Micro- and nanoplastic-mediated phototransformation and bioaccessibility of fluorinated liquid crystal monomer in aquatic environments†
Yiping
Feng
 *a,
Jingyi
Wu
a,
Wenhao
Lao
a,
Weibiao
Ye
a,
Danni
Guo
b,
Zhu
Wang
*b,
Xiaowei
Wu
*c and
Racliffe Weng Seng
Lai
d
*a,
Jingyi
Wu
a,
Wenhao
Lao
a,
Weibiao
Ye
a,
Danni
Guo
b,
Zhu
Wang
*b,
Xiaowei
Wu
*c and
Racliffe Weng Seng
Lai
d
aGuangdong Key Laboratory of Environmental Catalysis and Health Risk Control, School of Environmental Science and Engineering, Institute of Environmental Health and Pollution Control, Guangdong University of Technology, Guangzhou 510006, China. E-mail: ypfeng@gdut.edu.cn
bInstitute of Environmental Research at Greater Bay, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou 510006, China. E-mail: wangzhu@gzhu.edu.cn
cSchool of Environmental Science and Engineering, Nanjing University of Information Science and Technology, Nanjing 210044, China. E-mail: 003836@nuist.edu.cn
dDepartment of Ocean Science and Technology, Faculty of Science and Technology, University of Macau, Macau 999078, China
First published on 10th December 2024
Abstract
Micro- and nanoplastics are emerging pollutants that have attracted significant attention due to their potential to concentrate and transport coexisting organic pollutants in aquatic environments. Fluorinated liquid-crystal monomers (FLCMs) have also emerged as contaminants of concern, given their frequent occurrence, potential toxic effects, and propensity to co-occur with plastics in the environment. However, the influence of plastics on the environmental fate of FLCMs remains unclear yet. To address this knowledge gap, we investigated the accumulation of a key FLCM, 4-cyano-3-fluorophenyl 4-ethylbenzoate (CEB-F), on three common plastics, and examined the effects of nanoplastics on the phototransformation of CEB-F and its acute toxicity to Daphnia magna (D. magna). Our findings revealed that the adsorption capacity of CEB-F on different plastic materials followed the order: polystyrene (PS) < mixed cellulose ester (MCE) < polyamide (PA). The adsorption processes of CEB-F on the three plastics aligned more closely with the pseudo-first-order kinetic model and the Langmuir isotherm model, suggesting that the adsorption is primarily governed by physical diffusion. Theoretical calculations indicated that the adsorption of CEB-F on PS plastics is mainly driven by hydrophobic interactions. Additionally, PS nanoplastics (PSNPs) significantly enhanced the UV degradation of CEB-F, although the types of degradation intermediates did not change substantially, suggesting a limited impact on the degradation process and mechanism. Acute toxicity tests showed that PSNPs increased the toxicity of CEB-F to D. magna at lower concentrations, while the toxicity was reduced at higher concentrations. The obtained findings are of great significance to unraveling the plastic-mediated environmental fate and aquatic toxicity of FLCMs in natural waters.
Environmental significanceMicro- and nanoplastic contamination has received a lot of attention because of its ability to concentrate and transfer organic contaminants in aquatic settings. Fluorinated liquid-crystal monomers (FLCMs) have also emerged as contaminants of concern due to their widespread prevalence, potential hazardous effects, and proclivity to coexist alongside plastics in the environment. Thus, it is critical to investigate the impact of micro- and nanoplastics on the environmental fate of FLCMs. In this investigation, we discovered that FLCMs were readily absorbed by common micro- and nanoplastics, primarily due to hydrophobic interactions. Nanoplastics considerably increased the phototransformation of FLCMs while playing complex roles in their toxicity to D. magna. The current findings are of tremendous significance to unraveling the plastic-mediated environmental destiny and aquatic toxicity of organic contaminants. |
1. Introduction
Liquid crystal monomers (LCMs) are essential components of liquid crystal displays (LCDs), typically sealed between two glass substrates or other suitable materials to provide protection and support.1 Numerous studies have reported the widespread application of LCMs in the electronic industry, including computers, televisions, and smartphones. Global production of LCMs increased from 500 tons in 2011 to 1300 tons in 2021, with continued growth expected.2 Uncontrolled production and poor management of LCD products can lead to the release of abundant LCMs into the environment, particularly through e-waste recycling.3 Upon entry into the environments, released LCMs from LCDs are readily to undergo surface runoff, atmospheric deposition, and accumulation in global environmental matrices such as sediments,4 soil,5 and indoor dust.6 Additionally, due to their structural similarities with persistent organic pollutants like polybrominated diphenyl ethers (PBDEs) and organic phosphates (OPEs), LCMs can bioaccumulate in biota7 and potentially pose risks to human health and wildlife via food chains.2,8 From a public health and biodiversity protection perspective, it is crucial to understand the environmental fate and potential ecological risks associated with LCMs.Among the many LCM congeners synthesized and commercialized in current global markets, fluorinated liquid-crystal monomers (FLCMs) are particularly significant due to their high voltage retention, high resistivity, and good stability. FLCMs typically possess a biphenyl backbone structure with a range of chemical substitutions (i.e., alkyne, cycloethyl, cyano, ester, and fluorine substitutions), and have higher toxicity compared to other LCMs.9 This structure results in air–water partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kaw) ranging from −5 to −1 and octanol–water partition coefficients (log
Kaw) ranging from −5 to −1 and octanol–water partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) of the 27 FLCMs higher than 5, making FLCMs prone to widespread distribution and potential toxic risk to human health.10 However, the fate and toxic effects of FLCMs in the environment are still not fully understood, despite their ubiquitous presence, as indicated by previous research.
Kow) of the 27 FLCMs higher than 5, making FLCMs prone to widespread distribution and potential toxic risk to human health.10 However, the fate and toxic effects of FLCMs in the environment are still not fully understood, despite their ubiquitous presence, as indicated by previous research.
Micro- and nanoplastics, defined as plastic particles ranging from 1 μm to 5 mm and less than 1 μm, respectively, have garnered global concern due to their persistence in natural waters and toxic effects on biota, including human bodies.11 These plastic particles can readily adsorb organic and inorganic contaminants, such as heavy metals, dyes, pesticides, drugs, and antibiotics, forming plastic–contaminant complexes through electrostatic forces, hydrophobic interaction, hydrogen bonding, π–π conjugation, and micropore filling.12 Moreover, the universal presence of micro- and nanoplastics can alter the photochemical behavior of organic contaminants in aquatic environment.13,14 For example, the phototransformation of atorvastatin (ATV) has been shown to positively correlate with the carbonyl index of photoaged polystyrene (PS) microplastics, as aged microplastics with more oxygen-containing functional groups (e.g., alcohols, aldehydes, carboxylic acids, and esters) can produce more reactive oxygen species (ROS) like singlet oxygen (1O2) and triplet-excited state PS (3PS*), thus enhancing ATV phototransformation rates in water.15
Furthermore, it was also found that micro- and nanoplastics can also alter the toxicity of organic contaminants in water. For instance, it has been reported that PS nanoplastics reduced the toxicity of polycyclic aromatic hydrocarbons (PAHs), as PAHs adhered to the plastic surface, decreasing their bioavailability and subsequent toxicity.16 Given these insights, we hypothesize that micro- and nanoplastics can significantly influence the fate and toxicity of organic compounds including LCMs in aquatic environments. However, the mechanisms underlying the effects of micro- and nanoplastics on the phototransformation and toxicity of LCMs in water remain largely unexplored.
This study focused on the micro- and nanoplastic-mediated phototransformation and toxic effects of FLCMs in aquatic environments, since both of which have emerged as significant environmental concerns.7,17 Notably, various FLCMs have been detected in aquatic environments at concentrations of up to 1120 ng L−1,18 highlighting their substantial potential to interact with widespread plastic particles. Consequently, it is essential to examine the possible interactions between these contaminants and assess their environmental fate and risks when coexisting in aquatic systems. Using 4-cyano-3-fluorophenyl 4-ethylbenzoate (CEB-F) as a model LCM compound,19 this study aimed to: (i) explore the carrier effect of micro- and nanoplastics on CEB-F in water; (ii) elucidate the micro- and nanoplastic-mediated phototransformation of CEB-F, and (iii) investigate the bioaccessibility and toxic effects of the plastic–CEB-F complex. The findings will contribute to our understanding of the fate and potential ecological risks of CEB-F associated with plastic particles in natural waters.
2. Materials and methods
2.1. Chemicals and reagents
Mixed cellulose ester (MCE), polyamide (PA), and polystyrene (PS) plastic pellets used in this experiment were obtained from common plastic products in life. Nano-sized polystyrene (PSNPs, 0.05–0.1 μm) was purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). 4-Cyano-3-fluorophenyl-4-ethylbenzoate (CEB-F, >97% purity) was purchased from Bide Pharmatech Ltd. (Shanghai, China). Sodium sulfite (Na2SO4, ≥99% purity), sodium nitrate (NaNO3, ≥99% purity), sodium bicarbonate (NaHCO3, ≥99.8% purity), and sodium chloride (NaCl, ≥99.5% purity) of analytical grade were purchased from Shanghai Titanchem Co., Ltd (Shanghai, China). HPLC-grade acetic acid and acetonitrile were supplied by Aladdin (Shanghai, China). Deionized (DI) water prepared from a Millipore Milli-Q system (>18.2 MΩ cm−1) was used for solution preparation. All other chemicals and reagents were of analytical grade. A stock solution of CEB-F at 5.0 mM was prepared in acetonitrile and stored at 4 °C for further use.2.2. Characterization of micro- and nanoplastics
The surface morphology of MCE, PA, and PS plastics was examined through scanning electron microscopy (SEM, Hitachi S4800, Japan). The chemical structures of the four types of plastics were analyzed using Fourier-transform infrared spectroscopy (FT-IR, Thermo Nicolet 6700) via a pressurization method. The surface chemical composition and elemental binding energies were determined through X-ray photoelectron spectroscopy (XPS, Thermo VG ESCALAB 250, USA) with monochromatic Al Kα radiation. Zeta potential measurements at different pH levels and at 25 °C were determined using a Malvern Zetasizer Nano series Nano-ZS (MA, USA), which were computed using Zetasizer Software (Version 7.10, MA, USA) based on Henry's function and the Smoluchowski approximation. Additionally, the hydrophobic properties, represented by the water contact angle, were measured with a dynamic contact angle instrument (JY-82B Kruss DSA, Dataphysics OCA20, Shimadzu, Japan).2.3. Batch adsorption experiments
Regarding the adsorption kinetics of different types of plastics (i.e., MCE, PA, and PS) to CEB-F in water, we exposed 10 mg L−1 of plastics and 10 μM of CEB-F and incubated at 125 rpm in a shaker that operated at 25 °C. Then, 1 mL of the reaction solution was removed at 5, 10, 30, 45, 60, 90, 120, 180, 240, and 360 min, respectively, and determined the residue CEB-F within the reaction system. In addition, to illustrate the impact of water constituents (pH, anion, and salinity) on the sorption of PSNPs to CEB-F in water, we mixed 10 mg L−1 of PSNPs with different pH (3, 4, 5, 6, 7, 8, and 9, adjusted using 0.01 mol L−1 NaOH/HCl), 10 mM ions (Cl−, SO42−, NO3−, Mg2+, and Ca2+) and salinity (0.10%, 0.50%, 1.00%, 2.00%, and 3.00%).The sorption amount of plastics to CEB-F can be calculated as follows (eqn (1)),
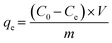 | (1) |
Pseudo-first- and second-order kinetic, as well as intraparticle diffusion models were applied for the sorption kinetic analysis of CEB-F to different types of plastics, with the equations as follows (eqn (2) to (4)),
| qt = qe(1 − e−k1t) | (2) |
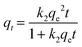 | (3) |
| qt = kit0.5 + c | (4) |
Adsorption isotherm of plastics to CEB-F was conducted by mixing 10 mg L−1 of PSNPs with 5, 10, 20, 40, and 80 μM of CEB-F and shaken for 240 min at 125 rpm and 25 °C. Then 1 mL of aquatic solution was removed from the reaction system and deterring residue CEB-F concentration. Langmuir (eqn (5)) and Freundlich (eqn (6)) models were utilized fitting the sorption amount of plastics to CEB-F in water, with the specific calculation strategies as follows (eqn (5) and (6)),
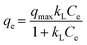 | (5) |
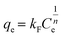 | (6) |
2.4. Phototransformation experiments
The phototransformation of CEB-F in water was conducted using a photochemical reactor (XPA-7, Xujiang, Nanjing) which coupled with an 800 W xenon lamp (UV intensity: 16.76 mW cm−2, wavelength range 200–1000 nm) and a 300 W medium pressure mercury lamp (UV intensity at 5.0 mW cm−2, with the wavelength range of 200–400 nm) to simulate sunlight and ultraviolet light irradiation, respectively. The reaction temperature was maintained at 25 °C using a cyclical cooling water system. PBS buffer was used to maintain the stability of the solution's pH. At 0, 1, 2, 4, 6, 10, 15, and 30 min of UV irradiation, 1 mL of the reaction solution was sampled and mixed with 2 mL of methanol to desorb any chemicals adhered to PSNPs. The mixture was then passed through a 0.45 μm membrane filter and analyzed using high-performance liquid chromatography (HPLC).The quantification of CEB-F was achieved by a Shimadzu LC-20AT HPLC (Shimadzu, Japan) equipped with a UV detector and a 4.6 × 250 mm Agilent Zorbax SB-C18 reverse-phase column (Agilent, USA) maintained at 35 °C. The UV detector wavelength was set at 254 nm, and the injection volume was set at 20 μL. The isocratic mobile phase was made up of 80% acetonitrile and 20% DI water (with 0.3% acetic acid) with a flow rate of 1.0 mL min−1. The phototransformation intermediates of CEB-F were identified using an ultra-high-performance liquid chromatography-tandem triple quadrupole mass spectrometer (UPLC-Q-Exactive Orbitrap-MS, Thermo, Bremen, Germany) with a Hypersil GOLD C18 column (100 × 2.1 mm, 1.9 μm). The mobile phase consisted of 0.1% formic acid in deionized water and acetonitrile (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v), with a flow rate of 0.3 mL min−1. The injection volume and the column temperature were maintained at 10 μL and 35 °C, respectively.
20, v/v), with a flow rate of 0.3 mL min−1. The injection volume and the column temperature were maintained at 10 μL and 35 °C, respectively.
2.5. Computational methods
The interaction and binding of CEB-F with PS plastics were calculated using the Forcite module in the Materials Studio software. The PS molecule was modeled with a polymerization degree of 20 repeating units, consisting of 160 carbon atoms and 162 hydrogen atoms. The initial model structure was optimized using the Smart algorithm, followed by molecular dynamics simulations. The simulations were carried out under NPT conditions at 298 K and 1 atm pressure, with a total equilibrium time of 100 ps. The COMPASS II force field was applied, using the Berendsen thermostat for temperature control and the Berendsen barostat for pressure control,20 with a time step of 1 fs. To ensure accurate simulation results, the system was considered equilibrated when temperature and energy fluctuations during the final NPT molecular dynamics simulation were within the range of 5% to 10%.212.6. Daphnia magna exposure experiments
The bioaccessibility and toxicity of CEB-F mediated by plastics to aquatic organisms were assessed using Daphnia magna as an indicator species, following the standard protocol outlined in OECD Guideline No. 202. Third-brood neonates (<24 h old) were used for the toxicity tests. Prior to the experiments, Daphnia magna was cultured at 24 °C with a daily light/dark cycle of 8 h/16 h. During the bioaccessibility and toxicity experiments, 10 daphnids were exposed to 20 mL of CEB-F at concentrations of 0, 0.2, 0.5, 1.0, 1.5, and 5 μM, with 0, 2 mg L−1, and 5 mg L−1 PSNPs, respectively. The survival rate was recorded after 96 hours of incubation without feeding. Catalase (CAT) and acetylcholinesterase (AChE) activities (expressed as U mg−1 protein) were measured using commercial kits from Beijing Boxbio Science & Technology Co., Ltd., following the manufacturer's instructions. Protein content was determined by the Bradford method.22,232.7. Statistical analysis
Each test was repeated at least three times, and finally presented as the mean ± standard deviation. Statistical analyses related to CEB-F sorption kinetics and isotherms were conducted using Origin 2018 software. Differences were statistically analyzed by one-way analysis of variance (ANOVA) followed by the LSD test for multiple comparisons (p < 0.05, n = 3) using SPSS 18.0 (IBM Company).3. Results and discussion
3.1. Characterization of micro- and nanoplastics
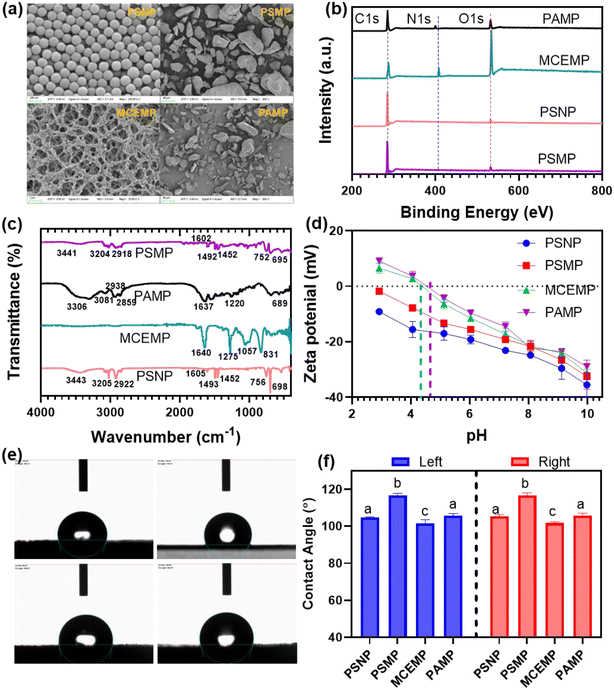
The surface morphology of PS, MCE, and PA microplastics (MP) and PS nanoplastics (NP) was analyzed using SEM, as shown in Fig. 1a. The purchased PSNPs are spherical particles with smooth surfaces and an average size of approximately 80 nm. Both PSMPs and PAMPs exhibited smooth bulk structures, while MCEMPs displayed a distinctive cross-linked porous structure. The size distributions for these plastics ranged from 1 to 300 μm. The surface elemental compositions of the four plastics were characterized via XPS (Fig. 1b). The survey spectra revealed the presence of C and O on the surfaces of PSNPs and PSMPs, whereas MCEMPs and PAMPs also contained N (Fig. 1b). High-resolution XPS spectra of C 1s, O 1s, and N 1s for all four plastics are shown in Fig. S1.† PSNPs and PSMPs exhibited three distinct C 1s peaks at 284.2, 285.8, and 291.6 eV (Fig. S1a and b†), corresponding to aromatic C–H, aliphatic C–H, and π–π* transitions, respectively.24,25 MCEMPs displayed four C 1s peaks at 284.6, 286.2, 287.0, and 288.1 eV (Fig. S1c†), attributed to aromatic C–H, aliphatic C–H, C–O/C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.26 Similarly, PAMPs showed three peaks at 284.4, 285.8, and 287.8 eV (Fig. S1d†), representing aliphatic C–H, C–N, and C
O groups.26 Similarly, PAMPs showed three peaks at 284.4, 285.8, and 287.8 eV (Fig. S1d†), representing aliphatic C–H, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.27 The O 1s peak fitting for four plastics is similar, showing all have –OH, –C–O, and –O–C
O groups.27 The O 1s peak fitting for four plastics is similar, showing all have –OH, –C–O, and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. MCEMPs showed three N 1s peaks at 398.5, 407.5, and 408.2 eV, corresponding to C–N, –NO2, and –ONO2 groups.26 For PAMPs, two peaks at 399.1 and 399.8 eV were attributed to N–H and C–N groups, respectively (Fig. S1d†).
O groups. MCEMPs showed three N 1s peaks at 398.5, 407.5, and 408.2 eV, corresponding to C–N, –NO2, and –ONO2 groups.26 For PAMPs, two peaks at 399.1 and 399.8 eV were attributed to N–H and C–N groups, respectively (Fig. S1d†).
Fig. 1c depicts the functional groups distribution of four plastics as characterized by FT-IR. PSNP and PSMP include 3440 (–OH), 3082–2849 (–CH2), 1740 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1600–1450 (C
O), 1600–1450 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1372 (C
C), 1372 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1069 (–C–O) cm−1, respectively. PAMPs has the peaks at 3306 (–OH, –NH), 3081–2859 (–CH2), 1637–1461 (C
O), 1069 (–C–O) cm−1, respectively. PAMPs has the peaks at 3306 (–OH, –NH), 3081–2859 (–CH2), 1637–1461 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1371 (C
C), 1371 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1, while MCEMP possess peaks at 3400 (–OH), 1748 (O–C
O) cm−1, while MCEMP possess peaks at 3400 (–OH), 1748 (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1640 (C
O), 1640 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1373 (C
O), 1373 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NO2), 1275 (C–N), 1069 (–C–O) cm−1, respectively.24–28 Zeta potential measurements in Fig. 1d revealed that both PSNP and PSMP carried a negative charge across a pH range of 2.0 to 10.0. In contrast, MCEMPs and PAMPs exhibited positive surface charges under acidic conditions, with zero-point charge (pHZPC) values of 4.18 and 4.35, respectively.
O, –NO2), 1275 (C–N), 1069 (–C–O) cm−1, respectively.24–28 Zeta potential measurements in Fig. 1d revealed that both PSNP and PSMP carried a negative charge across a pH range of 2.0 to 10.0. In contrast, MCEMPs and PAMPs exhibited positive surface charges under acidic conditions, with zero-point charge (pHZPC) values of 4.18 and 4.35, respectively.
Furthermore, water contact angle was measured to evaluate the hydrophobicity of the four plastics,15 as shown in Fig. 1e and calculated in Fig. 1f. Water contact angles of PSNP, PSMP, MCEMP, and PAMP were 104.7°, 116.6°, 101.5°, and 105.7°, respectively, indicating the hydrophobicity of these four plastics followed the order of PSMP > PSNP ≈ PAMP > MCEMP.
3.2. Carrier effect of micro- and nano-plastics to CEB-F in aqueous solution
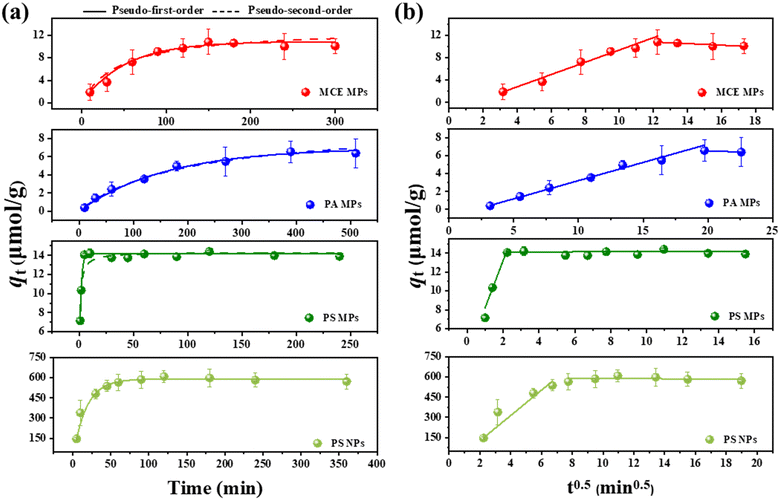
The adsorption behaviors of CEB-F by commonly present plastics, specifically PS, PA, and MCE were compared. As shown in Fig. 2a and Table S1,† the pseudo-first-order kinetic model provided higher correlation coefficients than the pseudo-second-order kinetic model for all three types of plastics, indicating that the adsorption of CEB-F was primarily driven by physical adsorption. The results from the intra-particle diffusion model (Fig. 2b) revealed that the linear fits did not pass through the origin for any of the three materials, suggesting that both surface adsorption and intra-particle diffusion played roles in the actual adsorption process of CEB-F on these plastics.Initially, approximately 80% of CEB-F was adsorbed onto the plastics, likely due to multiphase adsorption interactions such as hydrophobic interactions, covalent bonds, and van der Waals forces, which occupied the external activation sites. During this stage, the significant thickness of the plastic boundary layer highlighted the importance of surface adsorption in the process.29–31 Additionally, the qt and t0.5 did not pass through the origin, indicating that the rate-limiting step was influenced by both membrane diffusion and intra-particle diffusion.30 In the second stage, only 10% of CEB-F continued to adsorb on the plastic surface. This phase involved slow diffusion from the liquid film to the microporous surface. However, due to the relatively small diameter of the micropores and the large molecular size of CEB-F, the intra-particle diffusion rate was markedly slower than in the initial stage.29,30
The pseudo-first-order model's simulated sorption rate constants for CEB-F on the three plastic MPs (MCE, PA, PS) in water were 0.0193, 0.0062, and 0.6609 min−1, respectively. The pseudo-second-order model's simulated rate constants for three plastics were 0.0017, 0.0006, and 0.0914 μmol g−1 min−1, respectively. These results indicate that the sorption rate of CEB-F on PS MPs in water was higher than on MCE and PA MPs. However, according to the pseudo-first-order simulation results, the equilibrium sorption amounts of CEB-F on the three plastics were ranked as follows: PS (14.1503 μmol g−1) > MCE (10.8453 μmol g−1) > PA (6.9769 μmol g−1). These findings suggest that PS plastics have a high potential to adsorb CEB-F onto their surface in water, forming plastic–contaminant complexes.
Previous studies have estimated and demonstrated that the octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) values of most LCMs exceed 5, highlighting their high hydrophobicity. This property suggests a strong tendency for these compounds to adsorb onto surfaces with similar hydrophobic characteristics, such as sediments and fatty tissues in organisms.4,32,33 Furthermore, prior research has shown that the adsorption of hydrophobic pollutants, such as tylosin, onto microplastics like PE, PP, PS, or PVC is primarily driven by hydrophobic interactions.34 The higher adsorption of CEB-F onto PSMP can be explained by the fact that PSMP is more hydrophobic than PAMP and MCEMP, according to measurements of water contact angles (Fig. 1e and f). Additionally, the SEM image in Fig. 1a showed that MCEMPs possess a porous structure, which facilitates the capture of CEB-F, further contributing to its adsorption potential.35 In this study, the adsorption capacity of the three typical plastics for CEB-F reached the μmol g−1 level under simulated conditions, aligning with most studies on persistent organic pollutants (POPs).36 Despite environmental concentrations being lower than the modeled concentrations, plastics were still demonstrated to be significant carriers of FLCMs.
Kow) values of most LCMs exceed 5, highlighting their high hydrophobicity. This property suggests a strong tendency for these compounds to adsorb onto surfaces with similar hydrophobic characteristics, such as sediments and fatty tissues in organisms.4,32,33 Furthermore, prior research has shown that the adsorption of hydrophobic pollutants, such as tylosin, onto microplastics like PE, PP, PS, or PVC is primarily driven by hydrophobic interactions.34 The higher adsorption of CEB-F onto PSMP can be explained by the fact that PSMP is more hydrophobic than PAMP and MCEMP, according to measurements of water contact angles (Fig. 1e and f). Additionally, the SEM image in Fig. 1a showed that MCEMPs possess a porous structure, which facilitates the capture of CEB-F, further contributing to its adsorption potential.35 In this study, the adsorption capacity of the three typical plastics for CEB-F reached the μmol g−1 level under simulated conditions, aligning with most studies on persistent organic pollutants (POPs).36 Despite environmental concentrations being lower than the modeled concentrations, plastics were still demonstrated to be significant carriers of FLCMs.
Further comparison between the adsorption of CEB-F by PSMPs and PSNPs revealed that, although the simulated pseudo-first-order sorption rate constant for PSNPs (0.0570 min−1) was much lower than that for PSMPs (0.6609 min−1), the equilibrium sorption amount for PSNPs (589.41 μmol g−1) was significantly higher than that for PSMPs (14.15 μmol g−1). This indicates that nanosized plastics have a high potential to adsorb CEB-F onto their surface in water, forming plastic–contaminant complexes.
The sorption affinity of PS MPs and NPs for CEB-F in water was explored through adsorption isotherms constructed at different CEB-F concentrations. As shown in Fig. S2,† the sorption amount of CEB-F by both PS MPs and NPs increased continuously with rising CEB-F concentrations in water. The fitting results of the Langmuir, Freundlich, and Henry isotherm models for the adsorption of CEB-F by plastics (Table S2†) indicated that the Langmuir isotherm model best described the adsorption process for both PS MPs (R2 = 0.9998) and PS NPs (R2 = 0.9851), compared to the Freundlich and Henry isotherm models. This suggests that surface adsorption is the primary mechanism for CEB-F adsorption by PS MPs and NPs in water. Overall, the sorption kinetics and isotherm model results emphasize that PS MPs and NPs are crucial carriers for CEB-F in aquatic environments.
3.3. Influence of the initial pH, ions, and salinity on CEB-F adsorption by nanoplastics
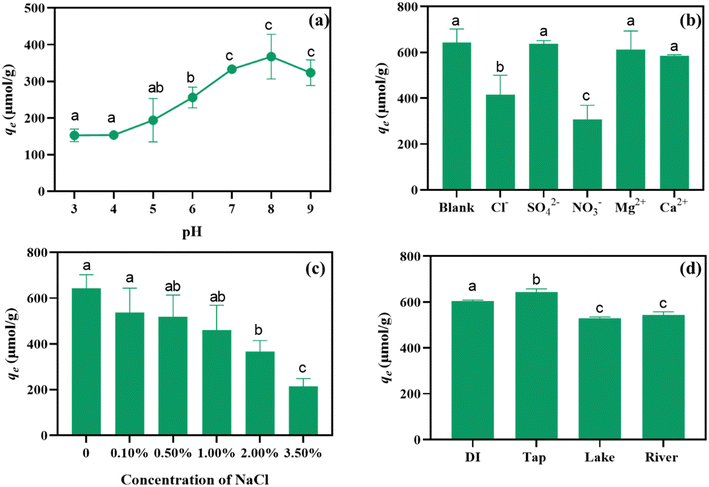
By using PSNPs as the represent, we further examined the influence of nature water constituents and properties (e.g., pH, ions, and salinity) on CEB-F adsorption by nanoplastics. pH is a crucial environmental factor that affects both the morphology of the adsorbent and its surface physicochemical properties.37 As shown in Fig. 3a, the adsorption capacity of PSNPs for CEB-F initially increases and then decreases as the pH rises from 3.0 (153 μmol g−1) to 9.0 (324 μmol g−1), with the maximum adsorption capacity observed at pH 8.0 (368 μmol g−1). Overall, the sorption affinity of CEB-F to PSNPs is higher in alkaline conditions (pH > 7) compared to acidic conditions (pH < 7). Zeta potential measurements in Fig. 1d indicate that PSNPs were negatively charged across the pH range of 2.0 to 10.0, with the zeta potential values gradually decreasing as pH increased. Under alkaline conditions, the surface of PSNPs exhibited a greater negative charge, which may enhance electronic interactions such as the π–π electron donor–acceptor effect and hydrogen bonding between PSNPs and CEB-F.38–40 Additionally, as the pH increased, the stronger negative charge on PSNPs likely reduced homoaggregation due to increased electrostatic repulsion. This reduction in aggregation is favorable for improving CEB-F adsorption onto PSNPs.41To unravel the effect of anions and cations in natural water on the adhesion of CEB-F to plastics, we compared the sorption capacity of PSNPs to CEB-F under 10 mM of Cl−, SO42−, NO3−, Mg2+, and Ca2+, respectively. In Fig. 3b, in comparison with blank controls (643 μmol g−1), the presence of Cl− and NO3− (415 and 307 μmol g−1) obviously inhibited the sorption capacity of PSNPs to CEB-F while the presence of SO42−, Mg2+, and Ca2+ (637, 613, and 586 μmol g−1) displayed exiguous effect on the sorption capacity of PSNPs to CEB-F.
In addition to pH and ions, solution salinity also affects the sorption affinity of PSNPs to CEB-F in water. As illustrated in Fig. 3c, the sorption capacity of PSNPs to CEB-F decreases continuously from 643 μmol g−1 to 215 μmol g−1 as the solution salinity increases from 0 to 3.5%. The mechanism behind salinity's impact on PSNPs adsorption is due to the increased solution salinity causing PSNP particles to aggregate, thereby reducing the specific surface area and the electric double layer of PSNPs. This aggregation decreases the available sorption sites for PSNPs CEB-F.42 For example, it has been observed that carboxylated polystyrene MPs with a particle size of 30 nm rapidly aggregate to sizes above 1000 nm in less than 30 minutes in seawater.43 Moreover, the presence of NaCl readily increases the viscosity and density of the solution, thereby inhibiting the mass transfer from the water phase to the solid phase.44 Consequently, higher aquatic salinity reduces the surface charge of PSNPs, diminishes the electrostatic interaction between CEB-F and PSNPs, and ultimately weakens the adsorption capacity of PSNPs for CEB-F in water. For better understanding the carrier effect of plastics particle on FLCMs in the aquatic environment, the adsorption ability of PSNPs to CEB-F in different natural water matrices (tap, river, and lake water, primary properties included in Table S3†) was examined. As shown in Fig. 3d, the adsorption amounts of CEB-F were not influenced by the mater matrix, confirming again that PSNPs are important carriers for FLCMs in natural surface water.
3.4. Sorption mechanism of PSNPs to CEB-F in water
To elucidate the sorption mechanism of PSNPs for CEB-F, FTIR was performed to assess changes in surface functional groups before and after adsorption. As depicted in Fig. S3,† pure CEB-F exhibits characteristic peaks at 1748 cm−1 (aryl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring vibrations), 1640 cm−1 (–C
C ring vibrations), 1640 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1275 cm−1 (C–F), 1057 cm−1 (C–O), and 839 cm−1 (C–H vibrations) cm−1, respectively. Pure PSNPs, on the other hand, display peaks at 3443 cm−1 (–OH), 3045–2922 cm−1 (–CH2), 1740 cm−1 (C
O), 1275 cm−1 (C–F), 1057 cm−1 (C–O), and 839 cm−1 (C–H vibrations) cm−1, respectively. Pure PSNPs, on the other hand, display peaks at 3443 cm−1 (–OH), 3045–2922 cm−1 (–CH2), 1740 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605–1452 cm−1 (C
O), 1605–1452 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1372 cm−1 (C
C), 1372 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1069 cm−1 (–C–O).45 After adoption of CEB-F, an increase in the characteristic peak at 1371 cm−1, was observed, suggesting the formation of C–N bonds,46 and an increase at 1067 cm−1, indicates the formation of C–F bonds. Additionally, the characteristic peak of PSNPs at 1605 cm−1 shifted to 1621 cm−1, possibly due to physical overlap resulting from CEB-F adsorption. However, due to the limited adsorption of CEB-F, the changes in these characteristic peaks were subtle. The surface interactions between PSNPs and CEB-F were further examined via high-resolution C 1s, O 1s, and F 1s XPS spectra of pure PSNPs, pure CEB-F, and the PSNP–CEB-F complex. The survey spectra confirmed the presence of C and O on PSNPs, while CEB-F exhibited C, O, N, and F (Fig. S4†). After adsorption, a weak F peak appeared, confirming the adsorption of CEB-F onto PSNPs. High-resolution XPS spectra of C 1s, O 1s, and F 1s are shown in Fig. S5.† PSNPs displayed three distinct C 1s peaks at 284.2, 285.8, and 291.6 eV, corresponding to aromatic C–H, aliphatic C–H, and π–π* transitions.24,25 Following adsorption, the π–π* transition peak at 291.6 eV shifted to a lower binding energy of 291.1 eV, indicating π–π electron donor–acceptor (EDA) interactions between the benzene rings of CEB-F and PSNPs.38–40 For O 1s spectra, the –OH, –C–O, and –O–C
O), and 1069 cm−1 (–C–O).45 After adoption of CEB-F, an increase in the characteristic peak at 1371 cm−1, was observed, suggesting the formation of C–N bonds,46 and an increase at 1067 cm−1, indicates the formation of C–F bonds. Additionally, the characteristic peak of PSNPs at 1605 cm−1 shifted to 1621 cm−1, possibly due to physical overlap resulting from CEB-F adsorption. However, due to the limited adsorption of CEB-F, the changes in these characteristic peaks were subtle. The surface interactions between PSNPs and CEB-F were further examined via high-resolution C 1s, O 1s, and F 1s XPS spectra of pure PSNPs, pure CEB-F, and the PSNP–CEB-F complex. The survey spectra confirmed the presence of C and O on PSNPs, while CEB-F exhibited C, O, N, and F (Fig. S4†). After adsorption, a weak F peak appeared, confirming the adsorption of CEB-F onto PSNPs. High-resolution XPS spectra of C 1s, O 1s, and F 1s are shown in Fig. S5.† PSNPs displayed three distinct C 1s peaks at 284.2, 285.8, and 291.6 eV, corresponding to aromatic C–H, aliphatic C–H, and π–π* transitions.24,25 Following adsorption, the π–π* transition peak at 291.6 eV shifted to a lower binding energy of 291.1 eV, indicating π–π electron donor–acceptor (EDA) interactions between the benzene rings of CEB-F and PSNPs.38–40 For O 1s spectra, the –OH, –C–O, and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of pure PSNPs were observed at 531.2, 532.0, and 532.9 eV, while for pure CEB-F, they were located at 531.8, 532.6, and 533.5 eV, respectively. After adsorption, these peaks for the PSNP–CEB-F complex shifted slightly to 531.6 eV (–OH), 532.5 eV (–C–O), and 533.2 eV (–O–C
O groups of pure PSNPs were observed at 531.2, 532.0, and 532.9 eV, while for pure CEB-F, they were located at 531.8, 532.6, and 533.5 eV, respectively. After adsorption, these peaks for the PSNP–CEB-F complex shifted slightly to 531.6 eV (–OH), 532.5 eV (–C–O), and 533.2 eV (–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), further confirming the interactions between CEB-F and the PSNP surface. The F 1s spectrum of CEB-F displayed a distinct C–F peak at 687.5 eV, which was absent in pure PSNPs. After adsorption, a weak C–F peak emerged at 687.8 eV, suggesting the possible formation of weak halogen bonds between PSNPs and CEB-F.34
O), further confirming the interactions between CEB-F and the PSNP surface. The F 1s spectrum of CEB-F displayed a distinct C–F peak at 687.5 eV, which was absent in pure PSNPs. After adsorption, a weak C–F peak emerged at 687.8 eV, suggesting the possible formation of weak halogen bonds between PSNPs and CEB-F.34
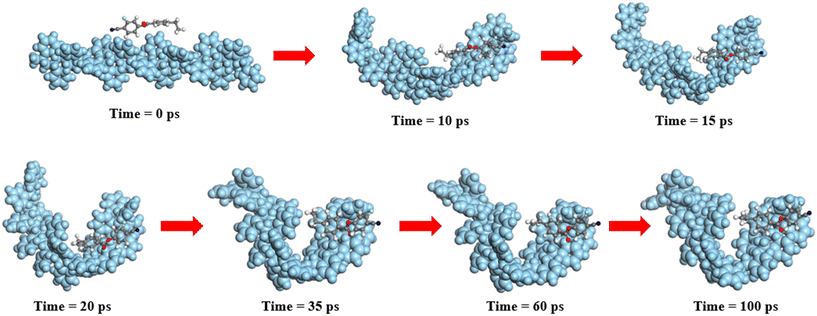
To further explore the sorption mechanism, molecular dynamic simulations of the interaction between PSNPs and CEB-F were conducted. The simulation ensured statistical equilibrium accuracy, with temperature (Fig. S6a†) and energy (Fig. S6b†) fluctuations maintained within 5–10%, confirming system equilibrium. Fig. 4 illustrates the molecular dynamic binding process between CEB-F and PSNPs. The simulation revealed that after 10 ps of interaction, CEB-F spontaneously moved to the PSNP surface, with a binding energy of −48.4075 kcal mol−1 (Table S4†). During the adsorption process, the PS polymer gradually bent and encapsulated CEB-F at 100 ps. The forces governing the PSNP–CEB-F interaction were primarily attributed to micropore filling and hydrophobic interactions, due to the hydrolytic nature of the carbon-based PSNP and CEB-F.
3.5. Plastic-mediated phototransformation of CEB-F in aquatic solutions
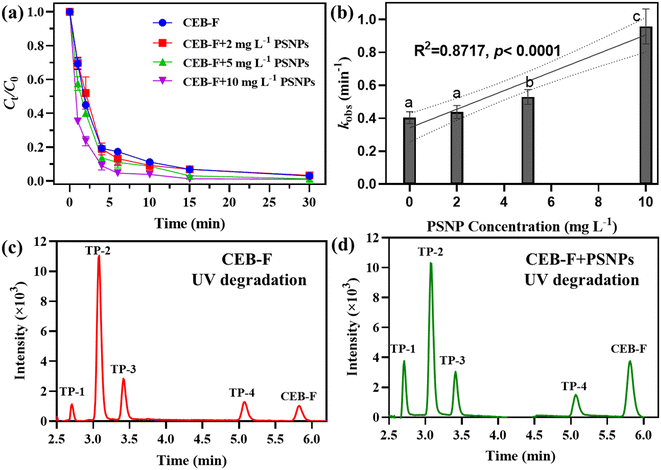
Since PSNPs showed the highest adsorption capacity toward CEB-F, we chose them as the subject of our investigation in order to better explain the plastic-mediated environmental behavior of CEB-F in natural waters. This allowed us to explore in greater detail the role of plastics in the phototransformation of CEB-F. As shown in Fig. 5a, 10 μM of CEB-F can be degraded under the existence of 0, 2, 5, and 10 mg L−1 PSNPs after 30 min of UV irradiation. The phototransformation of CEB-F with the presence of PSNPs can be well-described by a pseudo-first-order kinetics model with all R2 > 0.98. The pseudo-first-order rate constant (kobs) of the UV irradiation of CEB-F in the presence of PSNPs varying from 0 to 10 mg L−1 was calculated and is presented Fig. 5b. The phototransformation rate of CEB-F exhibited a positive correlation with the concentration of PSNPs (R2 = 0.87, p < 0.0001), indicating that PSNPs significantly promote the phototransformation of CEB-F in aquatic environments. As indicted in previous studies and in Fig. S7,† PSNPs exhibit strong ultraviolet absorption, which enables the photochemical generation of reactive species, such as free radicals and singlet oxygen. In addition, PSNPs can act as photosensitizers, facilitating the photochemical transformation of pollutants through energy transfer or electron transfer mechanisms.15,47 These factors likely contribute to the enhanced phototransformation of CEB-F observed in the presence of PSNPs.Furthermore, the presence of PSNPs facilitated the formation of CEB-F degradation by-products, as identified by LC-MS analysis. Four primary phototransformation products (P1–P4) were detected (Fig. 5c and S8†). These products are formed through reactive oxygen species attack, hydroxylation, and C–O/C–F bond cleavage.19,48,49 The distribution spectrum of CEB-F phototransformation products in the presence of PSNPs closely resembled that of the control group without PSNPs (Fig. 5d), indicating that the phototransformation pathways and mechanisms remain largely unchanged by the presence of PSNPs.
The temporal evolution of the four phototransformation products was tracked by recording their peak areas in the HPLC spectrum over the reaction time course (Fig. S9†). P1 showed maximum accumulation at 2 minutes, followed by a rapid decline and complete disappearance after 10 minutes, suggesting high reactivity and rapid degradation. The peak areas for P2, P3, and P4 initially increased rapidly within the first 10 minutes and then gradually decreased, indicating their propensity for further photolysis.
While the degradation rate of CEB-F increased in the presence of PSNPs, the phototransformation of intermediates was also affected. Notably, the generation and subsequent elimination of P1 were slower in the presence of PSNPs. For example, P1 vanished completely within 30 minutes in the absence of PSNPs but was still detectable in the presence of PSNPs. Conversely, the presence of PSNPs accelerated the elimination of P2. It is well-known that plastic particles, including PSNPs, exhibit photochemical reactivity and can degrade under long-term light irradiation, releasing dissolved organic matter and additives in the process.50–53 Consequently, the dynamic interactions between PSNPs and CEB-F as well as its intermediates significantly influence the rates and pathways of phototransformation, which should be highlighted in future investigations.
3.6. Toxicity change
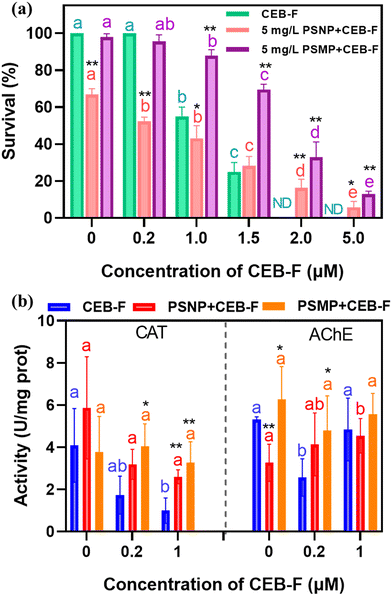
To evaluate the toxicity of CEB-F and plastic mixtures during photochemical reactions, we used Daphnia magna (D. magna) as a model aquatic organism. The survival data after 24 hours of exposure are shown in Fig. 6a. CEB-F exhibited significant acute toxicity to D. magna, with survival rates dropping to 50%, 25%, and 0% at concentrations of 1.0, 1.5, and 2.0 μM, respectively. PSNPs also demonstrated toxicity to D. magna; the survival rate decreased to 67% in the presence of 5 mg L−1 of PSNPs, whereas PSMPs at the same concentration did not significantly affect survival. Interestingly, PSMPs reduced the acute toxicity of CEB-F to D. magna. With 5 mg L−1 of PSMPs, the survival rate of D. magna increased from 55% to 87.8%, 25% to 69.5%, 0% to 32.8%, and 0% to 12.9% when exposed to 1.0, 1.5, 2.0, and 5.0 μM of CEB-F, respectively. This reduction in toxicity is likely due to the adsorption of CEB-F by PSMPs, while the reduced of PSMPs by D. magna due to its larger particle size compared to PSNPs, which hence reduced the intake of CEB-F by D. magna.However, the interaction between PSNPs and CEB-F presented a more complex toxicity profile. The presence of PSNPs at 5 mg L−1 significantly reduced the survival of D. magna from 100% to 52.3% and from 55% to 43.2% at 0.2 and 1.0 μM of CEB-F, respectively. Due to their small size, nanoplastics can accumulate in D. magna, disrupt mitochondrial metabolism, induce intracellular oxidative stress, and compromise cell membrane integrity.54–58 The accumulation of CEB-F on nanoplastics might extend the duration of CEB-F exposure within D. magna, potentially enhancing its toxicity. However, at higher concentrations of CEB-F (1.5, 2.0, and 5.0 μM), PSNPs alleviated the acute toxicity to D. magna. Our previous investigation found that CEB-F had significant acute toxicity on D. magna, with LC50 values of 1.0 μM after 24 hours.18 As a result, as CEB-F concentration increased, it began to dominate D. magna toxicity, whereas adsorption of CEB-F by PSNPs alleviated the acute toxicity to D. magna due to reduced bioavailability and bioaccessibility. At lower concentrations, PSNPs can enhance the toxicity of CEB-F by promoting its absorption and bioaccumulation.54,57,58 On the other hand, at higher concentrations, PSNPs may mitigate CEB-F toxicity by forming a protective barrier and triggering defense mechanisms in organisms.59,60
We further analyzed the activities of the enzymes catalase (CAT) and acetylcholinesterase (AChE) to clarify the role of PSMPs and PSNPs in mediating CEB-F aquatic toxicity. As shown in Fig. 6b, CAT activity significantly decreased with increasing concentrations of CEB-F from 0 to 1 μM. This reduction in CAT enzymatic activity indicates that elevated CEB-F exposure facilitates its penetration into D. magna, heightening oxidative stress and leading to an overproduction of H2O2 and HO˙. These reactive species exceed CAT's capacity to neutralize them, resulting in cellular damage.59 Interestingly, the presence of PSNPs mitigated the inhibition of CAT activity by CEB-F, thereby protecting D. magna from cellular damage caused by free radicals.60 Similarly, CEB-F exposure at 0.2 and 1.0 μM reduced AChE activity, indicating the neurotoxicity of CEB-F to D. magna.61,62 However, the presence of plastics, both PSMPs and PSNPs, increased AChE levels in the mixtures, suggesting a reduction in the neurological effects of CEB-F on D. magna. These results indicate that the adsorption of CEB-F by plastics may reduce its interaction with CAT and AChE, thus altering the overall toxicity profile when CEB-F coexists with plastics.63
4. Conclusions
This study explored the adsorption behavior, influencing factors, and phototransformation of CEB-F in the presence of micro- and nanoplastics (MPs and NPs) composed of polystyrene (PS), polyamide (PA), and mixed cellulose ester (MCE). The findings revealed that PS plastics, particularly PSNPs, have a high potential for adsorbing CEB-F due to their larger specific surface area and stronger hydrophobic interactions. The adsorption kinetics were best described by the pseudo-first-order model, and the adsorption process was primarily physical, involving surface adsorption and intra-particle diffusion. FTIR analysis and chemical calculations indicated that the adsorption mechanism involved hydrophobic interactions rather than chemical bonding. The presence of PSNPs was found to enhance the phototransformation of CEB-F under UV irradiation, increasing both the rate and extent of degradation. The phototransformation pathways remained consistent, although PSNPs altered the distribution and persistence of degradation by-products. Toxicity assessments using Daphnia magna demonstrated that CEB-F and PSNPs possess acute toxicity, with PSNPs exacerbating the toxic effects at lower CEB-F concentrations. However, PSMPs and PSNPs mitigated the toxicity at higher concentrations by reducing the bioavailability of CEB-F. Enzyme assays showed that PSNPs alleviated the inhibitory effects of CEB-F on CAT and AChE activities, suggesting a protective role against oxidative and neurotoxic damage. Considering the diverse interactions between plastics and CEB-F, as well as its degradation products, further evaluation is required to assess how the presence of plastic particles influences the toxicity of these products. Overall, the study highlights the significant role of MPs and NPs in modulating the environmental fate and toxicity of CEB-F, emphasizing the need for further research on the environmental impact of plastic–contaminant interactions.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
All authors contributed to the conduct of the research and agreed to submit the manuscript. Y. F.: conceptualization, writing – original draft, visualization, writing – review and editing. J. W.: methodology, visualization, review and editing; W. L.: visualization, methodology, and formal analysis. W. Y.: methodology and formal analysis. D. G. visualization, methodology, and formal analysis. X. W.: writing – review, editing and visualization; Z. W.: investigation, writing and editing. R. W. S. L.: writing – review and editing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (21707019), the Natural Science Foundation of Guangdong Province (2021A1515010019). X. W. acknowledges funding support from the National Natural Science Foundation of China (22376097, 41925031, 41630645, and 41521003) and the Startup Foundation for Introducing Talent of NUIST (1523142401062).References
- J. J. Feng, X. F. Sun and E. Y. Zeng, Emissions of liquid crystal monomers from obsolete smartphone screens in indoor settings: characteristics and human exposure risk, Environ. Sci. Technol., 2022, 56, 8053–8060 CrossRef CAS PubMed
.
- S. H. Zhang, Z. P. Cheng, M. Yang, Z. L. Guo, L. C. Zhao, M. Baqar, Y. Lu, L. Wang and H. W. Sun, Percutaneous penetration of liquid crystal monomers (LCMs) by In vitro three-dimensional human skin equivalents: Possible mechanisms and implications for human dermal exposure risks, Environ. Sci. Technol., 2023, 57, 4454–4463 CrossRef CAS PubMed
.
- M. J. Shen, Z. Q. Feng, X. X. Liang, H. Chen, C. Y. Zhu, B. B. Du, Q. Li and L. X. Zeng, Release and gas–particle partitioning behavior of liquid crystal monomers during the dismantling of waste Liquid crystal display panels in e-waste recycling facilities, Environ. Sci. Technol., 2022, 56, 3106–3116 CrossRef CAS PubMed
.
