Highly selective capture of palladium from acidic solution by sulfur-functionalized porous carbon microspheres: performance and mechanism†
Mingyue
Wang
 ab,
Ruiwen
Liang
ab,
Ruiwen
Liang
 ab,
Lanchao
Kou
ab,
Lanchao
Kou
 ab,
Xiukun
Cao
ab,
Xiukun
Cao
 c and
Dezhi
Chen
c and
Dezhi
Chen
 *ab
*ab
aNational-Local Joint Engineering Research Center of Heavy Metal Pollutant Control and Resource Utilization, Nanchang Hangkong University, Nanchang 330063, China. E-mail: chendz@nchu.edu.cn
bKey Laboratory of Jiangxi Province for Persistent Pollutants Prevention Control and Resource Reuse, Nanchang Hangkong University, Nanchang 330063, China
cJinChenBoKe Environmental Development Technology Co. Ltd, Tianjin 300384, China
First published on 15th November 2024
Abstract
Efficient recovery of palladium (Pd) from waste sources is of paramount importance due to its limited natural reserves and potential environmental hazards. Herein, a carbon sorbent, namely sulfur-functionalized porous carbon microspheres (SPCMs), was used to selectively capture Pd(II) from acidic solution. SPCMs exhibited high efficiency for the adsorption separation of Pd(II) from 0.5 M to 6 M HNO3 solution. The adsorption kinetics of Pd(II) matched well with the pseudo-second-order model. The adsorption reached equilibrium after 130 minutes and the adsorption capacity of Pd(II) was 79.3 mg g−1 in 1 M HNO3 solution. The Freundlich isotherm model exhibited a better description of the Pd(II) adsorption, suggesting that the Pd(II) adsorption is a multilayer adsorption process. SPCMs showed a high selectivity for the capture of Pd(II) in simulated acidic wastewater with 26 metal ions, and the selectivity increased with the increase of HNO3 concentration. The adsorption capacity per US dollar of Pd(II) by SPCMs from HNO3 solution is much higher than those of previously reported sorbents, exhibiting a high economic viability of SPCMs for Pd(II) capture from acidic solution. The adsorbed Pd(II) could be desorbed using 1.0 M thiourea and 0.1 M HNO3, and the SPCM sorbent maintained a high adsorption capacity after five adsorption–desorption cycles. Characterization and theoretical calculations revealed that the adsorption of Pd(II) on the SPCM sorbent is dominated by the coordination of [Pd(NO3)2] with O/S containing groups and some of the Pd(II) is reduced to Pd(0). The excellent adsorption performance of SPCMs provides a feasible and low-cost strategy for the selective recovery of Pd(II) from acidic wastewater.
Environmental significanceThe efficient recovery of Pd from high level liquid waste (HLLW) not only alleviates the scarcity of Pd but also substantially reduces environmental strains because the presence of Pd will inhibit the vitrification of HLLW. Adsorption is one of the most effective approaches for the separation of Pd from HLLW. Sulfur-functionalized porous carbon microspheres can efficiently and rapidly adsorb Pd from simulated HLLW based on the coordination of Pd(II) with O/S functional groups and the generation of Pd(0). The porous carbon material provides a feasible and low-cost strategy for the selective recovery of Pd from highly acidic wastewater. |
1. Introduction
Palladium (Pd), a precious metal with exceptional physical and chemical properties, is widely used in catalysts, sensors, electronic devices, jewellery, and coinage.1 However, Pd is a rare resource, with concentrations ranging from 0.1 to 3 ng g−1 within the Earth's crust, and its distribution is uneven globally.2 In recent years, the demand for Pd has increased rapidly, driven by the unprecedented growth in the mobile phone and automotive industries. It is anticipated that the shortage of Pd will become more severe. Consequently, there has been growing interest in separating and recovering Pd from waste sources, which not only can alleviate the scarcity of Pd but can also substantially reduce environmental strains, thus achieving a synergistic advantage for both resource preservation and ecological safeguarding.3,4Apart from Pd resources in discarded electronic equipment, deactivated catalysts, etc.,5,6 the spent fuel acidic wastewater, generated from the reprocessing of spent fuel employing the plutonium–uranium reduction extraction (PUREX) process, also contains a considerable amount of Pd.7,8 However, the valuable Pd is currently being vitrified into a glass matrix for storage along with other fission products, resulting in a huge loss of resources.9 Moreover, the presence of Pd has adverse effects on both the vitrification process and the durability of the vitreous state because of its low solubility in borosilicate glass.10 Therefore, the separation and recovery of Pd from the spent fuel acidic wastewater can help to enrich the limited Pd resources and solve the problems associated with vitrified nuclear waste storage technology.
At present, various technologies are available for the separation and recovery of Pd from the spent fuel acidic wastewater in the PUREX process, such as solvent extraction,11 ion exchange,12 membrane separation,13 electrochemical methods,14 and adsorption.15,16 Among the above methods, adsorption has been widely employed due to its advantages of high efficiency and ease of operation.17 As we know, adsorbents are key to the efficient adsorption process. Various sorbents, including resins,18 mesoporous silica,19 metal–organic frameworks (MOFs),20 and porous organic polymers (POPs),21 have been developed for the capture of Pd from acidic wastewater. However, the features of spent fuel acidic wastewater such as high acidity and high radioactivity22 will bring several significant deficiencies in the separation and recovery of Pd from the acidic wastewater using the above sorbents. For example, resin and mesoporous silica-based materials, while capable of exhibiting good selectivity and efficiency, often suffer from the serious problem of active species being easily leached.23,24 In addition, the poor stability of MOFs in aqueous solution,25 especially under highly acidic conditions, restricts their applicability in Pd recovery.26 As a new class of materials, POPs, which are mainly composed of C, H, O and N, have shown excellent adsorption performance and good chemical stability. However, the complicated synthesis process and the high cost of organic monomers as well as the use of toxic solvents seriously hinder their large-scale application for the recovery of Pd.27
Therefore, from a technical and economic point of view, current attention is focusing on low-cost adsorbents, such as porous carbon materials, which are popular because of their wide availability and high adsorption capacity.28–31 Furthermore, porous carbon materials offer many advantages such as high specific surface area and porosity, hierarchical and interconnected pore channels and good chemical and thermal stability, which make them have a wide range of applications in the separation and recovery of precious metal ions.29–31 To further enhance the performance of porous carbon sorbents, the doping of heteroatoms (such as N, B, S, etc.) or the modification of functional groups containing heteroatoms (–NH2, –NO2, –SH, –SO3H, etc.) are usually carried out.30–34 According to the theory of soft and hard acids and bases defined by Pearson, Pd is classified as a soft acid. Consequently, as a soft base, S functionalization is conducive to improving the adsorption performance of porous carbon for the recovery of Pd from acidic solution.
In addition, powder-like porous carbon has the problems of easy loss and difficulty in separating it from the solution after adsorption. Moreover, that which remains in the water environment will be desorbed under specific conditions, resulting in secondary environmental pollution.35 For the purpose of improving the adsorption performance and easy separation of adsorbents from wastewater, we first prepared S-functionalized porous carbon microspheres (SPCMs). The morphology, the pore structure, and the surface features of the as-prepared SPCMs were characterized. The adsorption behavior of Pd(II) on the SPCMs in HNO3 solution was evaluated through batch adsorption experiments including the effects of acidity, contact time, temperature, and initial ion concentration. Furthermore, the possible adsorption mechanisms of Pd(II) on the SPCMs were proposed. Finally, the selectivity and recycling capability of the SPCMs for the capture of Pd(II) were investigated.
