Advancing continuous flow techniques in effective trimethoprim oxidation: combatting bacterial resistance in wastewater†
Diana L.
Marques
a,
Giusi
Piccirillo
ab,
Fábio M. S.
Rodrigues
 a,
Rafael T.
Aroso
a,
Lucas D.
Dias
ac,
Gabriela J.
da Silva
d,
Mário J. F.
Calvete
a,
Rafael T.
Aroso
a,
Lucas D.
Dias
ac,
Gabriela J.
da Silva
d,
Mário J. F.
Calvete
 *a and
Mariette M.
Pereira
*a and
Mariette M.
Pereira
 *a
*a
aCQC-IMS, Department of Chemistry, University of Coimbra, Rua Larga, 3004-535, Coimbra, Portugal. E-mail: mcalvete@qui.uc.pt; mmpereira@qui.uc.pt
bBio4Plas – Biopolímeros, Lda. Zona Industrial Lote 61, 3064-197 Cantanhede, Portugal
cLaboratório de Novos Materiais, Universidade Evangélica de Goiás (UniEvangélica), Anápolis, Brazil
dFaculty of Pharmacy and Center for Neurosciences and Cell Biology, University of Coimbra, Azinhaga de Santa Comba, 3000-548, Coimbra, Portugal
First published on 26th October 2024
Abstract
This work describes an innovative catalytic process for aqueous trimethoprim degradation, using a fixed-bed continuous flow reactor packed with a manganese(III) meso-substituted porphyrin covalently immobilized functionalized aminopropyl silica gel as the catalyst and H2O2 as a green oxidant. It exhibits remarkable activity and stability, maintaining its performance over extended periods (up to 8 hours) and achieving significant reductions in total organic carbon (TOC = 80%). Importantly, microbiological assays confirmed that this degradation process effectively converts trimethoprim into non-resistance-inducing products.
The widespread use of antibiotics (AB) in human medicine and animal agriculture, while essential for treating infections, has become a double-edged sword due to its association with the emergence of antimicrobial resistance (AMR).1,2 AMR is a growing global health crisis, potentially sparking future pandemics.3,4 The persistence of antibiotics in surface and groundwater, as well as urban wastewater, highlights this concern. Therefore, developing sustainable methods to eliminate residual antibiotics from wastewater is crucial to safeguard public health and ensure access to clean water.
Conventional advanced oxidation processes (AOPs), such as homogeneous Fenton (Fe(II)–Fe(III)/H2O2),5 Fenton-like processes5 and direct UV/H2O2 treatment,6 are commonly used for the degradation of pharmaceuticals in wastewater. However, Fenton methods have disadvantages regarding ˙OH production, which requires narrow pH ranges to avoid excessive H2O2 consumption and accumulation of ferric ions in the sludge. Fenton-like processes require additional additives for the in situ production of hydrogen peroxide, typically through electrochemical or photochemical means. Additionally, UV/H2O2 treatment involves significant energy consumption. In contrast, reusable biomimetic immobilized metalloporphyrin oxidation catalysts are able to activate H2O2, without the need for costly light sources or additional additives. Additionally, these catalytic systems are highly efficient, operating effectively at very low catalyst-to-substrate ratios.7–11
However, for practical applications in degradation/oxidation systems, metalloporphyrins need immobilization onto solid supports.12,13 This approach enhances their stability and also enables catalyst reusability. In this context, the covalent bonding of porphyrin catalysts to silica gel represents a promising option given its ease of implementation and economical advantages.7,8,14 Additionally, continuous operating systems using heterogeneous catalysts in a fixed-bed reactor are desirable for practical applications because they avoid constant interruption for substrate and catalyst feeding.15,16 Continuous flow AOP research has demonstrated promising results in antibiotic degradation, including through photocatalysis,17 Fenton-based methods18 and ozonation,19 among others.20
However, no studies have investigated the use of immobilized metalloporphyrin catalysts within fixed-bed continuous flow reactors for the activation of H2O2, with the goal of developing stable and reusable methods for antibiotic degradation. Furthermore, existing continuous flow AOP research fails to address the potential for bacterial resistance emerging from the generated oxidation products.
Our collaboration with a local public oncology hospital clinic revealed trimethoprim (TMP) as the most prevalent antibiotic in wastewater effluent (Fig. S1, ESI†). This is coherent with TMP's status as one of the world's most widely prescribed antibiotics, with an estimated 10% prescription rate.21 This high usage translates to a significant presence in municipal and rural wastewaters, reaching concentrations as high as 2 μg L−1.22 These data highlight the urgency of developing studies aimed at the degradation of trimethoprim.
This work presents the development of the first covalently immobilized manganese(III) meso-substituted porphyrin catalyst on functionalized aminopropyl silica gel for the remediation of water contaminated with trimethoprim (TMP). Using a fixed-bed continuous flow reactor, this catalytic system employs hydrogen peroxide as a safe oxidant to effectively degrade the persistent antibiotic.
We synthesized the desired hybrid heterogeneous catalyst (Scheme S1, ESI†)14 by chlorosulfonation of 5,10,15,20-tetrakis(2,6-dichlorophenyl)porphyrin (TDCPPS), followed by its covalent immobilization onto amine-functionalized silica gel (NH2–SiO2), and complexation with Mn(II) acetate to afford the desired Mn(III)TDCPPS@NH-SiO2. The catalyst was characterized by solid-state UV-Vis, thermogravimetric analysis (TG), scanning electron microscopy (SEM), inductively coupled plasma and optical emission spectrometry (ICP-OES), with data in accordance with the literature (Fig. S2–S4, ESI†).14 TG and ICP-OES data analysis allowed the determination of an amount of 3.4 × 10−5 mol of immobilized porphyrin per g of functionalized silica, assuming 44% immobilization yield. This was corroborated by ICP-OES analysis, which revealed a Mn(III) value of 1.7% (w/w), corresponding to 3.1 × 10−5 mol of metalloporphyrin per g of material, being very similar to the TG result.
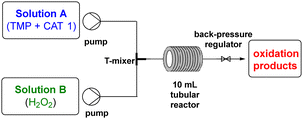
First, we assessed the ability of Mn(III)TDCPPS (CAT1) to promote the homogeneous oxidative degradation of trimethoprim (TMP), in the presence of H2O2 as a benign oxidant, in a continuous flow process, by varying the residence time and the volumetric ratio of TMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CAT1
CAT1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2, and the results are presented in Table 1 (see also Scheme S2, ESI†).
H2O2, and the results are presented in Table 1 (see also Scheme S2, ESI†).
| Entry | Residence time RT (min) | Volumetric ratio VR (TMP + CAT1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 H2O2 |
Degradation (%) |
|---|---|---|---|
| a H2O2 (3% v/v, 1.3 M). b In the absence of CAT1. c In the absence of H2O2 oxidant. d H2O2 (0.3% v/v, 0.13 M). e H2O2 (6% v/v, 2.6 M). f No catalyst. | |||
| 1a | 8.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15 |
2 |
| 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
7 | |
| 3a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
12 | |
| 4a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
40 | |
| 5a | 45.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
87 |
| 6b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
13 | |
| 7c | —f | Trace | |
| 8d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
32 | |
| 9e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
34 | |
To simulate realistic wastewater scenarios, we used a highly diluted TMP stock solution (6.68 μg mL−1). The residence time and the volumetric ratio A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B (VR) of the influent solutions were optimized according to the equipment specs. Initially, an aqueous solution of TMP (6.68 μg mL−1, 2.3 × 10−5 M) and CAT1 (2.3 × 10−7 M) was introduced into flask A, while a 3% v/v H2O2/H2O solution was placed in flask B. Subsequently, the volume ratio (VR) of solution A to solution B, as well as the residence time (RT), were optimized to determine the ideal flow conditions for the degradation of TMP. We set the RT to 8.7 minutes and the VR to 1
B (VR) of the influent solutions were optimized according to the equipment specs. Initially, an aqueous solution of TMP (6.68 μg mL−1, 2.3 × 10−5 M) and CAT1 (2.3 × 10−7 M) was introduced into flask A, while a 3% v/v H2O2/H2O solution was placed in flask B. Subsequently, the volume ratio (VR) of solution A to solution B, as well as the residence time (RT), were optimized to determine the ideal flow conditions for the degradation of TMP. We set the RT to 8.7 minutes and the VR to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 mL min−1 (solutions A and B) (entry 1). However, under these conditions, no significant degradation of TMP was observed (2%), prompting us to explore other volumetric ratios. Thus, experiments were conducted using A
0.15 mL min−1 (solutions A and B) (entry 1). However, under these conditions, no significant degradation of TMP was observed (2%), prompting us to explore other volumetric ratios. Thus, experiments were conducted using A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B volume ratios of 1
B volume ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3, 1
0.3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, and 1
0.6, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (entries 2–4). These results show a degradation rate correlation on the volumetric ratio used, with a maximum 40% degradation of TMP for VR = 1
1.2 (entries 2–4). These results show a degradation rate correlation on the volumetric ratio used, with a maximum 40% degradation of TMP for VR = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (entry 2). This led us to extend the RT to the maximum allowed by the equipment (RT = 45.5 min), under this volumetric ratio of 1
1.2 (entry 2). This led us to extend the RT to the maximum allowed by the equipment (RT = 45.5 min), under this volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, a TMP degradation value of 87% was achieved (entry 5). This substantial increase supports the expected direct correlation between degradation rate and residence time, at a constant volumetric ratio. To evaluate the factors affecting degradation, additional experiments were conducted. One experiment, conducted without the catalyst, resulted in only 13% TMP degradation (entry 6). Another experiment, conducted without the oxidant, resulted in 0% degradation (entry 7). These results confirm that both catalyst and oxidant must be present, during a selected RT, for effective TMP degradation (Fig. S5, ESI†). Then, to evaluate the impact of oxidant concentration, additional reactions were conducted, using 0.3% and 6% (v/v) H2O2 solutions (entries 8 and 9). At VR = 1
1.2, a TMP degradation value of 87% was achieved (entry 5). This substantial increase supports the expected direct correlation between degradation rate and residence time, at a constant volumetric ratio. To evaluate the factors affecting degradation, additional experiments were conducted. One experiment, conducted without the catalyst, resulted in only 13% TMP degradation (entry 6). Another experiment, conducted without the oxidant, resulted in 0% degradation (entry 7). These results confirm that both catalyst and oxidant must be present, during a selected RT, for effective TMP degradation (Fig. S5, ESI†). Then, to evaluate the impact of oxidant concentration, additional reactions were conducted, using 0.3% and 6% (v/v) H2O2 solutions (entries 8 and 9). At VR = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 and RT = 45.5 min, only 32% and 34% degradation were observed. Thus, optimal H2O2 concentration is also a key parameter to ensure the formation of Mn(III)TDCPPS oxo-species, which is the oxidant active catalytic species.11 These moderate degradation results obtained at higher H2O2 concentrations may be attributed to degradation of CAT1 as corroborated by UV-Visible spectroscopy (Fig. S6, ESI†).14 To avoid catalyst degradation and aiming at continuous reuse the research of TMP degradation with H2O2 as an oxidant shifted to evaluating the immobilized Mn(III)TDCPPS@NH-SiO2 heterogeneous catalyst (CAT2) under the previously optimized continuous flow conditions.
1.2 and RT = 45.5 min, only 32% and 34% degradation were observed. Thus, optimal H2O2 concentration is also a key parameter to ensure the formation of Mn(III)TDCPPS oxo-species, which is the oxidant active catalytic species.11 These moderate degradation results obtained at higher H2O2 concentrations may be attributed to degradation of CAT1 as corroborated by UV-Visible spectroscopy (Fig. S6, ESI†).14 To avoid catalyst degradation and aiming at continuous reuse the research of TMP degradation with H2O2 as an oxidant shifted to evaluating the immobilized Mn(III)TDCPPS@NH-SiO2 heterogeneous catalyst (CAT2) under the previously optimized continuous flow conditions.
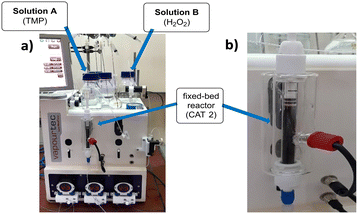
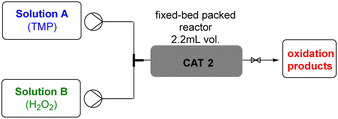
These studies began with the packing of the fixed-bed column reactor (Fig. 1) with 1.5 g of heterogeneous CAT2 (Scheme S3, ESI†).
Before initiating the TMP degradation experiments with H2O2 in this heterogeneous system, we carried out a blank adsorption experiment. The TMP solution was introduced through the fixed-bed reactor for 30 minutes and we observed that the concentration of TMP at the reactor output remained unchanged. This demonstrated that no TMP adsorption occurs and this point was consistently considered as time zero for all experiments. Building on the results obtained from homogeneous studies, we began by conducting an experiment with a TMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 volumetric ratio (VR = 1
H2O2 volumetric ratio (VR = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2), at the fixed-bed reactor's maximum residence time, which was set at RT = 10 minutes. Under these heterogeneous conditions, aqueous TMP solution A, and H2O2 aqueous solution B were pumped through the fixed-bed reactor containing CAT2 and TMP degradation reached 96% with [H2O2] = 3% (v/v) (entry 1, Table 2). Knowing that H2O2 concentration typically influences this type of reaction, we decided to lower [H2O2] = from 3% to 0.3% (v/v) and similar degradation results were observed (entry 2, Table 2 and Fig. S7, ESI†).
1.2), at the fixed-bed reactor's maximum residence time, which was set at RT = 10 minutes. Under these heterogeneous conditions, aqueous TMP solution A, and H2O2 aqueous solution B were pumped through the fixed-bed reactor containing CAT2 and TMP degradation reached 96% with [H2O2] = 3% (v/v) (entry 1, Table 2). Knowing that H2O2 concentration typically influences this type of reaction, we decided to lower [H2O2] = from 3% to 0.3% (v/v) and similar degradation results were observed (entry 2, Table 2 and Fig. S7, ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2) = 1
H2O2) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. Degradation results are given as average values of reactions performed in triplicate
1.2. Degradation results are given as average values of reactions performed in triplicate
The improved results observed in the flow system with heterogeneous catalysis, compared to similar conditions in the homogeneous phase, can be attributed to the well-known improved stability of immobilized porphyrins onto solid supports.11,14
As expected, reducing the residence time lowered the degradation yield, 90% (entry 3, Table 2). The decrease in yield observed for very short residence times (5 minutes) may be due to the lack of formation of the active oxo species with hydrogen peroxide and, consequently, the decrease of the TMP oxidation rate.14 Additionally, a blank experiment, conducted in a fixed-bed reactor packed with pristine SiO2 (described in ESI†) was also carried out and 20% TMP degradation was observed (entry 4, Table 2). This result may be explained by the H2O2 activation by the Si-Lewis acid.23 Under the same flow reaction conditions, we evaluated the degradation process using ten times higher concentration [TMP] = 66.8 μg mL−1 (2.3 × 10−4 M) and similar results were obtained (94%; entry 5, Table 2), which demonstrate the extremely high activity of the heterogeneous CAT2 under flow conditions (Fig. S8, ESI†).
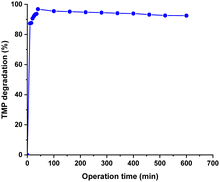
These results led us to assess the system's long-term effectiveness under continuous flow conditions, by implementing the previously described heterogeneous setup for TMP degradation ([TMP] = 66.8 μg mL−1, [H2O2] = 0.3% (v/v), VR = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (TMP
1.2 (TMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2)) in a continuous mode for 8 hours. The experiment achieved a high initial degradation rate, reaching nearly 96% TMP degradation after reaching steady-state at 40 minutes (Fig. 2; see also Fig. S9, ESI†) and stable 94% TMP degradation along 8 hours was observed.
H2O2)) in a continuous mode for 8 hours. The experiment achieved a high initial degradation rate, reaching nearly 96% TMP degradation after reaching steady-state at 40 minutes (Fig. 2; see also Fig. S9, ESI†) and stable 94% TMP degradation along 8 hours was observed.
The catalyst's high stability was confirmed by ICP-OES analysis, which revealed no change in manganese content compared to the initial CAT2 observed after 8 hours of reaction. Additionally, mineralization of TMP was determined by total organic content (TOC) removal analysis, before and after catalytic degradation in the flow system. The TOC value of the solution was measured before degradation (t = 0 min) at an average of 38 ± 2.5 mg C L−1. Following the flow process, the final TOC was measured at an average of 8 ± 2.5 mg L−1, signifying a ∼79% removal of carbon content in the sample. It should be highlighted that this value is substantially higher than the previously reported batch results.14
Beyond analysing the degradation products, we sought to assess this system's ability in generating TMP degradation products (DPs) with no antibacterial activity against E. coli and without inducing bacterial resistance development. We should once again emphasize the need to develop continuous-flow oxidative processes capable of degrading antibiotics in wastewater because it is well established that their presence, even in small quantities, leads to the development of multidrug-resistant bacteria. Our study makes a significant contribution to addressing this issue, but it is crucial to determine whether the 20% of remaining degradation products (DPs) (TOC values) continue to induce resistance to TMP. We consider that the best way to combat antimicrobial resistance and simultaneously avoid ecological risks is to reach high TOC removals.
In this regard, we determined (i) the minimum inhibitory concentration (MIC) of TMP and (ii) the inhibitory activity of a 1 mg mL−1 solution of DPs on E. coli growth. The obtained MIC value for TMP (0.488 μg mL−1) falls within the expected range for an E. coli strain susceptible to TMP.22 Notably, the DP solution exhibited no significant inhibitory activity against E. coli, due to the degradation of the antibiotic and to the absence of antibiotic activity by the degradation products.
To compare the potential of TMP degradation products (DPs) and TMP itself to induce E. coli resistance, we conducted a resistance-inducing assay over 9 days. We exploited the concept that continuous exposure to sub-inhibitory antibiotic concentrations is a major driver of bacterial resistance development (details in Fig. S10, ESI†). The assay compared the ability of E. coli to develop resistance to TMP when exposed to: (i) (MIC/2) of TMP (∼0.244 μg mL−1) and (ii) a solution containing 1 mg mL−1 of degradation products (DPs). The results, presented as MIC of TMP as a function of the number of days of E. coli exposure to TMP and DPs are shown in Fig. 3.
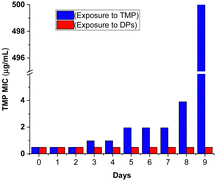 | ||
| Fig. 3 Resistance of E. coli to TMP after continuous exposure to sub-inhibitory concentrations of TMP (blue bars) and DPs (red bars). | ||
Our study demonstrates a progressive increase in TMP MIC values for bacteria exposed to sub-inhibitory TMP concentrations. Initially, the MIC remained constant at ∼0.49 μg mL−1 for the first two days. Then, a stepwise increase was observed, reaching ∼1.95 μg mL−1 by day 5, and ∼3.91 μg mL−1 by day 8. This culminated in a dramatic rise to ∼500.0 μg mL−1 by day 9. This finding aligns with previous reports highlighting the ability of TMP to induce bacterial resistance.21 Conversely, the group exposed to the DP solution exhibited no detectable resistance development throughout the 9-day experiment. Notably, by day 9, the MIC of bacteria exposed to TMP in the control group (group A – blue) was approximately 1000-fold higher compared to the bacteria exposed to DP (group B – red).
Thus, this study confirms that the high TOC levels, along with the presence of 20% degradation products, did not promote AMR development. Furthermore, no increase in ecotoxicity to V. fischeri was observed in our previous batch studies,14 which is consistent with findings from other researchers.24
In essence, this study established a highly efficient continuous flow system for trimethoprim degradation using green hydrogen peroxide as the oxidant. This system innovatively employs a fixed-bed continuous flow reactor packed with a covalently immobilized manganese(III) meso-substituted porphyrin immobilized on functionalized aminopropyl silica gel as the catalyst. The system demonstrates exceptional activity and stability, maintaining its activity over extended operation time (8 hours) and achieving an impressive TOC reduction of 80%. Notably, the degradation process effectively transforms TMP into products that lack the ability to induce bacterial resistance.
This study presents a promising approach for treating hospital wastewater. In collaboration with an engineering team and IPO Clinic, we are optimizing the reactor design for larger pre-treated effluent flows. Key parameters under evaluation include pressure drop, residence time, and mass and heat transfer, all essential for the efficient operation of scaled-up packed bed reactors.
FCT-Foundation for Science and Technology, I. P. (UIDB/00313/2020, UID/BIA/04004/2020); PRR-Recovery and Resilience Plan and by the European Union NextGeneration EU Funds through project 6979 – PRODUTECH R3 [Recuperação-Resiliência-Reindustrializacao]; G. P. thanks PhD grant (PD/BD/135532/2018). We thank IPO Coimbra for allowing the hospital wastewater analysis.
Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. P. Hays, M. J. Ruiz-Alvarez, N. Roson-Calero, R. Amin, J. Murugaiyan, M. B. M. van Dongen and A. M. R. I. A. N. Global, Infect. Dis. Ther., 2022, 11, 1315–1326 Search PubMed.
- V. Kasimanickam, M. Kasimanickam and R. Kasimanickam, Med. Sci., 2021, 9, 14 CAS.
- I. N. Okeke, M. E. A. de Kraker, T. P. Van Boeckel, C. K. Kumar, H. Schmitt, A. C. Gales, S. Bertagnolio, M. Sharland and R. Laxminarayan, Lancet, 2024, 403, 2426–2438 CrossRef CAS PubMed.
- M. L. Nadimpalli, C. W. Chan and S. Doron, Nat. Med., 2021, 27, 187–188 CrossRef CAS PubMed.
- N. A. Khan, A. H. Khan, P. Tiwari, M. Zubair and M. Naushad, J. Water Process Eng., 2021, 44, 102440 CrossRef.
- W.-L. Wang, Q.-Y. Wu, N. Huang, Z.-B. Xu, M.-Y. Lee and H.-Y. Hu, Water Res., 2018, 141, 109–125 CrossRef CAS PubMed.
- G. Piccirillo, R. T. Aroso, F. M. S. Rodrigues, R. M. B. Carrilho, S. M. A. Pinto, M. J. F. Calvete and M. M. Pereira, Catalysts, 2021, 11, 1335 CrossRef CAS.
- S. R. D. Gamelas, J. P. C. Tomé, A. C. Tomé and L. M. O. Lourenço, Catal. Sci. Technol., 2024, 14, 2352–2389 RSC.
- G. Piccirillo, N. Maldonado-Carmona, D. L. Marques, N. Villandier, C. A. Calliste, S. Leroy-Lhez, M. E. S. Eusébio, M. J. F. Calvete and M. M. Pereira, Catal. Today, 2023, 423, 113903 CrossRef CAS.
- V. A. Larson, B. Battistella, K. Ray, N. Lehnert and W. Nam, Nat. Rev. Chem., 2020, 4, 404–419 CrossRef CAS PubMed.
- M. M. Pereira, L. D. Dias and M. J. F. Calvete, ACS Catal., 2018, 8, 10784–10808 CrossRef CAS.
- P. D. Harvey, J. Mater. Chem. C, 2021, 9, 16885–16910 RSC.
- S. R. D. Gamelas, J. P. C. Tomé, A. C. Tomé and L. M. O. Lourenço, RSC Adv., 2023, 13, 33957–33993 RSC.
- G. Piccirillo, M. Moreira-Santos, M. Valega, M. E. S. Eusebio, A. M. S. Silva, R. Ribeiro, H. Freitas, M. M. Pereira and M. J. F. Calvete, Appl. Catal., B, 2021, 282, 195562 CrossRef.
- M. Guidi, P. H. Seeberger and K. Gilmore, Chem. Soc. Rev., 2020, 49, 8910–8932 RSC.
- Y. Saito, K. Nishizawa, B. Laroche, H. Ishitani and S. Kobayashi, Angew. Chem., Int. Ed., 2022, 134, e202115643 CrossRef.
- G. Vile, Catal. Sci. Technol., 2021, 11, 43–61 RSC.
- J. Leichtweis, E. Carissimi, U. Hagemann, M. Ulbricht and L. Fischer, Chem. Eng. J., 2023, 477, 160603 CrossRef.
- A. M. Gorito, A. R. L. Ribeiro, P. Rodrigues, M. F. R. Pereira, L. Guimaraes, C. M. R. Almeida and A. M. T. Silva, Water Res., 2022, 218, 118497 CrossRef CAS PubMed.
- S. Ahmad, W. Sebai, M. P. Belleville, N. Brun, A. Galarneau and J. Sanchez-Marcano, Catal. Today, 2021, 362, 62–71 CrossRef CAS.
- J. Domachowske and M. Suryadevara, Overview of Antibiotics, Springer Publishing, Cham, 2020 Search PubMed.
- N. Kraupner, S. Ebmeyer, M. Hutinel, J. Fick, C.-F. Flach and D. G. J. Larsson, Environ. Int., 2020, 144, 106083 CrossRef CAS.
- H. Targhan, P. Evans and K. Bahrami, J. Ind. Eng. Chem., 2021, 104, 295–332 CrossRef CAS.
- I. Michael, E. Hapeshi, V. Osorio, S. Perez, M. Petrovic, A. Zapata, S. Malato, D. Barceló and D. Fatta-Kassinos, Sci. Total Environ., 2012, 430, 167–173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Report on the presence of pharmaceuticals in effluents; catalyst syntheses and characterization, degradation experiments, HPLC chromatograms and microbiological studies. See DOI: https://doi.org/10.1039/d4cc04504a |
| This journal is © The Royal Society of Chemistry 2025 |