- H. J. Su, S. B. Shi, M. Zhu, J. H. Li and G. Y. Su, Liquid Crystal Monomers (LCMs) in Sediments: Method Validation and Detection in Sediment Samples from Three Typical Areas, Environ. Sci. Technol., 2021, 55, 2336–2345 CrossRef CAS PubMed
.
- J. Wu, R. Li and G. Su, Investigation of the Role of Distances from Liquid Crystal Monomer (LCM) Factories on Distribution of LCMs in Surface Soil Samples, Environ. Pollut., 2024, 356, 124285 CrossRef CAS PubMed
.
- S. Zhang, M. Yang, Y. Li, Y. Wang, Y. Lu, Z. Cheng and H. Sun, Occurrence, distribution, and human exposure of emerging liquid crystal monomers (LCMs) in indoor and outdoor dust: A nationwide study, Environ. Int., 2022, 164, 107295 CrossRef CAS PubMed
.
- J. Wang, J. Nan, M. Li, G. Yuan, Y. Zhao, J. Dai and K. Zhang, First evidence of contamination in aquatic organisms with organic light-emitting materials, Environ. Sci. Technol. Lett., 2022, 9, 739–746 CrossRef CAS
.
- Z. Cheng, Q. Shi, Y. Wang, L. Zhao, X. Li, Z. Sun, Y. Lu, N. Liu, G. Su and L. Wang, Electronic-waste-driven pollution of liquid crystal monomers: Environmental occurrence and human exposure in recycling industrial parks, Environ. Sci. Technol., 2022, 56, 2248–2257 CrossRef CAS PubMed
.
- X. Liang, R. Xie, C. Zhu, H. Chen, M. Shen, Q. Li, B. Du, D. Luo and L. Zeng, Comprehensive identification of liquid crystal monomers-Biphenyls, cyanobiphenyls, fluorinated biphenyls, and their analogues-in waste LCD panels and the first estimate of their global release into the environment, Environ. Sci. Technol., 2021, 55, 12424–12436 CrossRef CAS PubMed
.
- R. Yang, X. Wang, Q. Gao, C. Sang, Y. Zhao, Y. Niu and B. Shao, Dietary exposure and health risk of the emerging contaminant fluorinated liquid-crystal monomers, Environ. Sci. Technol., 2023, 57, 6309–6319 CrossRef CAS PubMed
.
- Z. Ouyang, Y. Yang, C. Zhang, S. Zhu, L. Qin, W. Wang, D. He, Y. Zhou, H. Luo and F. Qin, Recent advances in photocatalytic degradation of plastics and plastic-derived chemicals, J. Mater. Chem. A, 2021, 9, 13402–13441 RSC
.
- Y. Wang, Y. Yang, X. Liu, J. Zhao, R. Liu and B. Xing, Interaction of microplastics with antibiotics in aquatic environment: distribution, adsorption, and toxicity, Environ. Sci. Technol., 2021, 55, 15579–15595 CrossRef CAS PubMed
.
- W. Li, W. Zhao, H. Zhu, Z.-J. Li and W. Wang, State of the art in the photochemical degradation of (micro) plastics: From fundamental principles to catalysts and applications, J. Mater. Chem. A, 2023, 11, 2503–2527 RSC
.
- L. Wimberger, G. Ng and C. Boyer, Light-driven polymer recycling to monomers and small molecules, Nat. Commun., 2024, 15, 2510 CrossRef CAS
.
- H. Wang, P. Liu, M. Wang, X. Wu, Y. Shi, H. Huang and S. Gao, Enhanced phototransformation of atorvastatin by polystyrene microplastics: Critical role of aging, J. Hazard. Mater., 2021, 408, 124756 CrossRef CAS PubMed
.
- R. Trevisan, C. Voy, S. Chen and R. T. Di Giulio, Nanoplastics decrease the toxicity of a complex PAH mixture but impair mitochondrial energy production in developing zebrafish, Environ. Sci. Technol., 2019, 53, 8405–8415 CrossRef CAS PubMed
.
- K. L. Law and R. C. Thompson, Microplastics in the seas, Science, 2014, 345, 144–145 CrossRef CAS PubMed
.
- Q. Jin, D. Tao, Y. Lu, J. Sun, C. H. Lam, G. Su and Y. He, New insight on occurrence of liquid crystal monomers: A class of emerging e-waste pollutants in municipal landfill leachate, J. Hazard. Mater., 2022, 423, 127146 CrossRef CAS PubMed
.
- J. Wu, W. Ye, Y. Feng, W. Lao, J. Li, H. Lu, G. Liu, G. Su and Y. Deng, Aquatic photolysis of high-risk fluorinated liquid crystal monomers: Kinetics, toxicity evaluation, and mechanisms, Water Res., 2024, 255, 121510 CrossRef CAS PubMed
.
- H. J. C. Berendsen, J. P. M. Postma, W. F. Van Gunsteren, A. DiNola and J. R. Haak, Molecular dynamics with coupling to an external bath, J. Chem. Phys., 1984, 81, 3684–3690 CrossRef CAS
.
- R. Bo, J. Wang, C. Wang, Y. Wang, P. He and Z. Han, Selective distribution of BaTiO3 and graphene in PS/PVDF blends: Molecular dynamics simulations, Mater. Today Commun., 2023, 34, 105247 CrossRef CAS
.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed
.
- Y. Feng, J. Lao, J. Zou, Z. Zhu, D. Li, G. Liu and L. Mao, Interaction of graphitic carbon nitride with cell membranes: Probing phospholipid extraction and lipid bilayer destruction, Environ. Sci. Technol., 2022, 56, 17663–17673 CrossRef CAS PubMed
.
- H. Zhao, X. Huang, Y. Yang, L. Wang, X. Zhao, F. Yan, Y. Yang, P. Gao and P. Ji, The role of available nitrogen in the adsorption of polystyrene nanoplastics on magnetic materials, Water Res., 2023, 229, 119481 CrossRef CAS
.
- L. Zhou, R. Wang, Y. Liu, Y. Zhang, J. Zhou, G. Qu, S. Tang and T. Wang, Plasma-induced conversion of polystyrene nanoplastics in water: Intermediates release, toxicity, and disinfection byproducts formation, Chem. Eng. J., 2022, 433, 134543 CrossRef CAS
.
- A. Benkaddour, C. Journoux-Lapp, K. Jradi, S. Robert and C. Daneault, Study of the hydrophobization of TEMPO-oxidized cellulose gel through two routes: Amidation and esterification process, J. Mater. Sci., 2014, 49, 2832–2843 CrossRef CAS
.
- F. Liu, P. Dong, W. Lu and K. Sun, On formation of AlOC bonds at aluminum/polyamide joint interface, Appl. Surf. Sci., 2019, 466, 202–209 CrossRef CAS
.
- P. Liu, K. Lu, J. L. Li, X. W. Wu, L. Qian, M. J. Wang and S. X. Gao, Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates, J. Hazard. Mater., 2020, 384, 121193 CrossRef CAS PubMed
.
- A. N. Módenes, G. Bazarin, C. E. Borba, P. P. P. Locatelli, F. P. Borsato, V. Pagno, R. Pedrini, D. E. G. Trigueros, F. R. Espinoza-Quinones and F. B. Scheufele, Tetracycline adsorption by tilapia fish bone-based biochar: Mass transfer assessment and fixed-bed data prediction by hybrid statistical-phenomenological modeling, J. Cleaner Prod., 2021, 279, 123775 CrossRef
.
- N. Yao, X. P. Wang, Z. H. Yang, P. Q. Zhao and X. Meng, Characterization of solid and liquid carbonization products of polyvinyl chloride (PVC) and investigation of the PVC-derived adsorbent for the removal of organic compounds from water, J. Hazard. Mater., 2023, 456, 131687 CrossRef CAS PubMed
.
- J. Wang, D. Yue, D. Cui, L. Zhang and X. Dong, Insights into Adsorption of Humic Substances on Graphitic Carbon Nitride, Environ. Sci. Technol., 2021, 55, 7910–7919 CrossRef CAS PubMed
.
- M. Zhu, H. J. Su, Y. R. Bao, J. H. Li and G. Y. Su, Experimental determination of octanol-water partition coefficient (KOW) of 39 liquid crystal monomers (LCMs) by use of the shake-flask method, Chemosphere, 2022, 287, 132407 CrossRef CAS PubMed
.
- H. J. Su, S. B. Shi, M. Zhu, D. Crump, R. J. Letcher, J. P. Giesy and G. Y. Su, Persistent, bioaccumulative, and toxic properties of liquid crystal monomers and their detection in indoor residential dust, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 26450–26458 CrossRef CAS
.
- X. Guo, J. Pang, S. Chen and H. Jia, Sorption properties of tylosin on four different microplastics, Chemosphere, 2018, 209, 240–245 CrossRef CAS PubMed
.
- Y. Feng, G. Chen, Y. Zhang, D. Li, C. Ling, Q. Wang and G. Liu, Superhigh co-adsorption of tetracycline and copper by the ultrathin g-C3N4 modified graphene oxide hydrogels, J. Hazard. Mater., 2022, 424, 127362 CrossRef CAS PubMed
.
- L. Lin, S. Tang, X. Wang, X. Sun and Y. Liu, Sorption of tetracycline onto hexabromocyclododecane/polystyrene composite and polystyrene microplastics: Statistical physics models, influencing factors, and interaction mechanisms, Environ. Pollut., 2021, 284, 117164 CrossRef CAS PubMed
.
- C. Chen, X. Pang, Q. Chen, M. Xu, Y. Xiao, J. Wu, Y. Zhang, Y. Liu, L. Long and G. Yang, Tetracycline adsorption trajectories on aged polystyrene in a simulated aquatic environment: A mechanistic investigation, Sci. Total Environ., 2022, 851, 158204 CrossRef CAS PubMed
.
- K. Yokota, H. Waterfield, C. Hastings, E. Davidson, E. Kwietniewski and B. Wells, Finding the missing piece of the aquatic plastic pollution puzzle: Interaction between primary producers and microplastics, Limnol. Oceanogr., 2017, 2, 91–104 CrossRef
.
- S. Felsing, C. Kochleus, S. Buchinger, N. Brennholt, F. Stock and G. Reifferscheid, A new approach in separating microplastics from environmental samples based on their electrostatic behavior, Environ. Pollut., 2018, 234, 20–28 CrossRef CAS PubMed
.
- L. Zuo, Y. Guo, X. Li, H. Fu, X. Qu, S. Zheng, C. Gu, D. Zhu and P. J. J. Alvarez, Enhanced adsorption of hydroxyl- and amino-substituted aromatic chemicals to nitrogen-doped multiwall carbon nanotubes: A combined batch and theoretical calculation study, Environ. Sci. Technol., 2015, 50, 899–905 CrossRef PubMed
.
- Y. Feng, X. Liu, K. A. Huynh, J. M. McCaffery, L. Mao, S. Gao and K. L. Chen, Heteroaggregation of graphene oxide with nanometer-and micrometer-sized hematite colloids: Influence on nanohybrid aggregation and microparticle sedimentation, Environ. Sci. Technol., 2017, 51, 6821–6828 CrossRef CAS
.
- F. Wang, W. Yang, P. Cheng, S. Zhang, S. Zhang, W. Jiao and Y. Sun, Adsorption characteristics of cadmium onto microplastics from aqueous solutions, Chemosphere, 2019, 235, 1073–1080 CrossRef CAS PubMed
.
- O. S. Alimi, J. Farner Budarz, L. M. Hernandez and N. Tufenkji, Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport, Environ. Sci. Technol., 2018, 52, 1704–1724 CrossRef CAS PubMed
.
- M. A. Pascall, M. E. Zabik, M. J. Zabik and R. J. Hernandez, Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films, J. Agric. Food Chem., 2005, 53, 164–169 CrossRef CAS PubMed
.
- Z. Chen, J. Yang, D. Huang, S. Wang, K. Jiang, W. Sun, Z. Chen, Z. Cao, Y. Ren, Q. Wang, H. Liu, X. Zhang and X. Sun, Adsorption behavior of aniline pollutant on polystyrene microplastics, Chemosphere, 2023, 323, 138187 CrossRef CAS PubMed
.
- B. Gao, W. Zhang, Q. Lei and J. Men, Preparation and characterization of 8-hydroxyquinoline-functionalized polysulfone and preliminary study on luminescence property of its complex with Al(III), Macromol. Res., 2013, 21, 599–607 CrossRef CAS
.
- K. Zhu, H. Jia, Y. Sun, Y. Dai, C. Zhang, X. Guo, T. Wang and L. Zhu, Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species, Water Res., 2020, 173, 115564 CrossRef CAS PubMed
.
- Y. Feng, Q. Song, W. Lv and G. Liu, Degradation of ketoprofen by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices, Chemosphere, 2017, 189, 643–651 CrossRef CAS PubMed
.
- S. Hou, L. Ling, C. Shang, Y. Guan and J. Fang, Degradation kinetics and pathways of haloacetonitriles by the UV/persulfate process, Chem. Eng. J., 2017, 320, 478–484 CrossRef CAS
.
- E. Syranidou, K. Karkanorachaki, D. Barouta, E. Papadaki, D. Moschovas, A. Avgeropoulos and N. Kalogerakis, Relationship between the carbonyl index (CI) and fragmentation of polyolefin plastics during aging, Environ. Sci. Technol., 2023, 57, 8130–8138 CrossRef CAS PubMed
.
- L. Wang, J. Gao, W.-M. Wu, J. Luo, M. S. Bank, A. A. Koelmans, J. J. Boland and D. Hou, Rapid Generation of Microplastics and Plastic-Derived Dissolved Organic Matter from Food Packaging Films under Simulated Aging Conditions, Environ. Sci. Technol., 2024, 58, 20147–20159 CrossRef CAS
.
- Y. K. Lee, W. He, H. M. Guo, T. Karanfil and J. Hur, Effects of organic additives on spectroscopic and molecular-level features of photo-induced dissolved organic matter from microplastics, Water Res., 2023, 242, 120272 CrossRef CAS
.
- Y. Yu, M. Kumar, S. Bolan, L. P. Padhye, N. Bolan, S. Li, L. Wang, D. Hou and Y. Li, Various additive release from microplastics and their toxicity in aquatic environments, Environ. Pollut., 2023, 343, 123219 CrossRef PubMed
.
- X. Zhao, X. Wu, J. C. White, Z. Tang and F. Wu, Micro/nanoplastics contamination of the terrestrial environment: exposure routes, dose, and co-contaminants complicate the risk calculus, Carbon Res., 2023, 2, 24 CrossRef
.
- O. Pikuda, E. R. Dumont, S. Matthews, E. G. Xu, D. Berk and N. Tufenkji, Sub-lethal effects of nanoplastics upon chronic exposure to Daphnia magna, J. Hazard. Mater. Adv., 2022, 7, 100136 CAS
.
- O. O. Fadare, B. Wan, L.-H. Guo, Y. Xin, W. Qin and Y. Yang, Humic acid alleviates the toxicity of polystyrene nanoplastic particles to Daphnia magna, Environ. Sci.: Nano, 2019, 6, 1466–1477 RSC
.
- A. Reynolds, M. Giltrap and G. Chambers, Evaluation of non-invasive toxicological analysis of nano-polystyrene in relative in vivo conditions to D. magna, Environ. Sci.: Nano, 2019, 6, 2832–2849 RSC
.
- E. Besseling, B. Wang, M. Lurling and A. A. Koelmans, Nanoplastic affects growth of S. obliquus and reproduction of D. magna, Environ. Sci. Technol., 2014, 48, 12336–12343 CrossRef CAS
.
- X. Li, S. Chu, Z. Song, F. He, Z. Cui and R. Liu, Discrepancy of apoptotic events in mouse hepatocytes and catalase performance: Size-dependent cellular and molecular toxicity of ultrafine carbon black, J. Hazard. Mater., 2022, 421, 126781 CrossRef CAS PubMed
.
- C. Garcia-Gomez, M. Babin, S. Garcia, P. Almendros, R. A. Perez and M. D. Fernandez, Joint effects of zinc oxide nanoparticles and chlorpyrifos on the reproduction and cellular stress responses of the earthworm Eisenia andrei, Sci. Total Environ., 2019, 688, 199–207 CrossRef CAS PubMed
.
- G. Heredia-Garcia, G. A. Elizalde-Velazquez, L. M. Gomez-Olivan, H. Islas-Flores, S. Garcia-Medina, M. Galar-Martinez and O. Dublan-Garcia, Realistic concentrations of Bisphenol-A trigger a neurotoxic response in the brain of zebrafish: Oxidative stress, behavioral impairment, acetylcholinesterase inhibition, and gene expression disruption, Chemosphere, 2023, 330, 138729 CrossRef CAS
.
- Y. Wu, J. Wang, Y. Xia, K. Tang, J. Xu, A. Wang, S. Hu, L. Wen, B. Wang, W. Yao and J. Wang, Toxic effects of isofenphos-methyl on zebrafish embryonic development, Ecotoxicol. Environ. Saf., 2023, 254, 114723 CrossRef CAS
.
- C. C. Chen, Y. Shi, Y. Zhu, J. Zeng, W. Qian, S. Zhou, J. Ma, K. Pan, Y. Jiang, Y. Tao and X. Zhu, Combined toxicity of polystyrene microplastics and ammonium perfluorooctanoate to Daphnia magna: Mediation of intestinal blockage, Water Res., 2022, 219, 118536 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00723a |
| This journal is © The Royal Society of Chemistry 2025 |