2. Experimental section
2.1 Preparation of SPCMs
All chemicals were of analytical grade or higher and used without further purification (see Text S1† for details). The synthesis procedure for the SPCM adsorbent is depicted in Fig. S1.† Specifically, 40 mL of 1 M sucrose solution was transferred into a 100 mL autoclave and subjected to a reaction at 180 °C for 10 h. Once the autoclave had cooled down to room temperature, the precipitate was collected through filtration and subsequently washed with deionized water and anhydrous ethanol. After drying in an oven at 80 °C for 12 h, the obtained 1 g of carbon microspheres (CMs) were uniformly dispersed in 40 mL of deionized water including 2 g of KOH and a dosage of (NH4)2SO4. The above suspension was dried at 80 °C in an oven. The dried samples were ground uniformly and then placed in a tube furnace to be activated under an N2 atmosphere with a flow rate of 100 mL min−1 using a gradient activation program, including 450 °C for 0.5 h, 650 °C for 0.5 h, and 800 °C for 1 h with a heating rate of 5 °C min−1 from room temperature. The activated products were immersed in 10% HCl solution to remove any impurities and washed with deionized water until pH = 7.0. After drying at 60 °C for 12 h, the final SPCM samples were denoted as SPCM-1, SPCM-2, and SPCM-3 under the assistant activation of 0.3, 0.5, and 0.7 g of (NH4)2SO4, respectively. In addition, porous carbon microspheres (PCMs) were prepared using the above process without (NH4)2SO4.2.2 Characterization
The morphology and structure of the as-prepared carbon materials were observed using a scanning electron microscope (SEM, Zeiss Sigma 300) and a transmission electron microscope (TEM, FEI Talos F200X) equipped with an energy-dispersive X-ray spectrometer (EDS). The phase of the as-prepared carbon materials was analysed using an X-ray powder diffractometer (XRD, Bruker D8 Advance). The defects in the as-prepared carbon materials were evaluated using a Raman spectrometer (Renishaw inVia, UK) equipped with a laser of 532 nm. The functional groups on the as-prepared carbon materials were determined using a Fourier transform infrared spectrometer (FTIR, Bruker Optics VERTEX 70). The elemental composition was analysed using an organic elemental analyser (Elementar Vario EL III). The N2 adsorption–desorption isotherms of the as-prepared carbon materials were obtained from a specific surface area and pore size distribution analyser (Micromeritics, TriStar II 3020) at 77 K. The hydrophilicity of the material surface was determined using a contact angle analyser (DataPhysics OCA20). The elements and chemical states on the surface of the as-prepared carbon materials were investigated by X-ray photoelectron spectroscopy (XPS, Kratos Axis Ultra DLD). The C 1s line at 284.8 eV was used as a reference for correction. The concentrations of metal ions were determined using an inductively coupled plasma mass spectrometer (ICP-MS, iCAP Q, Thermo Fisher).2.3 Batch adsorption
All experiments were performed independently at least 3 times. The adsorption kinetics were carried out in a thermostatically controlled shaker at 35 °C with a speed of 180 rpm. 10 mg of SPCM sorbent was put into a 100 mL conical flask containing 50 mL of 40 mg L−1 Pd(II) in 1 M HNO3 solution. At certain intervals, 0.2 mL of the reaction solution was extracted using an extractor equipped with a 0.22 μm membrane filter, and then the residual Pd(II) was determined by ICP-MS.Isothermal adsorption of Pd(II) on the optimized sorbent was achieved in 50 mL of 1 M HNO3 solution containing different concentrations of Pd(II) from 20 to 200 mg L−1 at 35 °C. The effect of acidity on the adsorption of Pd(II) was investigated in 50 mL of 40 mg L−1 Pd(II) in HNO3 solution with different HNO3 concentrations of 0.5, 1, 2, 3, 4, and 6 M using 10 mg of sorbent. In addition, the impact of reaction temperature was assessed at 25, 35 and 45 °C in 50 mL of 40 mg L−1 Pd(II) in 1 M HNO3 solution. The adsorption selectivity study of the sorbent for Pd(II) was carried out using 10 mg of sorbent in 50 mL of simulated spent fuel acid wastewater, which was prepared using 1, 2 or 3 M HNO3 solution containing Li+, Na+, Mg2+, Al3+, K+, Ca2+, Ti4+, Fe3+, Nb5+, Mn2+, Zr4+, Ce3+, Mo6+, Cd2+, Cr3+, Nd3+, Ba2+, Ni2+, La3+, Co2+, Zn2+, Cu2+, Pb2+ and Pd2+ ions (their concentration is listed in Table S1†). After adsorption equilibrium, the residual metal ions were determined by ICP-MS.
The adsorption capacity (q, mg g−1), adsorption efficiency (Ads, %), desorption efficiency (Des, %), distribution coefficient (Kd, mL g−1), and selectivity coefficient (K) were calculated using the following equations:
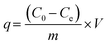 | (1) |
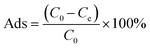 | (2) |
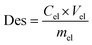 | (3) |
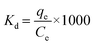 | (4) |
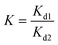 | (5) |
2.4 Regeneration and recycling of SPCMs
The desorption of adsorbed Pd(II) from SPCMs was carried out using an eluent consisting of 1 M thiourea and 0.1 M HNO3 in a shaker maintained at 35 °C and shaken at 180 rpm for a duration of 12 h. The concentration of Pd(II) in the eluent was subsequently determined by ICP-MS. After desorption, the eluted SPCM sorbent was collected and washed with deionized water and ethanol. After drying at 60 °C for 12 hours, the regenerated SPCMs were subsequently utilized as a sorbent to evaluate their reusability.2.5 DFT calculation
The adsorption mechanism for Pd(II) in the HNO3 solution was revealed by density functional theory (DFT) calculation. The DFT simulations of full geometry optimization and adsorption energy were carried out with the Dmol3 package36,37 in Materials Studio with the generalized gradient approximation (GGA)38 with the Becke–Lee–Yang–Parr (BLYP) exchange correlation. The calculation quality was set to customized. The Tkatchenko–Scheffler (TS) method was used for density functional theory with dispersion (DFT-D) correction. The core treatment chose DFT semi-core pseudopots. The basis set and basis file were a double numerical plus polarization basis set with a cutoff of 3.5 Å (DNP 3.5). The convergence criterion for the energy and maximum force for the optimization was set to 10−5 Ha and 0.002 Ha Å−1, respectively. The adsorption energy is defined as:| Eads = Etotal − Ecomponent | (6) |
3. Results and discussion
3.1 Characterization
Fig. 1 presents the typical SEM images of the prepared carbon samples. Fig. 1a shows that a hydrothermal CMs present a regular spherical structure with an average diameter of about 6 microns and have a very smooth and crack-free surface. As displayed in Fig. 1b, these preferred CMs are converted to rambutan-like structures modified by graphene-like carbon sheets under the activation effect of KOH. In addition, obvious pores can be observed on the surface of rambutan-like PCMs. Furthermore, we can see a clear honeycomb-like structure, which may originate from the broken PCMs. With the assistance of (NH4)2SO4, Fig. 1c–e show that the spherical morphology can still be seen in the as-obtained SPCM samples. With the increase of (NH4)2SO4 dose, the diameter of microspheres decreases. This is mainly attributed to the further etching of carbon microspheres by the gas generated by the decomposition of (NH4)2SO4. Fig. 1f shows the SEM image of the used SPCM-2. After Pd(II) adsorption in 1 M HNO3 solution, the microsphere morphology shows no change, suggesting that the as-prepared SPCM samples are stable in acidic solution.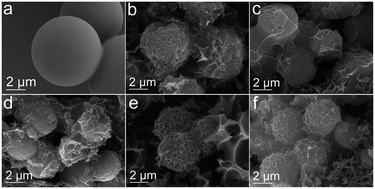 | ||
| Fig. 1 SEM images of a) a carbon microsphere (CM), b) porous carbon microspheres (PCMs), and S-functionalized porous carbon spheres c) SPCM-1, d) SPCM-2, e) SPCM-3, and f) used SPCM-2. | ||
To further observe the microstructure of the prepared SPCMs, we carried out TEM analysis using SPCM-2 as an example. Fig. 2a presents rambutan-like microspheres and a honeycomb-like structure in the SPCM-2 sample. As shown in Fig. 2b, these microspheres are wrapped by carbon nanosheets, and clear pores can be observed in these nanosheets. In addition, some carbon nanosheets are exfoliated from carbon microspheres and interwoven together to form a honeycomb-like structure. Fig. 2c displays the HRTEM image of carbon nanosheets on the surface of carbon microspheres in the SPCM-2 sample. The disordered lattice stripes indicate the amorphous feature of SPCM-2. The high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image presented in Fig. 2d shows the non-metallic features and abundant porosity of SPCM-2. EDS mapping images displayed in Fig. 2e–g indicate that C, O, and S elements are uniformly distributed in the SPCM-2 sample.
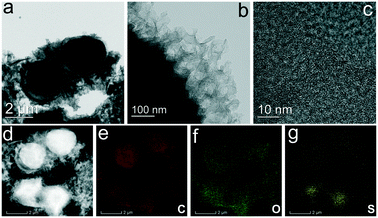 | ||
| Fig. 2 a and b) TEM, c) high resolution TEM, d) HAADF-STEM, and EDS mapping images of S-functionalized porous carbon spheres (SPCM-2), e) C, f) O, and g) S. | ||
Fig. 3a exhibits the XRD patterns of the as-prepared carbon samples. Two broad diffraction peaks at approximately 23° and 43° match well with the (002) and (101) planes of amorphous carbon, respectively.39 The obvious rise at the low diffraction angles implies the presence of abundant nanopores. In addition, no other diffraction peaks suggest the absence of other inorganic impurities. As depicted in Fig. 3b, the Raman spectra exhibit two characteristic peaks at 1320 and 1590 cm−1, which correspond to the D and G bands, respectively. The D band is indicative of defective or disordered sp3 carbon, while the G band is associated with graphitized sp2 carbon in carbon materials.40 Generally, the intensity ratio of the D band to the G band (ID/IG) is used to evaluate the degree of defects in carbon materials. The calculated ID/IG values for PCMs, SPCM-1, SPCM-2, and SPCM-3 are 1.03, 1.08, 1.16, and 1.09, respectively. The higher ID/IG value of SPCM-2 indicates higher concentration defects, which can provide more active sites for the adsorption reaction.
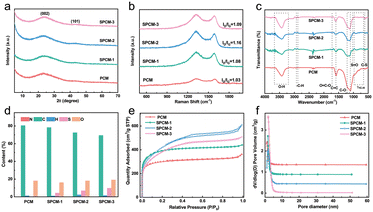 | ||
| Fig. 3 a) XRD patterns, b) Raman spectra, c) FTIR spectra, d) element content, e) N2 adsorption/desorption isotherms, and f) pore size distributions of the as-obtained carbon samples. | ||
Fig. 3c shows the FTIR spectra in the range of 4000 to 500 cm−1. The bands observed near 3430 cm−1 is attributed to the stretching vibrations of typical O–H groups. The band at 1720 cm−1 is associated with the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carboxyl group.41 The bands located at around 1580 and 1120 cm−1 are ascribed to the stretching vibrations of C
O in the carboxyl group.41 The bands located at around 1580 and 1120 cm−1 are ascribed to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–O, respectively.42,43 Compared to the PCMs, the SPCM samples exhibit two new bands at 1031 and 618 cm−1, which are assigned to the stretching vibrations of the S
C and C–O, respectively.42,43 Compared to the PCMs, the SPCM samples exhibit two new bands at 1031 and 618 cm−1, which are assigned to the stretching vibrations of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–S groups, respectively.44,45 This indicates that the S atoms are successfully doped into the prepared porous carbon microspheres. The bands in the range of 920–650 cm−1 are attributed to the out-of-plane bending vibration of aromatic C–H. Fig. 3d displays the percentage content of elements in the as-prepared carbon samples. C and O are the main elements in the PCMs. With the increase of (NH4)2SO4 dose, the content of the S element gradually increases in the prepared SPCM samples. Meanwhile, the content of O is stable at approximately 17.8%. The content of N is lower than 0.1% in the SPCM samples.
O and C–S groups, respectively.44,45 This indicates that the S atoms are successfully doped into the prepared porous carbon microspheres. The bands in the range of 920–650 cm−1 are attributed to the out-of-plane bending vibration of aromatic C–H. Fig. 3d displays the percentage content of elements in the as-prepared carbon samples. C and O are the main elements in the PCMs. With the increase of (NH4)2SO4 dose, the content of the S element gradually increases in the prepared SPCM samples. Meanwhile, the content of O is stable at approximately 17.8%. The content of N is lower than 0.1% in the SPCM samples.
Fig. 3e demonstrates the N2 adsorption/desorption isotherms of the as-obtained carbon samples, which are identical to the combination of typical I and IV isotherms from the IUPAC. The precipitous rise at low pressure (<0.05 P/P0) indicates the presence of micropores. The gentle elevation in the middle pressure of 0.1–0.8 P/P0 and the H4 hysteresis loop between 0.4 and 1.0 P/P0 suggest the presence of mesopores. In addition, the rise at high pressure near 1.0 P/P0 demonstrates the presence of macropores. The pore size distribution curves shown in Fig. 3f, calculated by the Horvath–Kawazoe (HK) method, also confirm the above results. The hierarchical pore structure is conducive to the adsorption of Pd(II) from solution. Micropores can provide a high specific surface area and offer a substantial number of active sites for the adsorption of Pd(II) ions. In addition, mesopores facilitate the Pd(II) ions into the pore channels for adsorption, and their diffusion rate is faster than that of micropores. Furthermore, although macropores do not possess adsorption capabilities themselves, they provide fast access for Pd(II) ions to the micropores and mesopores during the adsorption process.46,47
Table S2† shows the calculated specific surface area (SSA), average pore size, and pore volume. With the increase of (NH4)2SO4 dose, the SSA and pore volume of the SPCM samples significantly increase. This may be attributed to the gas generated by (NH4)2SO4 decomposition, which peels off the carbon layer and causes the activator to enter a deeper carbon layer to produce a richer pore structure.48 However, when the dose of (NH4)2SO4 is further increased to 0.7 g, the SSA and pore volume of SPCM-3 is reduced mainly because of the collapse of the pores caused by the excessive etching by the chemical activator. The hydrophilicity was determined by a contact angle measurement and the results are presented in Fig. S2.† All porous carbon samples show good hydrophilicity, and the contact angle of water droplets on the surface of the as-prepared carbon samples decreases with the increase of S doping, demonstrating that the introduction of S atoms exhibits a positive impact on the hydrophilicity of the SPCM samples.
3.2 Adsorption properties
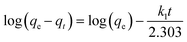 | (7) |
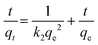 | (8) |
| qt = kit1/2 + C | (9) |
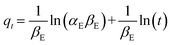 | (10) |
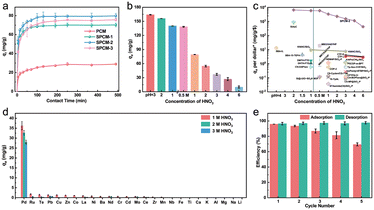 | ||
| Fig. 4 a) Adsorption curve of Pd(II) on the as-prepared porous carbon sorbents ([sorbent] = 200 mg L−1, Pd(II) = 40 mg L−1, [HNO3] = 1 M, T = 35 °C). b) Adsorption capacity of Pd(II) on S-functionalized porous carbon spheres (SPCM-2) at different HNO3 concentrations ([sorbent] = 200 mg L−1, Pd(II) = 1.0 mg L−1, T = 35 °C). c) Adsorption capacity per dollar of Pd(II) from HNO3 solution by SPCM-2 compared with previously reported sorbents (see Table S3† for details). d) Adsorption selectivity of Pd(II) by SPCM-2 in simulated acidic wastewater at different HNO3 concentrations ([sorbent] = 200 mg L−1, [HNO3] = 1/2/3 M, T = 35 °C). e) The cyclic stability of SPCM-2 ([sorbent] = 200 mg L−1, Pd(II) = 1.0 mg L−1, [HNO3] = 1 M, T = 35 °C). | ||
The fitting results are shown in Fig. S3 and Table S3,† in which the adsorption data of Pd(II) on the as-prepared porous carbon sorbents match better with the pseudo-second-order kinetic model compared to the pseudo-first-order kinetic model, the intra-particle diffusion kinetic model, and the Elovich kinetic model. It indicates that the adsorption behaviors of Pd(II) on these porous carbon samples are a chemical adsorption process, which is the rate control step of the reaction.49 The corresponding calculated qe values are 29.2, 70.5, 80.1 and 76.4 mg g−1 for the PCMs, SPCM-1, SPCM-2 and SPCM-3 sorbents, which are also closer to the experimental values. In addition, from the intra-particle diffusion model shown in Fig. S3c,† the linear fitting results do not pass through the origin and exhibit multiple linearities, demonstrating that the adsorption processes of Pd(II) on the surface of the as-prepared porous carbon samples are a multi-step process, mainly dominated by chemical adsorption.50
Fig. 4c presents the adsorption capacity per US dollar of Pd(II) by SPCM-2 compared with recently reported sorbents, and the corresponding details are shown in Table S4.† We can observe that our SPCM-2 sorbent possesses a clear advantage of low cost. The adsorption capacity per US dollar of Pd(II) by SPCM-2 from HNO3 solution is much higher than those of the reported mesoporous silica, MOF and POP sorbents, etc. It indicates that the as-prepared SPCM-2 has the potential for the recovery of Pd(II) from acidic wastewater.
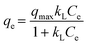 | (11) |
| qe = kFCe1/n | (12) |
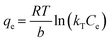 | (13) |
The fitted results and parameters of the adsorption isotherm model for Pd(II) on SPCM-2 are shown in Fig. S4a–d and Table S5.† The correlation coefficient R2 (0.961) of the Freundlich isotherm model is significantly higher than that of the Langmuir and Temkin isotherm models, which suggests that the adsorption of Pd(II) on SPCM-2 is more in line with the Freundlich model, indicating that the adsorption process may have occurred in multilayer adsorption rather than monolayer adsorption.51 In addition, the value of 1/n is generally between 0 and 1, and is usually used to evaluate the effect of concentration on the adsorption capacity.52 A smaller value of 1/n means a better adsorption performance. The obtained value of 1/n from the adsorption isotherms shown in Fig. S4b† is between 0.1 and 0.5, suggesting that the interaction between Pd(II) and the SPCM-2 sorbent is strong and the Pd(II) adsorption easily occurs on the surface of SPCM-2.53
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc | (14) |
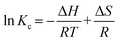 | (15) |
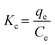 | (16) |
Fig. S5b† displays the linear fitting plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc to 1/T. According to the linear relationship between ln
Kc to 1/T. According to the linear relationship between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc and 1/T, the calculated adsorption thermodynamic parameters are summarized in Table S6.† The negative value of ΔH (−27.8 kJ mol−1) suggests that the adsorption of Pd(II) on SPCM-2 is an exothermic reaction, and lower temperatures are more favorable for the adsorption. The negative ΔS (−80.1 J mol−1 K−1) indicates a decrease in the randomness of the Pd(II) adsorption system. The negative ΔG at different temperatures indicates that the Pd(II) adsorption on SPCM-2 is a spontaneous process. This finding aligns with the literature that solute adsorption on adsorbents is an exothermic and entropy-decreasing process.55
Kc and 1/T, the calculated adsorption thermodynamic parameters are summarized in Table S6.† The negative value of ΔH (−27.8 kJ mol−1) suggests that the adsorption of Pd(II) on SPCM-2 is an exothermic reaction, and lower temperatures are more favorable for the adsorption. The negative ΔS (−80.1 J mol−1 K−1) indicates a decrease in the randomness of the Pd(II) adsorption system. The negative ΔG at different temperatures indicates that the Pd(II) adsorption on SPCM-2 is a spontaneous process. This finding aligns with the literature that solute adsorption on adsorbents is an exothermic and entropy-decreasing process.55
As observed in Fig. 4d, when the concentration of HNO3 is 1 M, the SPCM-2 sorbent exhibits superior adsorption capacity for Pd(II) compared to other competing ions. As the concentration of HNO3 increases, the adsorption capacity of Pd(II) on SPCM-2 gradually decreases because of the competitive adsorption of NO3− ions and the protonation of active sites in the adsorbent. Notably, the selectivity coefficients for Pd(II) increase with increasing HNO3 concentration, which is mainly attributed to the strong interaction between soft acid Pd(II) ions and soft base S functional groups. This suggests that the SPCM-2 sorbent maintains good separation ability for Pd(II) under high acidity conditions. Fig. S6† shows the capture of Pd(II) using the as-prepared SPCM-2 from actual industry acidic wastewater (pH = 0.69) containing Pd, Cu, Ni, Fe and Na metal ions and surfactants. It indicates that SPCM-2 exhibits high selectivity for the adsorption of Pd(II), further confirming its potential for the recovery of Pd(II) from acidic wastewater.
3.3 Adsorption mechanism
To elucidate the adsorption mechanism of Pd(II) on SPCM-2, characterization on the SPCM-2 sorbent before and after adsorption was carried out. Fig. S7† displays the pH-dependent zeta potential of SPCM-2 before and after Pd(II) adsorption. This indicates that the surface charge of the fresh SPCM-2 is positive in an acidic environment. The adsorption capacity of Pd(II) increases with the increase of the pH value of solution from 1 to 3, suggesting that the electrostatic reaction is conducive to the adsorption of Pd(II). However, Pd(II) ions usually appear as coordination complexes of [Pd(NO3)2]2−n in HNO3 solution, such as [Pd(NO3)2], [Pd(NO3)4]2−, etc.58,59 Therefore, the decrease of adsorption capacity for Pd(II) in high acidity demonstrates that the electrostatic adsorption is excluded in the adsorption of Pd(II) on SPCM-2. In addition, the zeta potentials of the used SPCM-2 after Pd(II) adsorption are lower than those of the fresh SPCM-2 at the pH values from 1 to 9, further implying that the coordination complexes of Pd(II) rather than the Pd2+ ions are involved in the adsorption process.XPS was employed to investigate the interaction between the adsorbate and the sorbent. Fig. 5a displays the survey XPS spectra of SPCM-2 before and after Pd(II) adsorption. The appearance of the Pd signal indicates that Pd is present on the surface of SPCM-2, and the decrease in the S signal implies that the S functional groups may participate in the adsorption of Pd(II). The Pd 3d spectrum shown in Fig. 5b exhibits two main peaks at 337.9 and 343.1 eV, corresponding to Pd 3d5/2 and Pd 3d3/2, respectively, which can be deconvoluted into four peaks, including 335.5 eV (Pd0 3d5/2), 337.9 eV (Pd2+ 3d5/2), 340.7 eV (Pd0 3d3/2) and 343.2 eV (Pd2+ 3d3/2).60 This indicates that a small amount of Pd(II) is reduced to Pd(0) on SPCM-2.
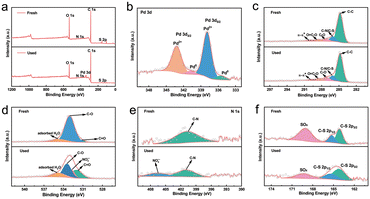 | ||
| Fig. 5 XPS spectra of the fresh S-functionalized porous carbon spheres (SPCM-2) and the used SPCM-2 after Pd(II) adsorption: a) survey, b) Pd 3d, c) C 1s, d) O 1s, e) N 1s, and f) S 2p. | ||
In addition, XPS spectra can reveal the interaction between coordination donors and metal ions based on the chemical shift of binding energy.61 As shown in Fig. 5c, the C 1s spectrum of the fresh SPCM-2 can be fitted into the five species C–C (284.8 eV), C–N/C–S (286.0 eV), C–O (287.2 eV), O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O (288.6 eV), and π–π* (290.1 eV). After adsorption of Pd(II), the binding energies of C–N/C–S, C–O, O
C–O (288.6 eV), and π–π* (290.1 eV). After adsorption of Pd(II), the binding energies of C–N/C–S, C–O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O and π–π* on the surface of the used SPCM-2 shift to 286.1, 287.1, 288.5 and 289.8 eV, respectively, suggesting that Pd(II) may interact with functional groups containing O, N and S during the adsorption process.62Fig. 5d shows the O 1s spectra of SPCM-2. The O 1s spectrum of the fresh SPCM-2 can be fitted to three peaks at 531.2, 533.0, and 534.0 eV, which are attributed to C
C–O and π–π* on the surface of the used SPCM-2 shift to 286.1, 287.1, 288.5 and 289.8 eV, respectively, suggesting that Pd(II) may interact with functional groups containing O, N and S during the adsorption process.62Fig. 5d shows the O 1s spectra of SPCM-2. The O 1s spectrum of the fresh SPCM-2 can be fitted to three peaks at 531.2, 533.0, and 534.0 eV, which are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and adsorbed H2O, respectively. After adsorption of Pd(II), four peaks at 532.0 (C
O, C–O, and adsorbed H2O, respectively. After adsorption of Pd(II), four peaks at 532.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 532.8 (NO3−), 533.4 (C–O), and 534.8 eV (adsorbed H2O) can be observed in the O 1s spectrum of the used SPCM-2. In addition, the content of C
O), 532.8 (NO3−), 533.4 (C–O), and 534.8 eV (adsorbed H2O) can be observed in the O 1s spectrum of the used SPCM-2. In addition, the content of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O increases, while C–O decreases after Pd(II) adsorption. This may be attributed to the electron transfer from C–O to the adsorbed Pd(II), resulting in the oxidation of C–O to C
O increases, while C–O decreases after Pd(II) adsorption. This may be attributed to the electron transfer from C–O to the adsorbed Pd(II), resulting in the oxidation of C–O to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the generation of Pd(0).
O and the generation of Pd(0).
The N 1s spectra of SPCM-2 before and after adsorption are shown in Fig. 5e; a new peak belonging to NO3− appears at 406.2 eV for the used SPCM-2 after Pd(II) adsorption, confirming that NO3− participates in the coordination with Pd(II) during the adsorption process. As presented in Fig. 5f, the S 2p spectra of the fresh SPCM-2 and the used SPCM-2 include peaks corresponding to S–C and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. After adsorption of Pd(II), the S 2p3/2 peak of S–C shifts from 164.2 to 163.2 eV and the S 2p1/2 peak from 165.4 to 164.4 eV, and the peak of S
O. After adsorption of Pd(II), the S 2p3/2 peak of S–C shifts from 164.2 to 163.2 eV and the S 2p1/2 peak from 165.4 to 164.4 eV, and the peak of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shifts from 169.1 to 168.3 eV. The obvious shifts are mainly attributed to the strong affinity between S functional groups and Pd(II) based on the hard and soft acid and base theory.
O shifts from 169.1 to 168.3 eV. The obvious shifts are mainly attributed to the strong affinity between S functional groups and Pd(II) based on the hard and soft acid and base theory.
Furthermore, the FTIR spectrum of the used SPCM-2 sorbent after Pd(II) adsorption was obtained. As shown in Fig. S8,† two new bands at 480 and 1384 cm−1 are attributed to Pd–O/S and NO3−, respectively, further demonstrating the coordination complexation between [Pd(NO3)2]2−n and O/S groups on SPCM-2. In addition, after Pd(II) adsorption, the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band is obviously higher than that of the C–O band due to the oxidation of C–O to C
O band is obviously higher than that of the C–O band due to the oxidation of C–O to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which is consistent with the O 1s XPS spectra presented in Fig. 5d.
O, which is consistent with the O 1s XPS spectra presented in Fig. 5d.
To further uncover the adsorption mechanism of Pd(II) on SPCM-2, the morphology and structure of the sorbent after Pd(II) adsorption were characterized. Fig. 6a displays the typical TEM image of Pd(II)-sorbed SPCM-2, which preserves the pristine morphology of the microspheres, suggesting that the SPCM-2 material exhibits excellent stability. Fig. 6b reveals that the honeycomb structure, consisting of carbon nanosheets, is present on the surface of these SPCM-2 microspheres. As shown in Fig. 6c, the HAADF-STEM image demonstrates the presence of crystalline nanoparticles. The corresponding HRTEM image in Fig. 6d shows that ordered lattice stripes can be observed on the surface of the disordered carbon nanosheets, further indicating the presence of crystalline nanoparticles. From Fig. 6e, the calculated fringe spacing is 2.25 Å, corresponding to the (111) crystal plane of the Pd(0) nanoparticles (JCPDS no. 46-1043).63 The EDS mapping images (Fig. 6f–i) indicate that the Pd element is uniformly adsorbed on the surface of SPCM-2.
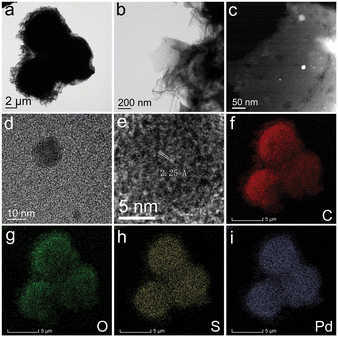 | ||
| Fig. 6 a and b) TEM, c) HAADF-STEM, d and e) HRTEM, and f–i) EDS mapping images of the used S-functionalized porous carbon spheres (SPCM-2) after Pd(II) adsorption. | ||
In addition, SPCM-2 after adsorption was analyzed by XRD. As shown in Fig. S9,† no diffraction peaks of metal Pd(0) are observed in the XRD pattern of the used SPCM-2 after Pd(II) adsorption. This indicates that the amount of Pd(0) on the surface of the sorbent is very small, which agrees well with the XPS spectrum shown in Fig. 5b. According to the above analysis, we propose that the adsorption of Pd(II) on SPCM-2 is dominated by the coordination interaction between the O/S containing groups of SPCM-2 and [Pd(NO3)n]2−n, and some of the Pd(II) is reduced to Pd(0).
In addition, the density-functional theory (DFT) calculations were used to gain insight into the adsorption mechanism of Pd(II) on SPCM-2. In the calculations, [Pd(NO3)2] was chosen as the adsorbate model for adsorbed Pd(II) since it has been previously demonstrated in the literature that the ratio of Pd2+ to NO3− of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is the lowest in energy and the most stable.58 Assuming that C–S, S
2 is the lowest in energy and the most stable.58 Assuming that C–S, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O
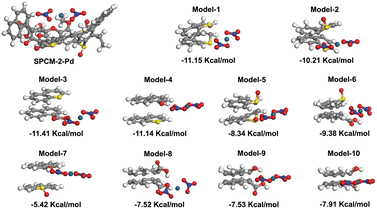
O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, and C–O are involved in the coordination between SPCM-2 and Pd(NO3)2, complexes and corresponding adsorption energies (Eads) were constructed after geometrical optimization, as shown in Fig. 7. According to the calculations, it is evident that the above binding sites on SPCM-2 can effectively interact with Pd(II) ions in nitrate medium. The Eads values from model 1 to model 10 are −11.15, −10.21, −11.41, −11.14, −8.34, −9.38, −5.42, −7.52, −7.53, and −7.91 kcal mol−1, respectively. A negative Eads value indicates that the adsorption process is exothermic. Usually, the higher the absolute value of Eads, the stronger the interaction between the adsorbent and the adsorbate, and the more stable the adsorption conformation.64,65 Therefore, for these 10 coordination models, the affinity of the corresponding models for Pd(II) ions is in the order 3 > 1 > 4 > 2 > 6 > 5 > 10 > 9 > 8 > 7, among which the 3-coordination model is the most stable and favorable adsorption conformation. According to the above results, the adsorption of Pd(II) on SPCM-2 is dominated by the coordination interaction between [Pd(NO3)2] and O/S containing groups and a small amount of Pd(II) is reduced to Pd(0) (Fig. 8).
C–O, and C–O are involved in the coordination between SPCM-2 and Pd(NO3)2, complexes and corresponding adsorption energies (Eads) were constructed after geometrical optimization, as shown in Fig. 7. According to the calculations, it is evident that the above binding sites on SPCM-2 can effectively interact with Pd(II) ions in nitrate medium. The Eads values from model 1 to model 10 are −11.15, −10.21, −11.41, −11.14, −8.34, −9.38, −5.42, −7.52, −7.53, and −7.91 kcal mol−1, respectively. A negative Eads value indicates that the adsorption process is exothermic. Usually, the higher the absolute value of Eads, the stronger the interaction between the adsorbent and the adsorbate, and the more stable the adsorption conformation.64,65 Therefore, for these 10 coordination models, the affinity of the corresponding models for Pd(II) ions is in the order 3 > 1 > 4 > 2 > 6 > 5 > 10 > 9 > 8 > 7, among which the 3-coordination model is the most stable and favorable adsorption conformation. According to the above results, the adsorption of Pd(II) on SPCM-2 is dominated by the coordination interaction between [Pd(NO3)2] and O/S containing groups and a small amount of Pd(II) is reduced to Pd(0) (Fig. 8).
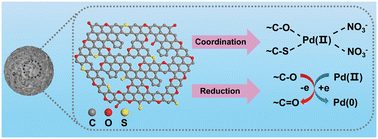 | ||
| Fig. 8 Possible adsorption mechanisms of Pd(II) on the S-functionalized porous carbon spheres (SPCM-2). | ||
4. Conclusions
S-functionalized porous carbon microsphere (SPCM) sorbents were prepared and exhibited high adsorption performance for Pd(II) capture from acidic solution due to their high specific surface area and high S doping. The optimized SPCM-2 sorbent demonstrated a high adsorption capacity of 79.3 mg g−1 and a fast adsorption equilibrium time of 130 min for Pd(II) in 1 M HNO3 solution, and its selectivity for Pd(II) adsorption increased with the increase of HNO3 concentration, resulting in the effective recovery of Pd(II) from a simulated acidic wastewater solution containing 26 metal ions. Kinetic and isotherm studies revealed that the adsorption behavior of Pd(II) on SPCM-2 agreed well with the pseudo-second-order and Freundlich models, indicating that the adsorption process is a multilayer chemical adsorption process. Additionally, the SPCM-2 sorbent exhibited high economic viability and good regeneration and reusability. The adsorption mechanisms of SPCM-2 for the capture of Pd(II) involve the coordination of [Pd(NO3)2] with O/S-containing groups and the reduction of Pd(II) to Pd(0). These impressive results suggest that the as-prepared SPCM sorbents have potential for the recovery of Pd(II) from acidic wastewater.Data availability
The data that support the findings of this study are available from the corresponding author, D Chen, E-mail: chendz@nchu.edu.cn, upon reasonable request.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation (20232ACB203010) of Jiangxi Province, China. We acknowledge the support from the Key Laboratory of Jiangxi Province for Persistent Pollutants Prevention Control and Resource Reuse (No. 2023SSY02061), Jiangxi Province, China. We are grateful to the High Performance Computing Center of Nanchang Hangkong University for the DFT calculations.Notes and references
- Y. Chen, Q. Qiao, J. Cao, H. Li and Z. Bian, Precious metal recovery, Joule, 2021, 5, 3097–3115 CrossRef CAS.
- S. C. Cao, D. Y. Li, A. A. Uliana, Y. L. Jiang, J. Y. Zhu, Y. T. Zhang and V. B. Bart, Multifunctional covalent organic framework membranes with an ultrathin recycled palladium nanolayer for efficient water decontamination, Appl. Catal., B, 2023, 323, 122175 CrossRef CAS.
- Y. Su, A. Berbille, X.-F. Li, J. Zhang, M. PourhosseiniAsl, H. Li, Z. Liu, S. Li, J. Liu, L. Zhu and Z. L. Wang, Reduction of precious metal ions in aqueous solutions by contact-electro-catalysis, Nat. Commun., 2024, 15, 4196 CrossRef CAS PubMed.
- A. Zupanc, J. Install, M. Jereb and T. Repo, Sustainable and selective modern methods of noble metal recycling, Angew. Chem., Int. Ed., 2022, 62, e202214453 CrossRef PubMed.
- Y. Chen, M. J. Xu, J. Y. Wen, Y. Wan, Q. F. Zhao, X. Cao, Y. Ding, Z. L. Wang, H. X. Li and Z. F. Bian, Selective recovery of precious metals through photocatalysis, Nat. Sustain., 2021, 4, 618–626 CrossRef.
- J. S. Xia and A. Ghahreman, Platinum group metals recycling from spent automotive catalysts: metallurgical extraction and recovery technologies, Sep. Purif. Technol., 2023, 311, 123357 CrossRef CAS.
- P. C. Wu, H. Liu, M. Q. Sun, Y. X. Zeng, J. W. Ye, S. Qin, Y. M. Cai, W. Feng and L. H. Yuan, Covalent triazine frameworks for the selective sorption of palladium from highly acidic radioactive liquid wastes, J. Mater. Chem. A, 2021, 9, 27320–27331 RSC.
- Y. Wang, Y. J. Wu, J. H. Li, Q. Li, P. H. Yang, S. D. Conradson, Y. M. Cai, W. Feng and L. H. Yuan, Ultra-selective and efficient static/dynamic palladium capture from highly acidic solution with robust macrocycle-based polymers, Adv. Funct. Mater., 2023, 33, 202304051 Search PubMed.
- S. Kancharla and K. Sasaki, Acid tolerant covalently functionalized graphene oxide for the selective extraction of Pd from high-level radioactive liquid wastes, J. Mater. Chem. A, 2019, 7, 4561–4573 RSC.
- Y. Y. Bai, L. Chen, L. W. He, B. Y. Li, L. X. Chen, F. Q. Wu, L. H. Chen, M. X. Zhang, Z. Y. Liu, Z. F. Chai and S. A. Wang, Precise recognition of palladium through interlaminar chelation in a covalent organic framework, Chem, 2022, 8, 1442–1459 CAS.
- A. Cieszynska and D. Wieczorek, Extraction and separation of palladium(II), platinum(IV), gold(III) and rhodium(III) using piperidine-based extractants, Hydrometallurgy, 2018, 175, 359–366 CrossRef CAS.
- J. Lee, W. Kurnia, H. Hong, K. W. Chung and S. Kim, Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review, Sep. Purif. Technol., 2020, 246, 116896 CrossRef CAS.
- S. Abdi, M. Nasiri and B. Van der Bruggen, Selective separation of gold and palladium using the improved Gemini Micellar-Enhanced ultrafiltration, Chem. Eng. J., 2022, 444, 136570 CrossRef CAS.
- A. V. Oladeji, J. M. Courtney, M. Fernandez-Villamarin and N. V. Rees, Electrochemical metal recycling: recovery of palladium from solution and in situ fabrication of palladium-carbon catalysts via impact electrochemistry, J. Am. Chem. Soc., 2022, 144, 18562–18574 CrossRef CAS PubMed.
- H. Chaudhuri, C. Lim and Y. Yun, Polyethylenimine functionalized sulfur-containing POSS-based dendritic adsorbent for highly efficient and selective capturing of precious metal ions, Desalination, 2023, 566, 116925 CrossRef CAS.
- S. Mincke, T. G. Asere, I. Verheye, K. Folens, F. Vanden Bussche, L. Lapeire, K. Verbeken, P. Van Der Voort, D. A. Tessema, F. Fufa, G. Du Laing and C. V. Stevens, Functionalized chitosan adsorbents allow recovery of palladium and platinum from acidic aqueous solutions, Green Chem., 2019, 21, 2295–2306 RSC.
- L. F. Peng, M. M. Zhang, Z. Dong, W. Qi, M. L. Zhai and L. Zhao, Efficient and selective adsorption of Pd(II) by amino acid-functionalized cellulose microspheres and their applications in palladium recovery from PCBs leaching solution, Sep. Purif. Technol., 2022, 301, 122037 CrossRef CAS.
- Q. Xiao, X. Y. Wang, L. J. Song, F. F. Li, Q. J. Li, L. L. He, Z. Shen, F. X. Luo and S. D. Ding, Highly efficient and selective adsorption of palladium(II) from simulated nuclear waste solution using Amberlite XAD-7 resin impregnated with a phenanthroline-derived diamide, Hydrometallurgy, 2023, 221, 106127 CrossRef CAS.
- H. Wu, S. Kim, M. Miwa and S. Matsuyama, Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste, J. Hazard. Mater., 2021, 411, 125136 CrossRef CAS PubMed.
- D. Saba, G. M. Mahbobeh, O. Ali Reza, K. Mostafa, N. Sergio, Â. Mercedes, G. M. Mansour, S. D. Hojat and G. Hermenegildo, A pyridyltriazol functionalized zirconium metal–organic gramework for selective and highly efficient adsorption of palladium, ACS Appl. Mater. Interfaces, 2020, 12, 25221–25232 CrossRef.
- B. Aguila, Q. Sun, H. C. Cassady, C. Shan, Z. Liang, A. M. Al-Enizic, A. Nafadyc, J. T. Wright, R. W. Meulenberg and S. Ma, A porous organic polymer nanotrap for efficient extraction of palladium, Angew. Chem., Int. Ed., 2020, 59, 19618–19622 CrossRef CAS.
- Y. Yuan, L. Han and N. Wang, Selective recovery of palladium from nuclear waste by covalent organic framework, Matter, 2022, 5, 3086–3088 CrossRef CAS.
- S. Sharifian and N.-H. L. Wang, Resin-based approaches for selective extraction and purification of rare earth elements: A comprehensive review, J. Environ. Chem. Eng., 2024, 12, 112402 CrossRef CAS.
- S. Sadjadi and M. M. Heravi, Current advances in the utility of functionalized SBA mesoporous silica for developing encapsulated nanocatalysts: state of the art, RSC Adv., 2017, 7, 30815–30838 RSC.
- Y. An, X. Lv, W. Jiang, L. Wang, Y. Shi, X. Hang and H. Pang, The stability of MOFs in aqueous solutions—research progress and prospects, Green Chem. Eng., 2024, 5, 187–204 CrossRef.
- S. S. A. Shah, M. Sohail, G. Murtza, A. Waseem, A. u. Rehman, I. Hussain, M. S. Bashir, S. S. Alarfaji, A. M. Hassan, M. A. Nazir, M. S. Javed and T. Najam, Recent trends in wastewater treatment by using metal-organic frameworks (MOFs) and their composites: A critical view-point, Chemosphere, 2024, 349, 140729 CrossRef CAS PubMed.
- D. Luo, T. Shi, Q.-H. Li, Q. Xu, M. Strømme, Q.-F. Zhang and C. Xu, Green, general and low-cost synthesis of porous organic polymers in sub-kilogram scale for catalysis and CO2 capture, Angew. Chem., Int. Ed., 2023, 62, e202305225 CrossRef CAS PubMed.
- S. Iftekhar, G. Heidari, N. Amanat, E. N. Zare, M. B. Asif, M. Hassanpour, V. P. Lehto and M. Sillanpaa, Porous materials for the recovery of rare earth elements, platinum group metals, and other valuable metals: a review, Environ. Chem. Lett., 2022, 20, 3697–3746 CrossRef CAS.
- K. Fu, X. Liu, X. Zhang, S. Zhou, N. Zhu, Y. Pei and J. Luo, Utilizing cost-effective pyrocarbon for highly efficient gold retrieval from e-waste leachate, Nat. Commun., 2024, 15, 6137 CrossRef CAS.
- L. Zhang, B. Li, P. Shao, X. Zhou, D. Li, Z. Hu, H. Dong, L. Yang, H. Shi and X. Luo, Selective capture of palladium from acid wastewater by thiazole-modified activated carbon: Performance and mechanism, Environ. Res., 2023, 238, 117253 CrossRef CAS PubMed.
- J. Fan, L. Duan, X. Zhang, Z. Li, P. Qiu, Y. He and P. Shang, Selective adsorption and recovery of silver from acidic solution using biomass-derived sulfur-doped porous carbon, ACS Appl. Mater. Interfaces, 2023, 15, 40088–40099 CrossRef CAS.
- X. Gang, M. Krishnamoorthy, W. Jiang, J. Pan, Z. Pan and X. Liu, A novel in-situ preparation of N-rich spherical porous carbon as greatly enhanced material for high-performance supercapacitors, Carbon, 2021, 171, 62–71 CrossRef CAS.
- K. Z. Elwakeel, A. S. Al-Bogami and E. Guibal, 2-Mercaptobenzimidazole derivative of chitosan for silver sorption – Contribution of magnetite incorporation and sonication effects on enhanced metal recovery, Chem. Eng. J., 2021, 403, 126265 CrossRef CAS.
- A. M. Elgarahy, A. S. Al-Bogami, A. Akhdhar, Z. A. Khan and K. Z. Elwakeel, Silver ions immobilized on thiourea/formaldehyde resin for solid phase extraction of iodide ions from aqueous solution, J. Mol. Liq., 2023, 376, 121474 CrossRef CAS.
- R.-s. Wang, Y. Li, X.-x. Shuai, R.-h. Liang, J. Chen and C.-m. Liu, Pectin/activated carbon-based porous microsphere for Pb2+ adsorption: Characterization and adsorption behaviour, Polymers, 2021, 13, 2453 CrossRef CAS.
- B. Delley, From molecules to solids with the DMol3 approach, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS.
- B. Delley, An all-electron numerical method for solving the local density functional for polyatomic molecules, J. Chem. Phys., 1990, 92, 508–517 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- Y. Hu, D. Z. Chen, R. Zhang, Y. Ding, Z. Ren, M. S. Fu, X. K. Cao and G. S. Zeng, Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway, J. Hazard. Mater., 2021, 419, 126495 CrossRef CAS.
- X. Y. Meng, C. C. Zhang, C. Z. Dong, W. J. Sun, D. Ji and Y. Ding, Carbon quantum dots assisted strategy to synthesize Co@NC for boosting photocatalytic hydrogen evolution performance of CdS, Chem. Eng. J., 2020, 389, 124432 CrossRef CAS.
- H. T. Yu, Y. Xu, K. Havener, M. Zhang, L. Zhang, W. J. Wu and K. Huang, Temperature-controlled selectivity of hydrogenation and hydrodeoxygenation of biomass by superhydrophilic nitrogen/oxygen co-doped porous carbon nanosphere supported Pd nanoparticles, Small, 2022, 18, 1–10 Search PubMed.
- Q. Ai, Q. Y. Fang, J. Liang, X. Y. Xu, T. S. Zhai, G. H. Gao, H. Guo, G. F. Han, L. j. Ci and J. Lou, Lithium-conducting covalent-organic-frameworks as artificial solid-electrolyte-interphase on silicon anode for high performance lithium ion batteries, Nano Energy, 2020, 72, 104657 CrossRef CAS.
- Q. Q. Ran, X. P. Wang, P. Ling, P. W. Yan, J. Xu, L. Jiang, Y. Wang, S. Su, S. Hu and J. Xiang, A thermal-assisted electrochemical strategy to synthesize carbon dots with bimodal photoluminescence emission, Carbon, 2022, 193, 404–411 CrossRef CAS.
- J. Zhao, Y. F. Wei, Y. F. Xia, Z. M. Wang, H. F. Tang, M. X. Tan, X. X. Liu, J. F. Shi and C. B. Liu, Rapid and effective removal of arsenite from water using a novel oxidation-sorption bifunctional MOF, Chem. Eng. J., 2023, 476, 146787 CrossRef CAS.
- Y. Wei, Y. Liu, T. Y. Wang, G. J. Zhang, L. W. Yang, C. S. He, Z. K. Xiong, Z. C. Pan and B. Lai, N, S co-doped porous carbon to accelerate Fe3+/Fe2+ redox cycle for peroxymonosulfate activation, Sep. Purif. Technol., 2024, 328, 125080 CrossRef CAS.
- H. Liu, L. Long, X. Weng, S. R. Zheng and Z. Y. Xu, Efficient removal of tetrabromobisphenol A using microporous and mesoporous carbons: The role of pore structure, Microporous Mesoporous Mater., 2020, 298, 110052 CrossRef CAS.
- R. L. Tseng and S. K. Tseng, Pore structure and adsorption performance of the KOH-activated carbons prepared from corncob, J. Colloid Interface Sci., 2005, 287, 428–437 CrossRef CAS.
- G. Q. Wu, H. Y. Wang, L. Z. Huang, L. Huang, J. Yan, X. X. Chen, Y. Xiao, X. J. Liu and H. G. Zhang, Gas exfoliation induced N, S-doped porous 2D carbon nanosheets for effective removal of copper ions by capacitive deionization, Desalination, 2023, 565, 116881 CrossRef CAS.
- X. X. Gao, B. S. Liu and X. D. Zhao, Thiol-decorated defective metal-organic framework for effective removal of mercury(II) ion, Chemosphere, 2023, 317, 137891 CrossRef CAS.
- J. Y. Tang, Y. F. Ma, C. Y. Zeng, L. Yang, S. Cui, S. Zhi, F. X. Yang, Y. Z. Ding, K. Q. Zhang and Z. L. Zhang, Fe-Al bimetallic oxides functionalized-biochar via ball milling for enhanced adsorption of tetracycline in water, Bioresour. Technol., 2023, 369, 128385 CrossRef CAS PubMed.
- Y. H. Li, M. Y. Liu, Y. W. Wei, C. C. Wang and P. Wang, Adsorption and photocatalytic desorption toward Cr(vi) over defect-induced hierarchically porous UiO-66-(OH)2: a sustainable approach, Environ. Sci.: Nano, 2023, 10, 672–682 RSC.
- S. S. Fan, Y. Wang, Z. Wang, J. Tang, J. Tang and X. D. Li, Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism, J. Environ. Chem. Eng., 2017, 5, 601–611 CrossRef CAS.
- X. G. Ji, Y. C. Liu, Z. L. Gao, H. Lin, X. Y. Xu, Y. Zhang, K. Zhu, Y. X. Zhang, H. W. Sun and J. M. Duan, Efficiency and mechanism of adsorption for imidacloprid removal from water by Fe-Mg co-modified water hyacinth-based biochar: Batch adsorption, fixed-bed adsorption, and DFT calculation, Sep. Purif. Technol., 2024, 330, 125235 CrossRef CAS.
- N. Dai, X. Liu, L. Yang, X. Huang, D. Song, S. Wang, K. Zhang, X. Liu, W. Dong and Y. Zhang, Cetyltrimethylammonium bromide-modified laponite@diatomite composites for enhanced adsorption performance of organic pollutants, Langmuir, 2024, 40, 8427–8439 CrossRef CAS.
- I. Ghosh, S. Kar, T. Chatterjee, N. Bar and S. K. Das, Removal of methylene blue from aqueous solution using Lathyrus sativus husk: Adsorption study, MPR and ANN modelling, Process Saf. Environ. Prot., 2021, 149, 345–361 CrossRef CAS.
- C. Zeng, P. Liu, Z. L. Xiao, Y. Li, L. B. Song, Z. Cao, D. X. Wu and Y. F. Zhang, Highly selective adsorption and recovery of palladium from spent catalyst wastewater by 1,4,7,10-tetraazacyclododecane-modified mesoporous silica, ACS Sustainable Chem. Eng., 2022, 10, 1103–1114 CrossRef CAS.
- Z. Wang, S. B. Kang and S. W. Won, Selective adsorption of palladium(II) from aqueous solution using epichlorohydrin crosslinked polyethylenimine-chitin adsorbent: Batch and column studies, J. Environ. Chem. Eng., 2021, 9, 105058 CrossRef CAS.
- H. R. Dong, S. Y. Ning, Z. Y. Li, S. Z. Xu, S. C. Zhang, X. P. Wang, Y. B. Wang, L. F. Chen, X. B. Yin, T. Fujita, M. F. Hamza and Y. Z. Wei, Efficient separation of palladium from high-level liquid waste with novel adsorbents prepared by sulfhydryl organic ligands containing imidazole, thiazole and oxazole composited with XAD7HP, J. Water Process Eng., 2023, 53, 103681 CrossRef.
- S. Watanabe, T. Sato, T. Yoshida, M. Nakaya, M. Yoshino, T. Nagasaki, Y. Inaba, K. Takeshita and J. Onoe, Spectroscopic and first-principles calculation studies of the chemical forms of palladium ion in nitric acid solution for development of disposal of high-level radioactive nuclear wastes, AIP Adv., 2018, 8, 045221 CrossRef.
- X. Jiang, Y. Zhou, H. O. Chen, R. Zhang, J. H. Yu, S. X. Wang, F. Z. Jiang, H. P. Bai and X. J. Yang, A novel hydrangea-like magnetic composite Fe3O4@MnO2@ZIF-67 for efficient selective adsorption of Pd(II) from metallurgical wastewater, Chemosphere, 2023, 344, 140432 CrossRef CAS PubMed.
- F. C. Wu, C. T. Yang, Y. Liu, S. Hu, G. Ye and J. Chen, Novel polyazamacrocyclic receptor impregnated macroporous polymeric resins for highly efficient capture of palladium from nitric acid media, Sep. Purif. Technol., 2020, 233, 115953 CrossRef CAS.
- K. Ning, M. Q. Wei, Z. L. Jiang, T. Jiang, G. Z. Zhao, L. Han, G. Zhu and Y. Y. Zhu, S and O doped porous carbon hollow bubble for Zn ion capacitors with enhanced energy density and long life, Mater. Lett., 2024, 355, 135316 CrossRef CAS.
- H. X. Liu, X. X. Yan, W. H. Luo, J. Liu and S. L. Ren, Effect of Pd crystal facet on the reaction of oxygen-promoted hydrogen evolution from formaldehyde driven by visible light, Colloids Surf., A, 2023, 673, 131820 CrossRef CAS.
- S. Pandey, B. Demaske, O. A. Ejegbavwo, A. A. Berseneva, W. Setyawan, N. Shustova and S. R. Phillpot, Electronic structures and magnetism of Zr-, Th-, and U-based metal-organic frameworks (MOFs) by density functional theory, Comput. Mater. Sci., 2020, 184, 109903 CrossRef CAS.
- C. Wang, C. Xiong, Y. L. He, C. Yang, X. T. Li, J. Z. Zheng and S. X. Wang, Facile preparation of magnetic Zr-MOF for adsorption of Pb(II) and Cr(VI) from water: Adsorption characteristics and mechanisms, Chem. Eng. J., 2021, 415, 128923 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00738g |
| This journal is © The Royal Society of Chemistry 2025 |