zhong, S. Zhang and Y. Wang, Single Pt atoms deposition on g-C3N4 nanosheets for photocatalytic H2 evolution or NO oxidation under visible light, Int. J. Hydrogen Energy, 2017, 42, 27043–27054 CrossRef CAS.
- X. N. Yang, L. T. Ren, D. C. Jiang, L. S. Yin, Z. J. Li and Y. P. Yuan, Strong Interfacial Chemical Bonding in Regulating Electron Transfer and Stabilizing Catalytic Sites in a Metal-Semiconductor Schottky Junction for Enhanced Photocatalysis, Small, 2023, 2308408, DOI:10.1002/smll.202308408.
- J. C. Shen, C. H. Luo, S. S. Qiao, Y. Q. Chen, Y. H. Tang, J. Q. Xu, K. X. Fu, D. W. Yuan, H. F. Tang, H. Zhang and C. B. Liu, Single-Atom Cu Channel and N-Vacancy Engineering Enables Efficient Charge Separation and Transfer between C3N4 Interlayers for Boosting Photocatalytic Hydrogen Production, ACS Catal., 2023, 13, 6280–6288 CrossRef CAS.
- Y. Zhu, T. Wang, T. Xu, Y. Li and C. Wang, Size effect of Pt co-catalyst on photocatalytic efficiency of g-C3N4 for hydrogen evolution, Appl. Surf. Sci., 2019, 464, 36–42 CrossRef CAS.
- P. Y. Dong, Y. Wang, A. C. J. Zhang, T. Cheng, X. G. Xi and J. L. Zhang, Platinum Single Atoms Anchored on a Covalent Organic Framework: Boosting Active Sites for Photocatalytic Hydrogen Evolution, ACS Catal., 2021, 11, 13266–13279 CrossRef CAS.
- G. G. Zhang, Z. A. Lan, L. H. Lin, S. Lin and X. C. Wang, Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents, Chem. Sci., 2016, 7, 3062–3066 RSC.
- P. Kuang, Y. Wang, B. Zhu, F. Xia, C.-W. Tung, J. Wu, H. M. Chen and J. Yu, Pt Single Atoms Supported on N-Doped Mesoporous Hollow Carbon Spheres with Enhanced Electrocatalytic H2-Evolution Activity, Adv. Mater., 2021, 33, 2008599 CrossRef CAS PubMed.
- X. Wu, H. Zhang, J. Dong, M. Qiu, J. Kong, Y. Zhang, Y. Li, G. Xu, J. Zhang and J. Ye, Surface step decoration of isolated atom as electron pumping: Atomic-level insights into visible-light hydrogen evolution, Nano Energy, 2018, 45, 109–117 CrossRef CAS.
- Y. J. Cao, D. H. Wang, Y. Lin, W. Liu, L. L. Cao, X. K. Liu, W. Zhang, X. L. Mou, S. Fang and X. Y. Shen, T. Yao. Single Pt Atom with Highly Vacant d-Orbital for Accelerating Photocatalytic H2 Evolution, ACS Appl. Energy Mater., 2018, 1, 6082–6088 CrossRef CAS.
- T. Cui, L. Ma, S. Wang, C. Ye, X. Liang, Z. Zhang, G. Meng, L. Zheng, H.-S. Hu, J. Zhang, H. Duan, D. Wang and Y. Li, Atomically Dispersed Pt-N3C1 Sites Enabling Efficient and Selective Electrocatalytic C-C Bond Cleavage in Lignin Models under Ambient Conditions, J. Am. Chem. Soc., 2021, 143, 9429–9439 CrossRef CAS PubMed.
- N. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. Xiao, R. Li, T.-K. Sham, L.-M. Liu, G. A. Botton and X. Sun, Platinum single-atom and cluster catalysis of the hydrogen evolution reaction, Nat. Commun., 2016, 7, 13638 CrossRef CAS PubMed.
- Z. Xue, M. Yan, X. Wang, Z. Wang, Y. Zhang, Y. Li, W. Xu, Y. Tong, X. Han, C. Xiong, W. Wang, M. Chen, B. Ye, X. Hong, L. Song, H. Zhang, L.-M. Yang and Y. Wu, Tailoring Unsymmetrical-Coordinated Atomic Site in Oxide-Supported Pt Catalysts for Enhanced Surface Activity and Stability, Small, 2021, 17, 2101008 CrossRef CAS PubMed.
- T. He, S. Chen, B. Ni, Y. Gong, Z. Wu, L. Song, L. Gu, W. Hu and X. Wang, Zirconium-Porphyrin-Based Metal-Organic Framework Hollow Nanotubes for Immobilization of Noble-Metal Single Atoms, Angew. Chem., Int. Ed., 2018, 57, 3493–3498 CrossRef CAS PubMed.
- Q. Liang, Z. Li, X. Yu, Z.-H. Huang, F. Kang and Q.-H. Yang, Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution, Adv. Mater., 2015, 27, 4634–4639 CrossRef CAS PubMed.
- X. Li, B. Kang, F. Dong, Z. Zhang, X. Luo, L. Han, J. Huang, Z. Feng, Z. Chen, J. Xu, B. Peng and Z. L. Wang, Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies, Nano Energy, 2021, 81, 105671 CrossRef CAS.
- E. C. Kohlrausch, H. A. Centurion, R. W. Lodge, X. L. Luo, T. Slater, M. J. L. Santos, S. L. Ling, V. R. Mastelaro, M. J. Cliffe, R. V. Goncalves and J. A. Fernandes, A high-throughput, solvent free method for dispersing metal atoms directly onto supports, J. Mater. Chem. A, 2021, 9, 26676–26679 RSC.
- X. Han, Q. A. Liu, A. Qian, L. Ye, X. Pu, J. C. Liu, X. Jia, R. J. Wang, F. Ju, H. Sun, J. G. Zhao and H. Ling, Transition-Metal Single Atom Anchored on MoS2 for Enhancing Photocatalytic Hydrogen Production of g-C3N4 Photocatalysts, ACS Appl. Mater. Interfaces, 2023, 15, 26670–26681 CrossRef CAS PubMed.
- T. Mahvelati-Shamsabadi, K. C. Bhamu, S. H. Lee, T. T. Dang, V. H. Khoi, S. H. Hur, W. M. Choi, S. G. Kang, T. J. Shin and J. S. Chung, Coordinatively unsaturated atomically dispersed Pt+2-N4 sites on hexagonal nanosheet structure of g-C3N4 for high-performance photocatalytic H2 production, Appl. Catal., B, 2023, 337, 122959 CrossRef CAS.
- D. W. Sun, C. C. Long and J. H. Huang, Highly dispersed platinum-anchored g-C3N4 nanotubes for photocatalytic hydrogen generation, Int. J. Hydrogen Energy, 2023, 48, 943–952 CrossRef CAS.
- H. Zhai, P. Tan, M. Jiang, M. Zhang, R. Ren, R. Sa and J. Pan, Electronic Regulation of Pt Single-Atom Catalysts via Local Coordination State Adjustment for Enhanced Photocatalytic Performance, ACS Catal., 2023, 13, 8063–8072 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |