Chemical reactivity of weathered nanoplastics and their interactions with heavy metals
Yingnan
Huang
,
Fei
Dang
 * and
Yujun
Wang
* and
Yujun
Wang

State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 211135, China. E-mail: fdang@issas.ac.cn
First published on 7th November 2024
Abstract
There is growing concern about the threat that nanoplastics (NPs) pose to ecosystems. However, a comprehensive risk assessment of NPs is currently constrained by the paucity of knowledge on the chemical reactivity of NPs, which were previously thought to be chemically inert. This review identifies the chemical reactivity of NPs that have undergone abiotic and biotic weathering, including the formation of free radicals, the increase in the content of oxygen-containing functional groups, and the release of plastic leachates. Their interaction with legacy contaminants, such as heavy metals (HMs), is then examined, highlighting their critical role in the oxidation and reduction of HMs, through free radical-mediated redox processes and electron shuttling by carbonyl groups. This review offers new insights into the risk of NPs, where their interaction with legacy contaminants determines the long-term exposure scenario for ecosystems. The unexpectedly large pool of reactive NPs in nature will not only affect their risks but also impact the biogeochemistry of HMs and other contaminants that could react with free radicals and carbonyl groups.
Environmental significanceNanoplastics (NPs) are ubiquitous and an emerging concern in the natural environment. The chemical reactivity of NPs has significant implications for their risks to human and ecosystem health. This review provides a comprehensive understanding of the chemical reactivity of NPs that have undergone abiotic and biotic weathering. We suggest that these reactive NPs are capable of triggering the transformation of legacy contaminants, such as heavy metals. The identification of the chemical reactivity of NPs offers new insights into their role in the geochemical cycling of heavy metals and the associated risks. |
1. Introduction
The rapid growth of plastic production, coupled with inefficient disposal and recycling systems, has resulted in the release of plastic waste into the environment.1–3 The accumulated plastics in the environment are subject to dynamic weathering via biotic (e.g., mediated by microorganisms) and/or abiotic processes (e.g., induced by ultraviolet light), resulting in the fragmentation of plastics into nanoplastics (NPs, <1000 nm).4–8 The presence of these NPs has been confirmed in a range of environmental samples. For instance, using pyrolysis gas chromatography-mass spectrometry (Py-GC-MS), Ter Halle et al. estimated that the spectrometric signal of the colloidal aromatic fraction (<1000 nm) of seawater in the North Atlantic subtropical gyre was attributable to a mixture comprising 73% polyvinyl chloride (PVC), 18% polyethylene glycol terephthalate (PET), and 9% polystyrene (PS).9 Wahl et al. observed polyethylene (PE), PS, and PVC within a 20–150 nm size range in water extracts from soil.10 Additionally, Materić et al. identified PET NPs at 5.4–27.4 ng mL−1 in surface snow samples from the Australian Alps.11 Despite these findings, the analysis of NPs in environmental samples is still emerging, with current protocols immature and needing further development. It is estimated that NPs are generally not intentionally designed and vary widely in shape, size, polydispersity, additives, adsorbed contaminants, surface properties, and composition due to different source materials, fragmentation pathways, and environmental exposure.6 The resulting physical and chemical heterogeneity of NPs may influence their reactivity and will certainly affect their interactions with natural components and organisms.6 Pieces of evidence show that NPs can interact with and cross biological barriers, including epithelial tissues and cell membranes,12,13 posing toxicity risks to organisms. Reported impacts include oxidative stresses, immobility, disrupted organ function, reduced reproduction, and potential bioaccumulation throughout food chains.14–17 However, biased risk assessments of NPs could be obtained by ignoring the coexistence of NPs and legacy contaminants, such as heavy metals (HMs), in nature.NPs and HMs co-occur either through the incorporation of HMs into NP manufacturing or through their common pathways to the environment. In particular, the improper handling of plastic products and the consequent escalating utilization of these materials has resulted in a substantial influx of NPs into the environment.18 The majority of HMs (e.g., Cd, Pb, Zn, Hg) serves a variety of functions in the production of plastic, acting as pigments, stabilizers, and fillers.19–21 These HMs are readily released following weathering processes. For example, 26.5% of Pb in PVC was released upon UV irradiation for 40 h,22 while 62.2% of Ni, 6.5% of Cd, and a range of 5.4–40.3% of Zn present in electronic plastics were released upon natural light irradiation for 112 days.23 In parallel, NPs inevitably interact with HMs in municipal wastewater plants, surface waters, sediments, and soils.24,25 The highly likely interaction between NPs and HMs may introduce an uncharacterized variable in the exposure of NPs, which results in toxicological effects that differ from those observed in NP exposure alone.
There is no consensus on the toxicological effects of NPs in the presence of HMs. Nanoplastics (e.g., PS at 300–9000 nm, PE at 50–533 nm) and HMs (e.g., Ag, Au, Cu, Zn, Pb, Cd) have been reported to exert synergistic toxic effects on a variety of organisms, including the lettuce Lactuca sativa L., the alga Chlorella vulgaris, Daphnia magna, and zebrafish embryos.26–29 Elsewhere, antagonistic interaction has been reported between NPs and HMs. For example, Alaraby et al. demonstrated that PS NPs (80 nm) reduced oxidative stress and genotoxicity in Drosophila in the presence of Ag(I), compared to the Ag(I) group alone.30 The discrepancy in the literature can be attributed to the differences in the physicochemical properties of NPs (e.g., size and type) and biological traits.31–33 Moreover, it is most likely that the interaction between NPs and HMs is not considered, where PS could induce the oxidation of Ag(0) to Ag(I).34 It is therefore crucial to examine the chemical reactivity of NPs and their interaction with HMs.
To gain insight into the interaction between NPs and HMs, we analyzed the literature of the past 34 years from the Web of Science database using Citespace (Fig. 1). A total of 263 publications were identified using the keywords “Nanoplastic* OR Nano plastic*” and “Heavy metals*”. The first publication on NPs and HMs appeared in the 1990s, focusing on the application of HMs in plastic production. From 2000 to 2010, most studies focused on the properties of plastics. In the past 10 years, there have been efforts to examine the absorption of HMs on NPs, and their combined effects. However, the chemical reactivity of NPs remains less understood.
This review begins by discussing the weathering and the resulting chemical reactivity of NPs. It then examines the role of weathered NPs in the adsorption and transformation of HMs. Finally, we identify areas requiring further research and suggest potential avenues for future studies. Understanding the chemical reactivity of weathered NPs, and thus their interaction with HMs, is crucial for determining the long-term exposure scenario for ecosystems as well as for developing a comprehensive risk assessment of NPs.
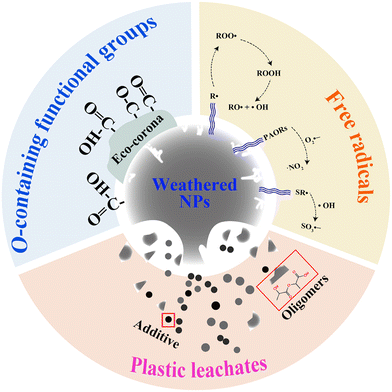
2. Chemical reactivity of weathered NPs
Weathered NPs undergo physicochemical modification, leading to the chemical reactivity, including the production of free radicals, an increase in the content of oxygen-containing functional groups, and the release of plastic leachates (Fig. 2).2.1 The generation of free radicals from weathered NPs
Most studies focus on sunlit surfaces, with little attention to non-sunlit environments. In fact, the majority of NPs persist in the dark environments, including deeper water or landfills.48,49 Whether and how free radicals would be produced during light-independent weathering remains largely unknown. Chen et al. have demonstrated that semiquinone radicals and H2O2 were produced from phenol-formaldehyde resins after hydrogen peroxide treatment in the dark.50 Future research on light-independent weathering of NPs is urgently needed.
The generation of free radicals from light-weathered NPs can be quantified using electron paramagnetic resonance (EPR) or chemical probe methods. After 60 days of light weathering, Li et al. used EPR to demonstrate that the levels of O2˙−, ·OH, and 1O2 produced from PS NPs (100 nm) increased by 8.6-, 15.3-, and 4.3-fold, respectively.53 Using chemical probe methods, Zhu et al. demonstrated that light-weathered PS (0.05 g) can generate O2˙− and ·OH at concentrations of 0.003 and 0.0003 μmol g−1 d−1, respectively.54 It is thus estimated that 500 g of light-weathered PS could induce O2˙− formation at a level of 1.5 μmol d−1. These concentrations of O2˙− are comparable to those detected in floodplain, riverine wetland, and river band soils (0.3–4 μmol d−1).55 Collectively, weathered NPs play a previously unrecognized role in contributing to total ROS production in nature, with significant implications for biogeochemical processes of elements.
2.2 The increase in oxygen-containing functional groups on weathered NPs
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) peak to a stable reference peak, has been utilized to measure the extent of polymer oxidation.46 For example, using the CI values as a measure, Luo et al. demonstrated that the CI values of PE increased rapidly in the initial stage of 2 weeks during thermal weathering but remained stable after 5 weeks.58 Additionally, Yu et al. observed a significant positive correlation between the CI values of PE and UV irradiation time, indicating that oxidation could be effectively predicted.59
O) peak to a stable reference peak, has been utilized to measure the extent of polymer oxidation.46 For example, using the CI values as a measure, Luo et al. demonstrated that the CI values of PE increased rapidly in the initial stage of 2 weeks during thermal weathering but remained stable after 5 weeks.58 Additionally, Yu et al. observed a significant positive correlation between the CI values of PE and UV irradiation time, indicating that oxidation could be effectively predicted.59
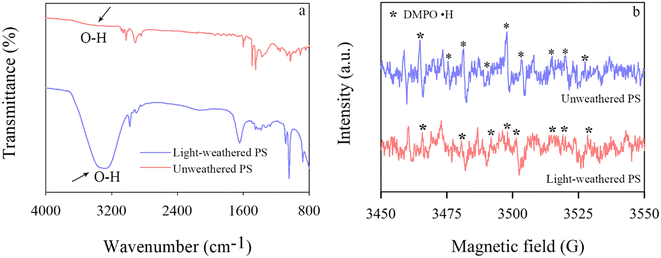
Abiotic oxidation of NPs is influenced by natural organic matter (NOM) and clay minerals. There is no consensus on the role of NOM in the oxidation of NPs. Suwannee River fulvic acid (SRFA) has been reported to facilitate the oxidation PS NPs (50–1000 nm) by generation of ·OH and O2˙− under light irradiation, resulting in an 8–11-fold increase in O/C ratios.60 In contrast, Cao et al. demonstrated that SRFA quenched ·OH and weakened the oxidation process, and the oxygen-containing functional groups did not vary significantly on PS NPs (100 nm).61 These disparate results could be attributed to the heterogeneity of PS NPs, which can be oxidized rather through laboratory weathering or during the polymer processing and/or storage. The latter was demonstrated by a small amount of oxygen atoms (2.21%) and oxygen-containing functional groups observed on unweathered PS.42 We have observed oxygen-containing functional groups in PS NPs (1000 nm) either before or after laboratory weathering under stimulated sunlight (Fig. 3a, unpublished data). Minerals are another important factor. Kaolinite and montmorillonite have been demonstrated to accelerate the oxidation of PVC and PET via the formation of ·OH, resulting in a 1.5–1.8-fold increase in the O/C ratio under light irradiation.62 Similar phenomena have also been observed with iron (hydr)oxides, whose O/C ratio increased by 1.6–2.2-fold under irradiation.63 Collectively, these studies demonstrate the impacts of NOM and clays on the oxidation of NPs under irradiation, with less consideration of other weathering processes.
In addition to abiotic pathways, biotic weathering can involve the oxidation of NPs by the formation of eco-corona, microbial degradation of NPs, and grinding NPs by animals. The biomolecular coating that often forms on NPs is often referred to as the eco-corona.64 This coating includes proteins as well as a diverse array of other biomolecules such as metabolites from cellular activity and/or NOM.64 The biomolecular coating is rich in functional groups, which provide oxygen-containing functional groups on NPs. For example, Zhu et al. demonstrated that the O/C ratio increased by 0.7–1.4 times and 0.3–0.8 times, respectively, with the formation of the eco-corona on weathered PS NPs (448 nm) and weathered HOOC-PS (476 nm).65
These biomolecules can trigger the degradation of NPs, which involves the oxidation of NPs. Enzymes derived from multiple microorganisms such as Bacillus subtilis, Thermobifida fusca, and Pseudomonas sp. have been demonstrated to degrade plastics, such as PET and polyurethane (PUR), primarily through hydrolysis of ester bonds.5,66–68 In the case of non-hydrolyzable polymers, such as PE and PS, enzymes degrade them through the oxidation occurring in-chain and at the termini, resulting in the formation of dicarboxylic acids, alcohols, fatty acids, ketones, aldehydes, and esters.69 Using Fourier-transform infrared (FTIR) spectroscopy, Gao et al. detected the formation of carbonyl groups on PE following the incubation with A. alternata FB1.70 Furthermore, Sathiyabama et al. demonstrated that lignolytic enzymes derived from Cladosporium sphaerospermum increased the content of carbonyl groups in weathered PE by 1.0–1.3 times compared to the unweathered counterparts.71 In addition to enzymes, biotic secretion may contribute to the oxidation of NPs. Using in situ FTIR, Zhang et al. demonstrated that PS NPs (20–1000 nm) incubated with secretions derived from amoeba D. discoideum exhibited a unique carbonyl group stretching vibration peak at 1730–1750 cm−1.72 In terms of NOM, they are capable of participating in the oxidation of PS NPs (1000 nm) on both sunlit surfaces and in the absence of light.73 This can be achieved through the formation of ROS, including O2˙−, ·OH, and 1O2, resulting in an increase of up to 10.7-fold in the O/C ratio in PS.73 Given that NPs are smaller than microorganisms, oxidation through colonization of microorganisms is unlikely to occur in NPs.74 Our current knowledge about microbial interactions with NPs is limited by the challenge of efficient extraction and analysis of NPs in environmental and biological matrices.75
Another consideration is grinding NPs by animals, rather than through the well-documented microbial degradation that occurs in their guts.76 Using confocal Raman microscopy (LCM-Raman) and scanning electron microscopy (SEM), Zhao et al. demonstrated that rotifers can grind the edges of PS, with irregular tear marks on weathered PS.77 These tear marks enable accessibility of oxygen to the internal layer of plastics, promoting the oxidation.56 Other animals species have been reported to grind plastics, such as Antarctic krill (Euphausia superba),78 yet there is a paucity of knowledge regarding the impact of such action on the degree of oxidation of NPs.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), carboxyl (–COOH), hydroxyl groups (O–H), and quinones.45,79,80 For example, oxidized phenol-formaldehyde resins had electron-donating capacity (0.264–1.15 mmol e− g−1) and electron-accepting capacity (0.120–0.300 mmol e− g−1) due to the quinone and carboxyl groups.50 These functional groups have been documented to facilitate the transformation of HMs, such as the reduction of Ag(I) and Cr(VI).81,82 Additionally, oxidized PS had electron-rich carbonyl groups, which were responsible for the transformation of HMs via complexing with charged HMs followed by electron shutting.83
O), carboxyl (–COOH), hydroxyl groups (O–H), and quinones.45,79,80 For example, oxidized phenol-formaldehyde resins had electron-donating capacity (0.264–1.15 mmol e− g−1) and electron-accepting capacity (0.120–0.300 mmol e− g−1) due to the quinone and carboxyl groups.50 These functional groups have been documented to facilitate the transformation of HMs, such as the reduction of Ag(I) and Cr(VI).81,82 Additionally, oxidized PS had electron-rich carbonyl groups, which were responsible for the transformation of HMs via complexing with charged HMs followed by electron shutting.83
The quantification of oxygen-containing functional groups on oxidized NPs can be achieved through the application of Boehm titration. The contents of carboxyl groups and phenolic hydroxyl on oxidized PVC were 0.15 mmol g−1 and 0.18 mmol g−1, respectively.84 These values can be approximately 1.9–7.5 times and 3.6–18 times greater than those observed in other carbon-containing materials, such as biochars, respectively.85 The functional groups on biochars play a role in contaminant transformation, either through direct electron transfer or as an electron shuttle between bacteria and elements.86,87 It is thus suggested that oxidized NPs can contribute significantly to the contaminant transformation, particularly in plastic-rich systems. Moreover, NPs are composed of carbon but also include other elements such as nitrogen in PA, chlorine in PVC, and sulfur in PPS. The capability of these NPs to produce redox-reactive functional groups remains less understood.
2.3 The release of plastic leachates
Plastic weathering triggers the release of leachates containing additives or organic substances derived from polymer degradation.88–90 Sheridan et al. showed that PE leachates were released at a level of 0.1 mg C per L into the freshwater in the field.91 Furthermore, accelerated weathering of PE in the laboratory resulted in the release of leachates at concentrations of 2.4–5.6 mg C per L.92,93 In this study, the leachates from weathered NPs are categorized into plastic additives and plastic oligomers.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 additives, such as phthalic esters and flame retardants, have been incorporated into plastics.94 In some cases, additives make up as much as 70% of the total weight of the plastic.95 These substances are physically bound to polymers and feasibly released into the environment during the plastic life cycle. In the field, plastic film released 11.3 tonnes of di-n-butyl phthalate and 25.3 tonnes of ethylhexyl phthalate into the soil, respectively.96 Additionally, PE can release 94.2% of tris(2,4-di-tert-butylphenyl) phosphate in the soil slurry within 2 days.97
000 additives, such as phthalic esters and flame retardants, have been incorporated into plastics.94 In some cases, additives make up as much as 70% of the total weight of the plastic.95 These substances are physically bound to polymers and feasibly released into the environment during the plastic life cycle. In the field, plastic film released 11.3 tonnes of di-n-butyl phthalate and 25.3 tonnes of ethylhexyl phthalate into the soil, respectively.96 Additionally, PE can release 94.2% of tris(2,4-di-tert-butylphenyl) phosphate in the soil slurry within 2 days.97
These additives are chemically reactive. For example, bisphenol A, a synthetic compound used in plastics and resins, can complex with Cr(VI) via its electron-rich groups.98 Additionally, our preliminary experiments have demonstrated that 2,6-di-tert-butylphenol, a common antioxidant used in plastics, facilitated the reduction of Ag(I) to Ag(0) (unpublished data). However, the chemical reactivity of additives that are released from weathered NPs remains largely unknown. A major challenge is the identification of these large amounts of additives in environmental and biological matrices.99 To address this, non-targeted analysis based on high-resolution mass spectrometry and computational tools will be an ideal approach to identify the chemicals in additives released from NPs.100 For example, using non-targeted analysis, Tang et al. identified 674 formulas of halogenated organic additives in the plastics of three electrical products. Of these, 166, 362, and 146 were identified as organochlorine, organobromine, and mixed chlorinated/brominated organic compounds, respectively.101 It is therefore necessary to differentiate the chemical reactivity of numerous additives present in the environments.
While the bioavailability and toxicity of plastic oligomers have been documented,107,108 their chemical reactivity remains unclear due to the challenges of characterization methodologies. Kwon et al. used benzene and dichloromethane to extract styrene oligomers from sand and seawater, respectively,109 while Yang et al. utilized ethanol to extract water-insoluble PET oligomer agglomerates.110 These analytical methods, however, cannot target all the oligomers released from plastic polymers. The structural diversity of oligomers results in a wide range of physical and chemical properties (e.g., solubility), even among oligomers derived from the same polymer. For example, 12-mer derived from PLA is hydrophobic, whereas the dimer derived from PLA is hydrophilic.102 Thus, ad hoc synthesis of oligomer standards with different chain lengths and stereochemistry is essential to analyze the oligomers in environmental and biological samples.
3. Interaction between weathered NPs and HMs
3.1 Adsorption of HMs on weathered NPs
The aforementioned evidence demonstrates that weathered NPs are chemically reactive, which can facilitate the adsorption of HMs. The adsorptive interaction between weathered NPs and HMs has been extensively studied and reviewed.36,40,111,112 Generally, HMs are adsorbed onto weathered NPs via physical (e.g., pore filling and electrostatic interaction) and chemical mechanisms (e.g., surface complexation, hydrogen bonding, and cation–π interaction) (Fig. 4a).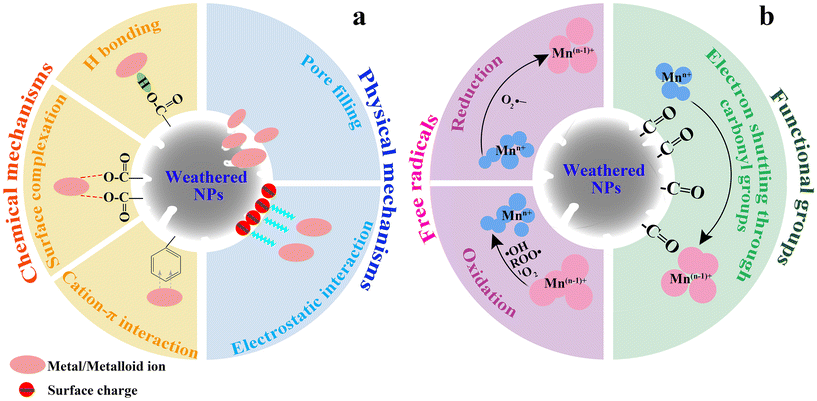 | ||
| Fig. 4 Interaction between weathered NPs and HMs. (a) Adsorption of HMs on weathered NPs. (b) Transformation of HMs by weathered NPs. | ||
Generated pores on weathered NPs can increase the adsorption of HMs compared to their unweathered counterparts. This can be attributed to the increase in the specific surface area and pore volume of weathered NPs. For example, SEM analysis demonstrated that light-weathered PS (100 nm) created surface pores and thus enhanced the adsorption of Pb(II), Cu(II), Cd(II), Ni(II), and Zn(II).113 In addition, weathering can increase the surface charges of NPs through the formation of functional groups such as carboxyl acids, thereby enhancing their electrostatic interaction with HMs.114 For example, light-weathered PP showed a 76% reduction in zeta potential compared to its unweathered counterparts, resulting in an 81% and 5.7% increase in the adsorption capacity of Cd(II) and Pb(II), respectively.115
More importantly, the oxygen-containing functional groups on the weathered NPs can complex with HMs via inner-sphere complexes. For example, Huang et al. demonstrated that the formation of carbonyl and hydroxyl groups on weathered PLA and PS enhanced the complexation of Pb(II) and Ag(I).83,116 Tang et al. revealed that amino N atoms within the amide group in polyamide 6 facilitated the adsorption of Cd(II), Pb(II), and Cd(II) via the formation of chemical coordination bonds, including Cu–N, Pb–N, and Cd–N.117 In addition, the hydrogen atom on the carboxyl group of weathered PS NPs (100–1000 nm) can form a hydrogen bond with the oxygen atom in the arsenic anion.118 Instead, a 33% decrease in the adsorption capacity of As(III) on PS NPs was observed when hydrogen bonds between them were broken at high temperatures.118 Furthermore, a significant increase in the ratio of aromatic C–H bands in light-weathered PS, coupled with an enhanced adsorption of Cd(II), suggested an important role of cation–π interaction in the adsorption of HMs on PS.119
The adsorption of HMs on weathered NPs can be influenced by the environmental factors, such as pH and NOM levels. The pH plays an important role in the adsorption of HMs by affecting the electronegativity of weathered plastics. For example, Holmes et al. demonstrated that PE had a negative charge at high pH, which increased the electrostatic attraction and thus increased the adsorption of Cd(II), Co(II), Ni(II) and Pb(II).120 Natural organic matter can compete with weathered NPs for adsorption of HMs, thereby reducing the adsorptive interaction between NPs and HMs. Specifically, NOM reduced the adsorption of Cd(II) on polyamide, 2-propenitrile, and PET by 22%, 43%, and 45%, respectively.121 In addition, the formation of eco-corona by NOM can alter the hydrophobicity, polarity, and surface charge of NPs, thereby influencing the adsorption of HMs. Oxygen-containing functional groups of NOM-induced eco-corona increased the surface charges of NPs, thereby enhancing the electrostatic interaction with Pb(II).122 In addition, Guo et al. indicated that the formation of a polymer–HM–NOM ternary complex can enhance the adsorption of Cd(II) on PE, PP, PS, and PVC by 1.2-, 1.3-, 1.3-, and 1.3-fold, respectively.123
3.2 Transformation of HMs by weathered NPs
Weathered NPs trigger the transformation of HMs, mainly due to the free radical-mediated redox processes (Fig. 4b). Chromium(VI) was reduced by light-weathered PS NPs (100, 1000 nm) via reductive O2˙−.124 However, long-term light weathering inhibited the reduction of Cr(VI) due to the increase in the amount of carbonyl groups on weathered PS NPs, resulting in the production of oxidative ·OH and 1O2 instead of O2˙−.124 Likewise, Ag(I) was reduced to Ag(0) in the presence of light-weathered PS NPs (100–1000 nm) via the formation of O2˙−.13 Through the generation of ROO· and O2˙−, the oxidation of Mn(II) was driven by light-weathered PS NPs (30–500 nm).125 In addition, Ag(0) and ZnO were oxidized in the presence of light-weathered PS NPs (100 nm) via the generation of ·OH, 1O2, or acids.34,126 The oxidation of metal sulfides can also be triggered by the presence of weathered NPs. For example, the oxidation of CdS and CuS was promoted by the light-weathered PS NPs (100 nm) through the production of ·OH and 1O2, resulting in the release of 30% and 2.5% of the total Cd and Cu, respectively.34,126 While it is well documented that weathered NPs produce both reductive and oxidative free radicals in the laboratory, it is still unclear which free radical dominates in the field.Notably, the observed Ag(I) reduction by light-weathered PS is not only due to the generation of free radicals, such as O2˙− production,34 but is also facilitated by electron shuttling through carbonyl groups. For example, Ag(I) reduction was observed in the presence of light-weathered PS in freshwater and sand matrices.127 The possibility of free radicals in Ag(I) reduction was excluded. Instead, a significant linear correlation was observed between the content of carbonyl groups on light-weathered PS and newly formed Ag(0).127 The addition of Cu(II) (as an oxidizer of carbonyl groups) induced a concentration-dependent inhibition of Ag(I) reduction, suggesting a critical role of carbonyl groups on light-weathered PS in Ag(I) reduction.127 These distinct mechanisms through either radical-mediated transformation or electron shuttling through carbonyl groups may be attributed to the chemical reactivity of PS induced before or after laboratory weathering. The former primarily results from polymer processing and/or storage.42 For example, we have identified the formation of free radicals on PS (1000 nm) prior to or after laboratory weathering (Fig. 3b).
In addition to the hitherto described ROS and carbonyl groups, other free radicals and functional groups derived from weathered NPs may provide new insights into their interaction with HMs. For example, the redox reactive SO3˙−, with an oxidation potential of 0.7 V, can be produced from light-weathered PPS.128 This species could influence the transformation of HMs, which deserves further investigation. In addition, recent studies have shown that after sulfur weathering (i.e., incubating with Na2S solution), sulfur-containing functional groups were formed on the surface of plastics.129 Whether sulfur-weathered NPs would drive the transformations of HMs, such as sulfidation, is largely unknown.
While the aforementioned NP-induced transformation of HMs is constrained in sunlit conditions, other environmental factors, including oxygen level, pH level, and cations, have been demonstrated to influence the processes. Oxygen, in particular, is often observed to facilitate these transformation processes directly and indirectly. Directly, oxygen serves as an oxidative agent, participating in the oxidative dissolution of HMs.130 For example, oxygen can oxidize Ag(0) and CdS, releasing Ag(I) and Cd(II).34 Indirectly, oxygen acts as a prerequisite for the formation of oxygen-containing groups or promotes ROS generation, which in turn indirectly influences the transformation of HMs. For example, the oxidative dissolutions of Ag(0) and CdS are strongly related to ROS produced from weathered NPs in an oxygen-rich atmosphere.34,131 The pH level similarly influences NP-induced HM transformation, both directly and indirectly. For example, low pH conditions facilitate Ag(0) dissolution, whereas elevated pH conditions favor Ag(I) reduction. However, the reduction of Ag(I) induced by weathered PS NPs (100 nm) was hindered in low pH conditions.132 This is because O2˙− produced from weathered PS NPs can react with protons under acidic conditions to yield O2·H, whose reductive ability is inferior to that of O2˙−.133 Cations, such as Ca(II), also exert an influence on NP-induced HM transformation via a reduction in the stability of NPs. Zhang et al. observed a decrease in the rate of Ag(I) reduction induced by PS NPs (100 nm) as Ca(II) was introduced.132 This is attributed to the promotion of (hetero-)aggregation of PS NPs and/or newly formed Ag(0) via the complexation of Ca(II) with oxygen-containing functional groups on weathered PS, which subsequently inhibited the ROS produced from PS.132
More importantly, weathered NPs can fulfill the function of a driver of the transformation of HMs in a similarly effective manner to that of NOM. In the reduction processes, the observed Ag(I) reduction rates induced by weathered PS NPs (100 nm) ranged from 2.3 × 10−3 h−1 to 6.8 × 10−2 h−1,83,132 which are within the documented values for NOM (8.1 × 10−4–0.3 h−1).134–136 Similarly, the Cr(VI) reduction rate (2.3 × 10−3 h−1) induced by weathered PS NPs (100–1000 nm)124 is comparable with documented values for reduced-NOM (9.0 × 10−3 h−1).137 In the oxidation dissolution process, the CdS dissolution rates triggered by weathered PS NPs (100 nm) were 0.101–0.144 h−1,131 aligning with the reported value ranges for NOM (3.9 × 10−2–0.199 h−1).138 Furthermore, Ag(0) dissolution rates induced by weathered PS NPs (100 nm) were documented to be 6.0 × 10−3–1.4 × 10−2 h−1,132 which were even more significant than that observed in certain natural water systems (5.0 × 10−3).139 Collectively, these results suggest that weathered NPs are therefore significant, yet an unrecognized driver in nature to alter HM cycling.
4. Perspective
Despite the increasing number of studies on the risk assessment of NPs, their chemical reactivity has long been overlooked. This review identifies the chemical reactivity of NPs and clarifies their critical role in the transformation of HMs. However, many fundamental questions remain to be addressed, including those related to the weathering processes under environmental conditions, the quantitation of reactive NPs, and the risk of NPs in realistic environments.4.1 Evaluating the weathering processes in realistic environments
While the chemical reactivity of NPs induced by weathering processes has been identified, the majority of investigations have been conducted under laboratory-simulated conditions. It is controversial whether the laboratory-simulated conditions represent environmentally relevant systems. For example, the light weathering of NPs in laboratory settings is conducted using a xenon lamp with a light intensity of 55 mW cm−2, which is 1.6–3.3-fold greater than the daily solar irradiance received on Earth (16.4–34 mW cm−2).34,140 The intensity and spectral distribution of sunlight are dependent on a number of factors, including latitude, longitude, solar elevation angle, and meteorological conditions.120 Furthermore, NPs undergo a range of weathering processes that simultaneously occur in nature, which introduces complexity and uncertainty to the mechanisms involved. It is of great importance to gain a comprehensive understanding of the chemical reactivity of NPs by focusing on the weathering in large-scale field studies.4.2 Estimating the pool of chemically reactive NPs in nature
The chemical relativity of weathered NPs can be quantitated by the levels of free radicals, functional groups, and released leachates, which can lead to an estimation of pool of chemically reactive NPs in nature. The unexpectedly large pool of reactive NPs may have significant consequences for the cycling of HMs as well as for carbon. For example, the formation of eco-corona on reactive NPs via adsorbing NOM could allow NPs to have a larger impact on carbon storage and transport.141 Based on the oxidation rate under light irradiation, we estimated a pool of reductive PS during light weathering in the natural environment (18.1–39.4 MT per day). However, this estimation does not account for the heterogeneity of NPs and their surroundings, where they experience varying degrees of weathering in distinct environmental matrixes, such as light weathering on the surface water but light-independent weathering in landfills and sediments. More work is needed to evaluate and differentiate the pool of reactive NPs in different environmental systems.4.3 Focusing on the risk of NPs in nature
The observed complexity in the toxicity of NPs can be attributed to their chemical reactivity and intricate interaction with other chemical pollutants, such as HMs. A meta-analysis revealed that the combined exposure of micro(nano)plastics and HMs resulted in a significant reduction in root length, whereas exposure to micro(nano)plastics alone had no impact.142 This finding suggests that the toxicological effects observed in empirical studies focusing on NP particles may differ from those in realistic environmental conditions, where NPs are subjected to weathering and are highly likely to interact with HMs. It is therefore proposed that in order to obtain an unbiased result in the risk assessment of NPs, chemical reactivity can be regarded as a significant feature of NPs, along with their other properties, such as mass concentrations and size.Data availability
Data availability is not applicable to this review article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (42207457), the National Key Research and Development Program of China (2023YFC3711400), and the Natural Science Foundation of Jiangsu Province (BK20220092).References
- M. MacLeo, H. P. H. Arp, M. B. Tekman and A. Jahnke, The global threat from plastic pollution, Science, 2021, 373, 61–65 CrossRef.
- A. C. Ryan, D. Allen, S. Allen, V. Maselli, A. LeBlanc, L. Kelleher, S. Krause, T. R. Walker and M. Cohen, Transport and deposition of ocean-sourced microplastic particles by a North Atlantic hurricane, Commun. Earth Environ., 2023, 4, 442 CrossRef.
- S. Allen, D. Allen, V. R. Phoenix, G. Le Roux, P. Durántez Jiménez, A. Simonneau, S. Binet and D. Galop, Atmospheric transport and deposition of microplastics in a remote mountain catchment, Nat. Geosci., 2019, 12, 339–344 CrossRef CAS.
- K. Min, J. D. Cuiffi and R. T. Mathers, Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure, Nat. Commun., 2020, 11, 727 CrossRef CAS PubMed.
- A. Ganesh Kumar, K. Anjana, M. Hinduja, K. Sujitha and G. Dharani, Review on plastic wastes in marine environment – Biodegradation and biotechnological solutions, Mar. Pollut. Bull., 2020, 150, 110733 CrossRef.
- J. Gigault, H. El Hadri, B. Nguyen, B. Grassl, L. Rowenczyk, N. Tufenkji, S. Feng and M. Wiesner, Nanoplastics are neither microplastics nor engineered nanoparticles, Nat. Nanotechnol., 2021, 16, 501–507 CrossRef CAS.
- J. P. G. L. Frias and R. Nash, Microplastics: Finding a consensus on the definition, Mar. Pollut. Bull., 2019, 138, 145–147 CrossRef CAS PubMed.
- F. Dang, Q. Wang, Y. Huang, Y. Wang and B. Xing, Key knowledge gaps for One Health approach to mitigate nanoplastic risks, Eco-Environ. Health, 2022, 1, 11–22 CrossRef PubMed.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono, L. Brach and J. Gigault, Nanoplastic in the North Atlantic Subtropical Gyre, Environ. Sci. Technol., 2017, 51, 13689–13697 CrossRef CAS.
- A. Wahl, C. Le Juge, M. Davranche, H. El Hadri, B. Grassl, S. Reynaud and J. Gigault, Nanoplastic occurrence in a soil amended with plastic debris, Chemosphere, 2021, 262, 127784 CrossRef CAS PubMed.
- D. Materić, A. Kasper-Giebl, D. Kau, M. Anten, M. Greilinger, E. Ludewig, E. van Sebille, T. Röckmann and R. Holzinger, Micro- and nanoplastics in alpine snow: a new method for chemical identification and (semi) quantification in the nanogram range, Environ. Sci. Technol., 2020, 54, 2353–2359 CrossRef.
- Y. Guo, A. M. O'Brien, T. F. Lins, R. S. Shahmohamadloo, X. O. Almirall, C. M. Rochman and D. Sinton, Effects of hydrogen peroxide on cyanobacterium microcystis aeruginosa in the presence of nanoplastics, ACS ES&T Water, 2021, 1, 1596–1607 Search PubMed.
- G. Rossi, J. Barnoud and L. Monticelli, Polystyrene nanoparticles perturb lipid membranes, J. Phys. Chem. Lett., 2014, 5, 241–246 CrossRef CAS.
- E. Besseling, B. Wang, M. Lürling and A. A. Koelmans, Nanoplastic affects growth of S. obliquus and reproduction of D. magna, Environ. Sci. Technol., 2014, 48, 12336–12343 CrossRef CAS.
- S. Rist, A. Baun and N. B. Hartmann, Ingestion of micro- and nanoplastics in Daphnia magna – Quantification of body burdens and assessment of feeding rates and reproduction, Environ. Pollut., 2017, 228, 398–407 CrossRef CAS PubMed.
- L. J. Feng, J. W. Li, E. G. Xu, X. D. Sun, F. P. Zhu, Z. J. Ding, H. Y. Tian, S. S. Dong, P. F. Xia and X. Z. Yuan, Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria, Environ. Sci.: Nano, 2019, 6, 3072–3079 RSC.
- Y. Chae, D. Kim, S. W. Kim and Y.-J. An, Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain, Sci. Rep., 2018, 8, 284 CrossRef.
- Y. Xue, K. Song, Z. Wang, Z. Xia, R. Li, Q. Wang and L. Li, Nanoplastics occurrence, detection methods, and impact on the nitrogen cycle: a review, Environ. Chem. Lett., 2024, 22, 2241–2255 CrossRef CAS.
- F. Tamaddon and W. Hogland, Rview of cadmium in plastic waste in, Waste Manage. Res., 1993, 11, 287–295 CrossRef CAS.
- M. Filella and A. Turner, Observational study unveils the extensive presence of hazardous elements in beached plastics from lake Geneva, Front. Environ. Sci., 2018, 6, 1 CrossRef.
- A. Turner and M. Filella, Hazardous metal additives in plastics and their environmental impacts, Environ. Int., 2021, 156, 106622 CrossRef CAS PubMed.
- J. X. Ren, T. L. Wu, C. Liu, P. X. Cui, F. Dang, Q. Yang and Y. J. Wang, UV-irradiation facilitating Pb release from recycled PVC microplastics, Bull. Environ. Contam. Toxicol., 2021, 107, 748–753 CrossRef CAS PubMed.
- J. Zhang, S. Yu, Z. Xu, R. Qi, Y. Chi, L. Wang, L. Liu and Y. Tang, Metal leaching accompanied with natural photo-aging behavior of e-waste plastic derived microplastics in aquatic environment, J. Environ. Sci., 2024 DOI:10.1016/j.jes.2024.06.044 , in press.
- M. Pujari and D. Kapoor, 1 - Heavy metals in the ecosystem: Sources and their effects, Heavy metals in the environment, ed. V. Kumar, A. Sharma and A. Cerdà, Elsevier, 2021, pp. 1–7 Search PubMed.
- X. Lin, S. Zhang, S. Yang, R. Zhang, X. Shi and L. Song, A landfill serves as a critical source of microplastic pollution and harbors diverse plastic biodegradation microbial species and enzymes: Study in large-scale landfills, China, J. Hazard. Mater., 2023, 457, 131676 CrossRef CAS PubMed.
- F. Abdolahpur Monikh, M. G. Vijver, Z. Guo, P. Zhang, G. K. Darbha and W. J. G. M. Peijnenburg, Metal sorption onto nanoscale plastic debris and trojan horse effects in Daphnia magna: Role of dissolved organic matter, Water Res., 2020, 186, 116410 CrossRef CAS.
- W. S. Lee, H.-J. Cho, E. Kim, Y. H. Huh, H. J. Kim, B. Kim, T. Kang, J. S. Lee and J. Jeong, Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos, Nanoscale, 2019, 11, 3173–3185 RSC.
- J. Rong, C. Yuan, X. Yin, X. Wu, F. He, Y. Wang, K. S. Y. Leung and S. Lin, Co-exposure of polystyrene nanoplastics and copper induces development toxicity and intestinal mitochondrial dysfunction in vivo and in vitro, Sci. Total Environ., 2024, 930, 172681 CrossRef CAS PubMed.
- G. Xu, X. Lin and Y. Yu, Different effects and mechanisms of polystyrene micro- and nano-plastics on the uptake of heavy metals (Cu, Zn, Pb and Cd) by lettuce (Lactuca sativa L.), Environ. Pollut., 2023, 316, 120656 CrossRef CAS PubMed.
- M. Alaraby, D. Abass, A. Villacorta, A. Hernández and R. Marcos, Antagonistic in vivo interaction of polystyrene nanoplastics and silver compounds. A study using Drosophila, Sci. Total Environ., 2022, 842, 156923 CrossRef CAS.
- R. M. Town and H. P. van Leeuwen, Uptake and release kinetics of organic contaminants associated with micro- and nanoplastic particles, Environ. Sci. Technol., 2020, 54, 10057–10067 CrossRef PubMed.
- B. Huang, Z. B. Wei, L. Y. Yang, K. Pan and A. J. Miao, Combined toxicity of silver nanoparticles with hematite or plastic nanoparticles toward two freshwater algae, Environ. Sci. Technol., 2019, 53, 3871–3879 CrossRef CAS PubMed.
- F. Wang, C. S. Wong, D. Chen, X. Lu, F. Wang and E. Y. Zeng, Interaction of toxic chemicals with microplastics: A critical review, Water Res., 2018, 139, 208–219 CrossRef CAS PubMed.
- L. Tong, P. Duan, X. Tian, J. Huang, J. Ji, Z. Chen, J. Yang, H. Yu and W. Zhang, Polystyrene microplastics sunlight-induce oxidative dissolution, chemical transformation and toxicity enhancement of silver nanoparticles, Sci. Total Environ., 2022, 827, 154180 CrossRef CAS.
- R. Mao, M. Lang, X. Yu, R. Wu, X. Yang and X. Guo, Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals, J. Hazard. Mater., 2020, 393, 122515 CrossRef CAS.
- Y. Xu, Q. Ou, J. P. van der Hoek, G. Liu and K. M. Lompe, Photo-oxidation of micro- and nanoplastics: Physical, chemical, and biological effects in Environments, Environ. Sci. Technol., 2024, 58, 991–1009 CrossRef CAS.
- Y. Yu, A. F. Astner, T. M. Zahid, I. Chowdhury, D. G. Hayes and M. Flury, Aggregation kinetics and stability of biodegradable nanoplastics in aquatic environments: Effects of UV-weathering and proteins, Water Res., 2023, 239, 120018 CrossRef CAS PubMed.
- Y. Sun, J. Huang, Z. Wang, P. Duan and W. Zhang, Phototransformation and toxicity enhancement of silver chloride nanoparticles by polystyrenemicroplastics under sunlit, Environ. Chem. Lett., 2024 DOI:10.1007/s10311-024-01783-7 , in press.
- L. Wang and J. Yu, Chapter 1 Principles of photocatalysis, Interface Science and Technology, ed. J. Yu, L. Zhang and L. Wang, Elsevier, 2023, pp. 1–52 Search PubMed.
- J. Duan, N. Bolan, Y. Li, S. Ding, T. Atugoda, M. Vithanage, B. Sarkar, D. C. W. Tsang and M. B. Kirkham, Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments, Water Res., 2021, 196, 117011 CrossRef CAS PubMed.
- V. Kholodovych and W. J. Welsh, Thermal-oxidative stability and degradation of polymers, Physical Properties of Polymers Handbook, ed. J. E. Mark, Springer New York, New York, NY, 2007, pp. 927–938 Search PubMed.
- K. Zhu, H. Jia, S. Zhao, T. Xia, X. Guo, T. Wang and L. Zhu, Formation of environmentally persistent free radicals on microplastics under light irradiation, Environ. Sci. Technol., 2019, 53, 8177–8186 CrossRef CAS.
- E. Yousif, J. Salimon and N. Salih, New stabilizer for polystyrene based on 2-Thioacetic Acid Benzothiazol complexes, J. Appl. Polym. Sci., 2012, 125, 1922–1927 CrossRef CAS.
- E. Pearce, Photodegradation, photo-oxidation and photostabilization of polymers, B. Ranby and J. F. Rabek, Wiley-Interscience, New York, 1975, 573 pp, J. Polym. Sci., Polym. Lett. Ed., 1975, 13, 621–622 CrossRef.
- J. Duan, Y. Li, J. Gao, R. Cao, E. Shang and W. Zhang, ROS-mediated photoaging pathways of nano- and micro-plastic particles under UV irradiation, Water Res., 2022, 216, 118320 CrossRef CAS PubMed.
- K. Zhu, H. Jia, W. Jiang, Y. Sun, C. Zhang, Z. Liu, T. Wang, X. Guo and L. Zhu, The First Observation of the Formation of persistent aminoxyl radicals and reactive nitrogen species on photoirradiated nitrogen-containing microplastics, Environ. Sci. Technol., 2022, 56, 779–789 CrossRef CAS PubMed.
- W. Jiang, K. Zhu, H. Ma, J. Liu, C. Zhang, Y. Dai and H. Jia, Sulfur-containing persistent free radicals and reactive species on photoaged microplastics: Identification and the formation mechanism, Environ. Sci. Technol., 2023, 57, 8680–8690 CrossRef CAS.
- C. Martin, F. Baalkhuyur, L. Valluzzi, V. Saderne, M. Cusack, H. Almahasheer, P. K. Krishnakumar, L. Rabaoui, M. A. Qurban, A. Arias-Ortiz, P. Masqué and C. M. Duarte, Exponential increase of plastic burial in mangrove sediments as a major plastic sink, Sci. Adv., 2020, 6, eaaz5593 CrossRef CAS PubMed.
- X. Fei, Y. Guo, Y. Wang, M. Fang, K. Yin and H. He, The long-term fates of land-disposed plastic waste, Nat. Rev. Earth Environ., 2022, 3, 733–735 CrossRef.
- L. Chen, D. Wang, T. Sun, T. Fan, S. Wu, G. Fang, M. Yang and D. Zhou, Quantification of the redox properties of microplastics and their effect on arsenite oxidation, Fundam. Res., 2023, 3, 777–785 CrossRef CAS PubMed.
- S. Suman, D. Bagal, D. Jain, S. Ragini, I. K. Singh and A. Singh, Chapter 14 - Biotic stresses on plants: reactive oxygen species generation and antioxidant mechanism, Frontiers in Plant-Soil Interaction, ed. T. Aftab and K. R. Hakeem, Academic Press, 2021, pp. 381–411 Search PubMed.
- X. Wang and L. Zhang, Kinetic study of hydroxyl radical formation in a continuous hydroxyl generation system, RSC Adv., 2018, 8, 40632–40638 RSC.
- H. Li, Y. Gu, Y. Jiang, P. Ding, X. Chen, C. Chen, R. Pan, C. Shi, S. Wang and H. Chen, Environmentally persistent free radicals on photoaged nanopolystyrene induce neurotoxicity by affecting dopamine, glutamate, serotonin and GABA in Caenorhabditis elegans, Sci. Total Environ., 2024, 906, 167684 CrossRef CAS.
- K. Zhu, H. Jia, Y. Sun, Y. Dai, C. Zhang, X. Guo, T. Wang and L. Zhu, Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species, Water Res., 2020, 173, 115564 CrossRef CAS.
- F. Liu, Y. Ding, J. Liu, J. Latif, J. Qin, S. Tian, S. Sun, B. Guan, K. Zhu and H. Jia, The effect of redox fluctuation on carbon mineralization in riparian soil: An analysis of the hotspot zone of reactive oxygen species production, Water Res., 2024, 265, 122294 CrossRef CAS PubMed.
- P. Liu, L. Qian, H. Wang, X. Zhan, K. Lu, C. Gu and S. Gao, New insights into the aging behavior of microplastics accelerated by advanced oxidation processes, Environ. Sci. Technol., 2019, 53, 3579–3588 CrossRef CAS PubMed.
- Y. K. Song, S. H. Hong, M. Jang, G. M. Han, S. W. Jung and W. J. Shim, Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type, Environ. Sci. Technol., 2017, 51, 4368–4376 CrossRef CAS.
- H. Luo, Y. Zhao, Y. Li, Y. Xiang, D. He and X. Pan, Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids, Sci. Total Environ., 2020, 714, 136862 CrossRef CAS PubMed.
- F. Yu, Q. Y. Qin, X. C. Zhang and J. Ma, Characteristics and adsorption behavior of typical microplastics in long-term accelerated weathering simulation, Environ. Sci.: Processes Impacts, 2024, 26, 882–890 RSC.
- Y. N. Zhang, F. Cheng, T. Zhang, C. Li, J. Qu, J. Chen and W. J. G. M. Peijnenburg, Dissolved organic matter enhanced the aggregation and oxidation of nanoplastics under simulated sunlight irradiation in water, Environ. Sci. Technol., 2022, 56, 3085–3095 CrossRef CAS.
- R. Cao, X. Liu, J. Duan, B. Gao, X. He, B. Nanthi and Y. Li, Opposite impact of DOM on ROS generation and photoaging of aromatic and aliphatic nano- and micro-plastic particles, Environ. Pollut., 2022, 315, 120304 CrossRef CAS PubMed.
- L. Ding, X. Yu, X. Guo, Y. Zhang, Z. Ouyang, P. Liu, C. Zhang, T. Wang, H. Jia and L. Zhu, The photodegradation processes and mechanisms of polyvinyl chloride and polyethylene terephthalate microplastic in aquatic environments: Important role of clay minerals, Water Res., 2022, 208, 117879 CrossRef CAS PubMed.
- L. Ding, X. Guo, S. Du, F. Cui, Y. Zhang, P. Liu, Z. Ouyang, H. Jia and L. Zhu, Insight into the photodegradation of microplastics boosted by iron (Hydr)oxides, Environ. Sci. Technol., 2022, 56, 17785–17794 CrossRef CAS PubMed.
- K. E. Wheeler, A. J. Chetwynd, K. M. Fahy, B. S. Hong, J. A. Tochihuitl, L. A. Foster and I. Lynch, Environmental dimensions of the protein corona, Nat. Nanotechnol., 2021, 16, 617–629 CrossRef CAS.
- M. Zhu, Z. Zhang, T. Zhang, T. Hofmann and W. Chen, Eco-Corona dictates mobility of nanoplastics in saturated porous media: The critical role of preferential binding of macromolecules, Environ. Sci. Technol., 2023, 57, 331–339 CrossRef CAS PubMed.
- E. Marten, R. J. Müller and W.-D. Deckwer, Studies on the enzymatic hydrolysis of polyesters. II. Aliphatic–aromatic copolyesters, Polym. Degrad. Stab., 2005, 88, 371–381 CrossRef CAS.
- R. Koshti, L. Mehta and N. Samarth, Biological recycling of polyethylene terephthalate: A Mini-Review, J. Polym. Environ., 2018, 26, 3520–3529 CrossRef CAS.
- S. S. Kothawale, L. Kumar and S. P. Singh, Role of organisms and their enzymes in the biodegradation of microplastics and nanoplastics: A review, Environ. Res., 2023, 232, 116281 CrossRef CAS.
- J. Jin, J. Arciszewski, K. Auclair and Z. Jia, Enzymatic polyethylene biorecycling: Confronting challenges and shaping the future, J. Hazard. Mater., 2023, 460, 132449 CrossRef CAS PubMed.
- R. Gao, R. Liu and C. Sun, A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene, J. Hazard. Mater., 2022, 431, 128617 CrossRef CAS PubMed.
- M. Sathiyabama, R. V. Boomija, T. Sathiyamoorthy, N. Mathivanan and R. Balaji, Mycodegradation of low-density polyethylene by Cladosporium sphaerospermum, isolated from platisphere, Sci. Rep., 2024, 14, 8351 CrossRef CAS.
- S. Zhang, Z. He, C. Wu, Z. Wang, Y. Mai, R. Hu, X. Zhang, W. Huang, Y. Tian, D. Xia, C. Wang, Q. Yan, Z. He and L. Shu, Complex bilateral interactions determine the fate of polystyrene micro- and nanoplastics and soil protists: Implications from a soil amoeba, Environ. Sci. Technol., 2022, 56, 4936–4949 CrossRef CAS.
- X. Qiu, S. Ma, J. Zhang, L. Fang, X. Guo and L. Zhu, Dissolved organic matter promotes the aging process of polystyrene microplastics under dark and ultraviolet light conditions: The crucial role of reactive oxygen species, Environ. Sci. Technol., 2022, 56, 10149–10160 CrossRef CAS PubMed.
- M. Junaid and J. Wang, Interaction of nanoplastics with extracellular polymeric substances (EPS) in the aquatic environment: A special reference to eco-corona formation and associated impacts, Water Res., 2021, 201, 117319 CrossRef CAS PubMed.
- A. ter Halle and J. F. Ghiglione, Nanoplastics: A Complex, polluting terra incognita, Environ. Sci. Technol., 2021, 55, 14466–14469 CrossRef CAS PubMed.
- S. Yang, W. Wu, F. Bertocchini, M. Benbow, S. Devipriya, H. Cha, B. Y. Peng, M. Ding, L. He, M. Li, C. H. Cui, S. N. Shi, H. J. Sun, J. Pang, D. He, Y. Zhang, J. Yang, D. Hou, D. F. Xing and C. Criddle, Radical innovation breakthroughs of biodegradation of plastics by insects: history, present and future perspectives, Front. Environ. Sci. Eng., 2024, 18, 78 CrossRef CAS.
- J. Zhao, R. Lan, Z. Wang, W. Su, D. Song, R. Xue, Z. Liu, X. Liu, Y. Dai, T. Yue and B. Xing, Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution, Nat. Nanotechnol., 2024, 19, 406–414 CrossRef CAS.
- A. L. Dawson, S. Kawaguchi, C. K. King, K. A. Townsend, R. King, W. M. Huston and S. M. Bengtson Nash, Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill, Nat. Commun., 2018, 9, 1001 CrossRef PubMed.
- T. Jia, J. Cai, S. He, Z. Mao, X. Zhang, A. Geng, H. Yang, S. Jiang and P. Huang, UV-aged polystyrene nanoplastics aggravate intestinal barrier damage by overproduction of ROS, Environ. Toxicol. Pharmacol., 2024, 108, 104448 CrossRef CAS.
- G. Cao, W. Wang, J. Zhang, P. Wu, X. Zhao, Z. Yang, D. Hu and Z. Cai, New evidence of rubber-derived quinones in water, air, and soil, Environ. Sci. Technol., 2022, 56, 4142–4150 CrossRef CAS PubMed.
- Y. N. Huang, T. T. Qian, F. Dang, Y.-G. Yin, M. Li and D.-M. Zhou, Significant contribution of metastable particulate organic matter to natural formation of silver nanoparticles in soils, Nat. Commun., 2019, 10, 3775 CrossRef PubMed.
- J. Xu, Y. Dai, Y. Shi, S. Zhao, H. Tian, K. Zhu and H. Jia, Mechanism of Cr(VI) reduction by humin: Role of environmentally persistent free radicals and reactive oxygen species, Sci. Total Environ., 2020, 725, 138413 CrossRef CAS.
- Y. Huang, F. Dang, Y. Yin, G. Fang, Y. Wang, G. Yu, D. Zhou and B. Xing, Weathered microplastics induce silver nanoparticle formation, Environ. Sci. Technol. Lett., 2022, 9, 179–185 CrossRef CAS.
- L. Zhou, T. Wang, G. Qu, H. Jia and L. Zhu, Probing the aging processes and mechanisms of microplastic under simulated multiple actions generated by discharge plasma, J. Hazard. Mater., 2020, 398, 122956 CrossRef CAS.
- W. Suliman, J. B. Harsh, N. I. Abu-Lail, A. M. Fortuna, I. Dallmeyer and M. Garcia-Perez, Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties, Biomass Bioenergy, 2016, 84, 37–48 CrossRef CAS.
- A. Kappler, M. L. Wuestner, A. Ruecker, J. Harter, M. Halama and S. Behrens, Biochar as an electron shuttle between bacteria and Fe(III) minerals, Environ. Sci. Technol. Lett., 2014, 1, 339–344 CrossRef CAS.
- M. Chen, X. Chen, X. Xu, Z. Xu, Y. Zhang, B. Song, D. C. W. Tsang, N. Xu and X. Cao, Biochar colloids facilitate transport and transformation of Cr(VI) in soil: Active site competition coupling with reduction reaction, J. Hazard. Mater., 2022, 440, 129691 CrossRef CAS PubMed.
- P. Pfohl, M. Wagner, L. Meyer, P. Domercq, A. Praetorius, T. Hüffer, T. Hofmann and W. Wohlleben, Environmental degradation of microplastics: How to measure fragmentation rates to secondary micro- and nanoplastic fragments and dissociation into dissolved organics, Environ. Sci. Technol., 2022, 56, 11323–11334 CrossRef CAS PubMed.
- Y. Lai, L. Dong, X. Sheng, Q. Li, P. Li, Z. Hao, S. Yu and J. Liu, Swelling-induced fragmentation and polymer leakage of nanoplastics in seawater, Environ. Sci. Technol., 2022, 56, 17694–17701 CrossRef CAS PubMed.
- H. Chen, X. Shan, X. Qiu, L. Ding, X. Liang and X. Guo, High-Resolution mass spectrometry combined with reactive oxygen species reveals differences in photoreactivity of dissolved organic matter from microplastic sources in aqueous environments, Environ. Sci. Technol., 2024, 58, 10334–10346 CrossRef CAS.
- E. A. Sheridan, J. A. Fonvielle, S. Cottingham, Y. Zhang, T. Dittmar, D. C. Aldridge and A. J. Tanentzap, Plastic pollution fosters more microbial growth in lakes than natural organic matter, Nat. Commun., 2022, 13, 4175 CrossRef CAS PubMed.
- C. Romera-Castillo, R. Mallenco-Fornies, M. Saá-Yánez and X. A. Álvarez-Salgado, Leaching and bioavailability of dissolved organic matter from petrol-based and biodegradable plastics, Mar. Environ. Res., 2022, 176, 105607 CrossRef PubMed.
- Y. K. Lee, W. He, H. Guo, T. Karanfil and J. Hur, Effects of organic additives on spectroscopic and molecular-level features of photo-induced dissolved organic matter from microplastics, Water Res., 2023, 242, 120272 CrossRef CAS PubMed.
- H. Wiesinger, Z. Wang and S. Hellweg, Deep dive into plastic monomers, additives, and processing aids, Environ. Sci. Technol., 2021, 55, 9339–9351 CrossRef CAS PubMed.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS.
- Q. Q. Zhang, Z. R. Ma, Y.-Y. Cai, H.-R. Li and G.-G. Ying, Agricultural plastic pollution in China: Generation of plastic debris and emission of phthalic acid esters from agricultural films, Environ. Sci. Technol., 2021, 55, 12459–12470 CrossRef CAS PubMed.
- X. Gong, W. Zhang, S. Zhang, Y. Wang, X. Zhang, Y. Lu, H. Sun and L. Wang, Organophosphite antioxidants in mulch films are important sources of organophosphate pollutants in farmlands, Environ. Sci. Technol., 2021, 55, 7398–7406 CrossRef CAS.
- Y. Lan, X. Wang, Y. Chen, J. Xu and Y. Zhang, Simultaneous removal of bisphenol A and Cr(VI) by Pd–TiO2: Significantly improve the photocatalytic activity through synergistic effect, Solid State Sci., 2023, 146, 107346 CrossRef CAS.
- Y. Li, Z. Lu, D. P. Abrahamsson, W. Song, C. Yang, Q. Huang and J. Wang, Non-targeted analysis for organic components of microplastic leachates, Sci. Total Environ., 2022, 816, 151598 CrossRef CAS PubMed.
- K. E. Manz, A. Feerick, J. M. Braun, Y.-L. Feng, A. Hall, J. Koelmel, C. Manzano, S. R. Newton, K. D. Pennell, B. J. Place, K. J. Godri Pollitt, C. Prasse and J. A. Young, Non-targeted analysis (NTA) and suspect screening analysis (SSA): a review of examining the chemical exposome, J. Exposure Sci. Environ. Epidemiol., 2023, 33, 524–536 CrossRef CAS PubMed.
- C. Tang, R. Zheng, Q. Huang, S. Xiong, Y. Zhu, Y. Liang, Y. H. Zeng, X. J. Luo and B. X. Mai, Nontarget and comprehensive analyses of halogenated organic additives in electrical product plastics by GC-NCI-Q-Orbitrap-HRMS and Cl/Br-screening algorithms, Anal. Chem., 2023, 95, 10052–10060 CrossRef CAS.
- C. Shi, M. Wang, Z. Wang, G. Qu, W. Jiang, X. Pan and M. Fang, Oligomers from the synthetic polymers: Another potential iceberg of new pollutants, Environ. Health, 2023, 1, 228–235 CAS.
- V. Vermylen, P. Lodefier, J. Devaux, R. Legras, W. A. Mac Donald, R. Rozenberg and E. De Hoffmann, Study of the thermal evolution of the cyclic-oligomer formation in a cyclic-oligomer-free PET, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 416–422 CrossRef CAS.
- M. Hoppe, P. de Voogt and R. Franz, Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates – A review, Trends Food Sci. Technol., 2016, 50, 118–130 CrossRef CAS.
- M. Hoppe, P. de Voogt and R. Franz, Oligomers in polyethylene naphthalate and polybutylene terephthalate–Identification and exploring migration, Food Packag. Shelf Life, 2018, 17, 171–178 CrossRef.
- J. Alberto Lopes and E. D. Tsochatzis, Poly(ethylene terephthalate), poly(butylene terephthalate), and polystyrene pligomers: Occurrence and analysis in food contact materials and food, J. Agric. Food Chem., 2023, 71, 2244–2258 CrossRef CAS PubMed.
- M. Wang, Q. Li, C. Shi, J. Lv, Y. Xu, J. Yang, S. L. Chua, L. Jia, H. Chen, Q. Liu, C. Huang, Y. Huang, J. Chen and M. Fang, Oligomer nanoparticle release from polylactic acid plastics catalysed by gut enzymes triggers acute inflammation, Nat. Nanotechnol., 2023, 18, 403–411 CrossRef CAS PubMed.
- K. I. Ohyama, F. Nagai and Y. Tsuchiya, Certain styrene oligomers have proliferative activity on MCF-7 human breast tumor cells and binding affinity for human estrogen receptor, Environ. Health Perspect., 2001, 109, 699–703 CAS.
- B. G. Kwon, K. Koizumi, S.-Y. Chung, Y. Kodera, J.-O. Kim and K. Saido, Global styrene oligomers monitoring as new chemical contamination from polystyrene plastic marine pollution, J. Hazard. Mater., 2015, 300, 359–367 CrossRef CAS PubMed.
- T. Yang, Y. Xu, G. Liu and B. Nowack, Oligomers are a major fraction of the submicrometre particles released during washing of polyester textiles, Nat. Water., 2024, 2, 151–160 CrossRef.
- G. Kutralam-Muniasamy, F. Pérez-Guevara, I. E. Martínez and V. C. Shruti, Overview of microplastics pollution with heavy metals: Analytical methods, occurrence, transfer risks and call for standardization, J. Hazard. Mater., 2021, 415, 125755 CrossRef CAS.
- B. Liu, S. Zhao, T. Qiu, Q. Cui, Y. Yang, L. Li, J. Chen, M. Huang, A. Zhan and L. Fang, Interaction of microplastics with heavy metals in soil: Mechanisms, influencing factors and biological effects, Sci. Total Environ., 2024, 918, 170281 CrossRef CAS PubMed.
- X. Gao, I. Hassan, Y. Peng, S. Huo and L. Ling, Behaviors and influencing factors of the heavy metals adsorption onto microplastics: A review, J. Cleaner Prod., 2021, 319, 128777 CrossRef CAS.
- H. Lyu, H. Zhang, J. Dong, B. Shen, Z. Cheng, J. Yu, R. Li, N. Shao and J. Tang, Pyrolysis temperature matters: Biochar-derived dissolved organic matter modulates aging behavior and biotoxicity of microplastics, Water Res., 2024, 250, 121064 CrossRef CAS.
- X. Fan, Z. Ma, Y. Zou, J. Liu and J. Hou, Investigation on the adsorption and desorption behaviors of heavy metals by tire wear particles with or without UV ageing processes, Environ. Res., 2021, 195, 110858 CrossRef CAS PubMed.
- W. Huang, J. Deng, J. Liang and X. Xia, Comparison of lead adsorption on the aged conventional microplastics, biodegradable microplastics and environmentally-relevant tire wear particles, Chem. Eng. J., 2023, 460, 141838 CrossRef CAS.
- S. Tang, X. Yang, T. Zhang, Y. Qin, C. Cao, H. Shi and Y. Zhao, Adsorption mechanisms of metal ions (Pb, Cd, Cu) onto polyamide 6 microplastics: New insight into environmental risks in comparison with natural media in different water matrices, Gondwana Res., 2022, 110, 214–225 CrossRef CAS.
- Y. Dong, M. Gao, Z. Song and W. Qiu, As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism, Chemosphere, 2020, 239, 124792 CrossRef CAS PubMed.
- L. Wang, C. Guo, Q. Qian, D. Lang, R. Wu, S. Abliz, W. Wang and J. Wang, Adsorption behavior of UV aged microplastics on the heavy metals Pb(II) and Cu(II) in aqueous solutions, Chemosphere, 2023, 313, 137439 CrossRef CAS.
- L. A. Holmes, A. Turner and R. C. Thompson, Interactions between trace metals and plastic production pellets under estuarine conditions, Mar. Chem., 2014, 167, 25–32 CrossRef CAS.
- Y. Zhou, Y. Yang, G. Liu, G. He and W. Liu, Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene, Water Res., 2020, 184, 116209 CrossRef CAS PubMed.
- Q. Fu, X. Tan, S. Ye, L. Ma, Y. Gu, P. Zhang, Q. Chen, Y. Yang and Y. Tang, Mechanism analysis of heavy metal lead captured by natural-aged microplastics, Chemosphere, 2021, 270, 128624 CrossRef CAS.
- X. Guo, G. Hu, X. Fan and H. Jia, Sorption properties of cadmium on microplastics: The common practice experiment and A two-dimensional correlation spectroscopic study, Ecotoxicol. Environ. Saf., 2020, 190, 110118 CrossRef CAS.
- J. Zhang, J. Wei, T. Hu, L. Du, Z. Chen, Y. Zhang and W. Zhang, Polystyrene microplastics reduce Cr(VI) and decrease its aquatic toxicity under simulated sunlight, J. Hazard. Mater., 2023, 445, 130483 CrossRef CAS.
- Z. Gao, P. I. Chou, J. Liu, Y. Zhu and Y.-S. Jun, Oxidative roles of polystyrene-based nanoplastics in inducing manganese oxide formation under light illumination, ACS Nano, 2022, 16, 20238–20250 CrossRef CAS.
- L. Tong, K. Song, Y. Wang, J. Yang, J. Ji, J. Lu, Z. Chen and W. Zhang, Zinc oxide nanoparticles dissolution and toxicity enhancement by polystyrene microplastics under sunlight irradiation, Chemosphere, 2022, 299, 134421 CrossRef CAS PubMed.
- E. Yousif and R. Haddad, Photodegradation and photostabilization of polymers, especially polystyrene: review, Springerplus, 2013, 2, 398 CrossRef PubMed.
- T. N. Das, R. E. Huie and P. Neta, Reduction Potentials of SO3·-, SO5·-, and S4O6·3- Radicals in Aqueous Solution, J. Phys. Chem. A, 1999, 103, 3581–3588 CrossRef CAS.
- Y. Qiu, Z. Zhang, T. Zhang and P. Zhang, Sulfide modifies physicochemical properties and mercury adsorption of microplastics, Sci. Total Environ., 2022, 848, 157802 CrossRef CAS.
- M. Huang, C. Liu, P. Cui, T. Wu, X. Feng, H. Huang, J. Zhou and Y. Wang, Facet-dependent photoinduced transformation of cadmium sulfide (CdS) nanoparticles, Environ. Sci. Technol., 2021, 55, 13132–13141 CAS.
- Y. Sun, J. Zhang, Z. Jiang, Y. Wang, P. Duan, W. Min and W. Zhang, Polystyrene microplastics enhance oxidative dissolution but suppress the aquatic acute toxicity of a commercial cadmium yellow pigment under simulated irradiation, J. Hazard. Mater., 2024, 463, 132881 CrossRef CAS.
- W. Zhang, K. Song, R. Ding, H. Han, L. Yao, M. Ji, Z. Chen, H. Yu, C. Wu and T. Fang, Role of polystyrene microplastics in sunlight-mediated transformation of silver in aquatic environments: Mechanisms, kinetics and toxicity, J. Hazard. Mater., 2021, 419, 126429 CrossRef CAS.
- Y. Nosaka and A. Y. Nosaka, Generation and detection of reactive oxygen species in photocatalysis, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS.
- Y. Yin, J. Liu and G. Jiang, Sunlight-induced reduction of ionic Ag and Au to metallic nanoparticles by dissolved organic matter, ACS Nano, 2012, 6, 7910–7919 CrossRef CAS.
- S. Yu, Y. Yin, X. Zhou, L. Dong and J. Liu, Transformation kinetics of silver nanoparticles and silver ions in aquatic environments revealed by double stable isotope labeling, Environ. Sci.: Nano, 2016, 3, 883–893 RSC.
- W. C. Hou, B. Stuart, R. Howes and R. G. Zepp, Sunlight-driven reduction of silver ions by natural organic matter: Formation and transformation of silver nanoparticles, Environ. Sci. Technol., 2013, 47, 7713–7721 CrossRef CAS PubMed.
- B. Li, P. Liao, L. Xie, Q. Li, C. Pan, Z. Ning and C. Liu, Reduced NOM triggered rapid Cr(VI) reduction and formation of NOM-Cr(III) colloids in anoxic environments, Water Res., 2020, 181, 115923 CrossRef CAS PubMed.
- E. Shang, Y. Li, J. Niu, Y. Zhou, T. Wang and J. C. Crittenden, Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles, Water Res., 2017, 124, 595–604 CrossRef CAS PubMed.
- M. Sikder, J. R. Lead, G. T. Chandler and M. Baalousha, A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV–Vis, Sci. Total Environ., 2018, 618, 597–607 CrossRef CAS.
- R. Lindsey, Climate and earth's energy budget, earthobservatory nasa.gov, 2009.
- W. Huang and X. Xia, Element cycling with micro(nano)plastics, Science, 2024, 385, 933–935 CrossRef CAS.
- Q. An, C. Wen and C. Yan, Meta-analysis reveals the combined effects of microplastics and heavy metal on plants, J. Hazard. Mater., 2024, 476, 135028 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |