Open Access Article
Open Access ArticleLayered double hydroxide modified bismuth vanadate as an efficient photoanode for enhancing photoelectrochemical water splitting
Md. Masum Billah
 ab and
Go Kawamura
*a
ab and
Go Kawamura
*a
aDepartment of Electrical and Electronic Information Engineering, Toyohashi University of Technology, 1-1 Hibarigaoka, Tempaku-cho, Toyohashi, 441-8580, Aichi, Japan. E-mail: kawamura.go.km@tut.jp
bDepartment of Chemistry, Comilla University, Cumilla-3506, Bangladesh
First published on 10th January 2025
Abstract
Photoelectrochemical (PEC) water splitting has attracted significant interest as a promising approach for producing clean and sustainable hydrogen fuel. An efficient photoanode is critical for enhancing PEC water splitting. Bismuth vanadate (BiVO4) is a widely recognized photoanode for PEC applications due to its visible light absorption, suitable valence band position for water oxidation, and outstanding potential for modifications. Nevertheless, sluggish water oxidation rates, severe charge recombination, limited hole diffusion length, and inadequate electron transport properties restrict the PEC performance of BiVO4. To surmount these constraints, incorporating layered double hydroxides (LDHs) onto BiVO4 photoanodes has emerged as a promising approach for enhancing the performance. Herein, the latest advancements in employing LDHs to decorate BiVO4 photoanodes for enhancing PEC water splitting have been thoroughly studied and outlined. Initially, the fundamental principles of PEC water splitting and the roles of LDHs are summarized. Secondly, it covers the development of different composite structures, including BiVO4 combined with bimetallic and trimetallic LDHs, as well as other BiVO4-based composites such as BiVO4/metal oxide, metal sulfide, and various charge transport layers integrated with LDHs. Additionally, LDH composites incorporating materials like graphene, carbon dots, quantum dots, single-atom catalysts, and techniques for surface engineering and LDH exfoliation with BiVO4 are discussed. The research analyzes the design principles of these composites, with a specific focus on how LDHs enhance the performance of BiVO4 by increasing the efficiency and stability through synergistic effects. Finally, challenges and perspectives in future research toward developing efficient and stable BiVO4/LDHs photoelectrodes for PEC water splitting are described.
Wider impactThis review presents recent advancements in layered double hydroxide (LDH) modified BiVO4 photoanodes for efficient PEC water splitting. First, we outline the fundamental principles of photoelectrochemical (PEC) water splitting and the roles of LDHs in improving efficiency and stability. The development of various composite structures, including BiVO4 with bimetallic and trimetallic LDHs, and BiVO4-based composites with metal oxides, metal sulfides, and charge transport layers is summarized. Additionally, we discuss LDH composites incorporating graphene, carbon dots, quantum dots, and single-atom catalysts, along with techniques for surface engineering and LDH exfoliation with BiVO4. This research contributes to scalable green hydrogen generation, decarbonizing industries, ensuring energy security, and mitigating climate change, aligning with global sustainability goals (SDGs 7 and 13), advancing the transition to sustainable society. Despite progress in coupling LDHs with BiVO4 photoanodes for PEC water splitting, challenges like interfacial recombination, stability, and conductivity remain. Future research will focus on interface engineering, optimized LDH loading, and exploring intercalated anions. Advanced tandem systems using LDH modified BiVO4 photoanodes will offer promising, cost-effective pathways for improving PEC performance, shaping the development of next generation techniques and driving innovations in energy conversion, storage, and environment conservation. |
1. Introduction
Due to the rapid increase in world population and industrialization, energy demand is expected to increase from 20 TW to 40 TW by 2050.1–3 Currently, about 85% of our energy comes from nonrenewable energy sources, such as coal, oil, and natural gas.4,5 Nevertheless, these sources are unsustainable and release carbon dioxide (CO2), carbon monoxide (CO), nitrogen oxides (NOx), sulfur oxides (SOx), and other pollutants, which have serious consequences for the environment and human health, ultimately resulting in catastrophic climate change and global warming.6–9To address these challenges, research into sustainable and renewable energy generation technologies is crucial. Solar energy, among all sustainable sources, is the most plentiful, inexhaustible, and widely distributed renewable energy source on Earth.10 Every year, about 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TW of solar energy comes from the sun, of which approximately 36
000 TW of solar energy comes from the sun, of which approximately 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TW reaches the earth.11 It is suggested that the amount of solar radiation that reaches the Earth's surface per h is sufficient to meet the planet's energy needs for a whole year.12,13 Nonetheless, the effectiveness of solar energy conversion and storage encounters obstacles due to its erratic nature, as well as seasonal and regional variations.14–17 Therefore, it is essential to develop technologies that can effectively capture and utilize solar energy to produce clean and sustainable fuel. Among the various solar energy conversion strategies, solar-driven water splitting is an effective approach for converting solar energy into hydrogen (H2).18 H2 produced by the overall solar water splitting process is referred to as green H2 because it does not emit any carbon dioxide.19,20 As a fuel with high density, it possesses the advantageous characteristics of being both storable and transportable.21 Moreover, it finds extensive application in various industries as chemical feedstock, particularly in the production of ammonia, the reduction of carbon dioxide into liquid synthetic fuel and in fuel cells to generate electricity.22–24 As a green and renewable energy source, solar hydrogen can also meet future energy needs and address environmental concerns.25
000 TW reaches the earth.11 It is suggested that the amount of solar radiation that reaches the Earth's surface per h is sufficient to meet the planet's energy needs for a whole year.12,13 Nonetheless, the effectiveness of solar energy conversion and storage encounters obstacles due to its erratic nature, as well as seasonal and regional variations.14–17 Therefore, it is essential to develop technologies that can effectively capture and utilize solar energy to produce clean and sustainable fuel. Among the various solar energy conversion strategies, solar-driven water splitting is an effective approach for converting solar energy into hydrogen (H2).18 H2 produced by the overall solar water splitting process is referred to as green H2 because it does not emit any carbon dioxide.19,20 As a fuel with high density, it possesses the advantageous characteristics of being both storable and transportable.21 Moreover, it finds extensive application in various industries as chemical feedstock, particularly in the production of ammonia, the reduction of carbon dioxide into liquid synthetic fuel and in fuel cells to generate electricity.22–24 As a green and renewable energy source, solar hydrogen can also meet future energy needs and address environmental concerns.25
Currently, the three most relevant solar hydrogen production processes are (i) photovoltaic assisted electrolysis (PV-E), (ii) photocatalysis (PC), and (iii) photoelectrochemical (PEC) water splitting. Non-integrated PV-E technology, with its high solar-to-hydrogen (STH) efficiency and mature technological base, is the most practical configuration and has been optimized over decades.26 However, hydrogen production using this method is too expensive due to the high complexity of the cell design.27,28 In contrast, solar energy photocatalysis of water provides a potentially easier and economical approach to hydrogen production. Nonetheless, the efficiency of this process, particularly in converting solar energy into hydrogen, remains a significant challenge due to its insufficient light conversion rates.29 To address these issues, researchers explored the integration of photocatalysis and electrocatalysis in PEC water splitting.30–32 In comparison to PC, PEC generates H2 and O2 on separate photoelectrodes, preventing gas mixing and reversal reactions.33 The self or external bias can mitigate charge carrier recombination in PEC, allowing for effective charge separation and migration, resulting in significantly higher efficiency compared to PC.34
In 1972, Fujishima and Honda pioneered PEC water splitting on a TiO2 photoanode under ultraviolet irradiation, which has since become a cornerstone in the solar energy conversion and storage revolution.35,36 A typical PEC system comprises a photo cathode, a photoanode, an electrolyte, and a membrane for product separation.37 The PEC water splitting process entails two half-cell reactions: the oxygen evolution reaction (OER) on the photoanode and the hydrogen evolution reaction (HER) on the photocathode. The photocathode utilizes two electrons to produce hydrogen gas, while the photoanode employs four holes per oxygen (O2) molecule to liberate O2 gas. The OER is more challenging than the HER because of its higher energy barrier and slower reaction kinetics.38,39 Consequently, developing efficient photoanode is crucial to enhance the activity of O2 evolution and boost the efficiency for solar energy conversion in PEC water splitting.
To be an economically and commercially viable photoanode, semiconductor materials must meet several criteria simultaneously such as an appropriate band gap for sufficient absorption of visible range of solar spectrum, suitable band alignment and effective separation of charge carriers at the semiconductor/aqueous interface, low overpotential for performing water splitting half oxidation/reduction reaction, electrochemical and chemical stability, and cost-effectiveness.40–42 In this regard, a wide range of materials based on oxides,43–47 chalcogenides,48–51 nitrides,52 and phosphides53 have been explored for water splitting processes. Among these materials, monoclinic BiVO4 stands out as a highly promising photoanode because of its suitable bandgap (2.3–2.5 eV) for absorbing visible light and advantageous band edge potential positions for efficient PEC water splitting.54,55 Moreover, BiVO4 has a high theoretical photocurrent density of 7.5 mA cm−2 and a STH conversion efficiency of 9.2% under AM 1.5 solar light irradiation (100 mW cm−2), making it suitable for practical applications.56 However, the unmodified BiVO4 photoanode exhibits lower photocurrent densities and efficiencies than theoretically predicted.57,58 This discrepancy can be attributed to several factors, including its short hole diffusion length, excessive electron hole recombination and slow O2 evolution kinetics.59 Therefore, various strategies, including morphology control,60–62 metal or non-metal doping,63–65 crystal facet engineering,66,67 heterojunction construction,68–70 and cocatalyst loading,71–80 have been developed to enhance the PEC performance of BiVO4.
Particularly, the deposition of oxygen-evolution cocatalysts (OECs) on BiVO4 has been recognized as an advantageous and efficient method to enhance PEC water splitting by lowering the energy barrier, increasing active sites, and accelerating the surface OER.80–82 Although IrO2 and RuO2 are now regarded as the most efficient OECs for water splitting and have been used to improve the PEC performance of BiVO4, their costly price, scarcity, and insufficient stability restrict their practical application.73,83 Therefore, there is significant interest in designing an effective photoanode comprised of potential BiVO4 materials and highly efficient, low cost, non-noble-metal OER electrocatalysts.
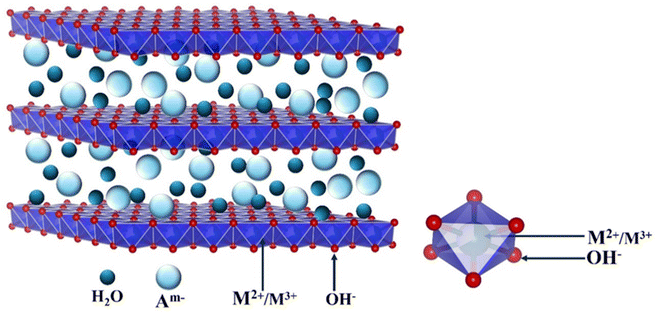
Layered double hydroxides (LDHs) (Fig. 1) have been recognized as one of the most promising non noble metal O2 evolution electrocatalysts due to their distinct physicochemical characteristics, and ease of synthesis in nanostructures, making them attractive for various applications, particularly in energy conversion and storage.84–86 LDHs, also known as a hydrotalcite-like compound, are 2D layer anionic clays. The basic structure of LDH is represented as [M1−x2+Mx3+(OH)2]x+[Ax/mm−·nH2O], where M2+ and M3+ are divalent and trivalent metals, respectively, and ‘A’ is the exchangeable interlayered anion.87 Generally, LDHs exhibit a 2D nanosheet structure, which offers numerous advantages. Firstly, it enables the inclusion of a variety of metal species and the ability to change their ratios within the interlayer structure. Secondly, the large interlayer spacing of LDHs enhances the rate of ion diffusion. Furthermore, the hierarchical porosity of LDHs offers an extensive surface area and numerous active sites that facilitate charge transfer at the electrolyte interface, thereby boosting the performance of PEC systems.88–90 Considering these collective attributes, LDHs present themselves as appealing co-catalysts for fabricating the BiVO4 photoanode, which may lead to exceptional PEC performance.
Recently, several comprehensive reviews on BiVO4 photoelectrodes have thoroughly explored synthesis methods and modification techniques, including morphology control, diverse nanostructures, heterostructures and cocatalyst integration. However, a detailed review focusing solely on LDHs combined with BiVO4 is still lacking, thus the missing part must be addressed by discussing the latest advancements in using LDHs to enhance BiVO4 photoanodes. In this review, the basic principles of PEC water splitting and the roles of LDHs are firstly summarized. Secondly, it covers the development of different composite structures, including BiVO4 combined with bimetallic and trimetallic LDHs, as well as other BiVO4-based composites such as BiVO4/metal oxide, metal sulfide, and various charge transport layers integrated with LDHs. Additionally, LDH composites incorporating materials like graphene, carbon dots (CDs), quantum dots (QDs), single-atom catalysts, and techniques for surface engineering and LDH exfoliation with BiVO4 are discussed. The design principles of these composites, with a specific focus on how LDHs enhance the performance of BiVO4 by increasing efficiency and stability through synergistic effects, are also analyzed. Finally, some challenges and perspectives in future research toward developing efficient and stable BiVO4/LDHs photoelectrodes for PEC water splitting are described.
2. Principle of photoelectrochemical water splitting
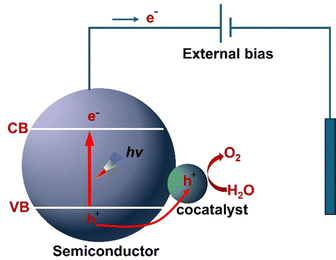
PEC processes are at the forefront of renewable energy research, offering an appealing approach for converting solar energy into high-density chemical energies, notably H2 in water splitting. Generally, a PEC cell harnesses photon energy to facilitate chemical reaction that dissociates water into H2 and O2 gases.42,90,91 The PEC cell typically involves an anode and a cathode in an electrolyte, connected by an external circuit. A semiconductor photoanode is commonly employed as the working electrode, which absorbs photon energy. The cathode, usually composed of platinum (Pt), serves as the counter electrode and an external circuit is utilized to facilitate the transfer of electrons from the anode to the cathode (Fig. 2a).92,93 In general, water splitting in the PEC reaction involves three fundamental physicochemical processes:94 (i) the absorption of light by a semiconductor photoanode, which leads to the generation of electron–hole pairs. (ii) the separation and transportation of the photogenerated charge carriers and (iii) the water splitting reaction by the photoexcited electrons–holes at the electrode–electrolyte interface. The charge transfer mechanism at the electrode–electrolyte interface is critical for enhancing the overall PEC water splitting efficiency, as it governs the water oxidation and reduction half reactions, as shown in Fig. 2b.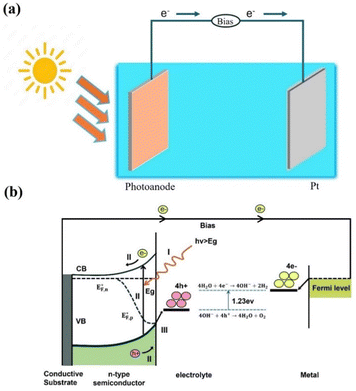 | ||
| Fig. 2 (a) A schematic representation of a typical PEC water splitting cell based on photoanodes. (b) Schematic illustration of a basic PEC cell comprising an n-type semiconducting photoanode coupled with a counter electrode under an external bias. On the photoanode, 4OH− + 4H+ → 2H2O + O2 occurs under alkaline conditions, and a bias is necessary, as the location of the conduction band (CB) is too positive to facilitate water reduction. The basic processes are: (I) light absorption; (II) photogenerated charge carrier separation and transfer and (III) surface water splitting redox reactions. Reproduced from ref. 94, CC. BY 3.0. | ||
When a semiconductor material is immersed in an electrolyte in the absence of light, charge (electron or hole) transfer occurs between the semiconductor and the electrolyte to align the Fermi level (EF) of the semiconductor with the redox potential (ERedox) of the electrolyte.95 This equilibration causes band bending at the semiconductor interface. The band bending generates an electric field near the semiconductor surface as a result of the disparity in electrochemical potential between the semiconductor and the electrolyte, creating a space charge layer (SCL). In this region, electrons or holes accumulate at the surface, while the bulk of the semiconductor remains electrically neutral.96,97 Thus, the space charge layers induce an internal electric field, which plays a vital role in the separation of the photogenerated electrons and holes.95,98,99
When an n-type semiconductor i.e. photoanode is in equilibrium with ERedox, band bending occurs upward, forming a positively charged depletion layer in the SCL, while a negatively charged Helmholtz layer is generated on the photoanode's surface. Similarly, when the p-type photocathode is immersed in the electrolyte, the band bends downwards.30,100–102 Upon illumination by solar light, a semiconductor photoanode absorbs photons with energy equal to or exceeding its bandgap, generating electron–hole pairs, where excited electrons in the valence band (VB) are transferred to the conduction band (CB), resulting in holes being left in the VB. When an external bias voltage is applied, electrons migrate through the external circuit to the surface of the counter electrode, where they take part in the HER. Meanwhile the holes transfer to the photoanode surface to undergo the OER.103,104 The water splitting reactions at the photoanode and counter electrode at different pH are as follows:34,105
Acidic solution:
| OER: 2H2O → O2 + 4H+ + 4e− (E° = −1.23 V vs. NHE) |
| HER: 4H+ + 4e− → 2H2 (E° = 0.00 V vs. NHE) |
Basic solution:
| OER: 4OH− → O2 + 2H2O + 4e− (E° = −0.40 V vs. NHE) |
| HER: 4H2O + 4e− → 2H2 + 4OH− (E° = −0.83 V vs. NHE) |
Overall reaction
| H2O → 1/2 O2 + H2 (ΔG = +237.2 kJ mol−1) |
The water splitting reaction is an endothermic process that requires a Gibbs free energy of ΔG = 237.2 kJ mol−1 to split one molecule of water into H2 and 1/2 O2. This energy corresponds to a photon energy of 1.23 V per electron transfer.105 Therefore, the semiconductor photoanode must capture light radiation with photon energy exceeding 1.23 eV and utilize this energy to split water. Moreover, to effectively reduce and oxidize water, the CB edge has a more negative value than the H2 evolution potential (0 V vs. NHE), while the VB edge should have a more positive value than the oxygen evolution potential from water (1.23 V vs. NHE).106,107 An extra over potential is necessary to compensate for energy losses caused by the movement of photogenerated holes within the space charge region and the transfer of electrons through the external circuit to the counter electrode to drive the HER and OER reactions.94 Hence, an ideal semiconductor photoanode is required to have an energy range of approximately 1.6 to 2.4 eV to harness solar energy for water splitting.108,109 Photoanodes should possess excellent light absorption, rapid electron–hole separation, and minimal over potential for efficient water splitting. However, no single material has yet met these criteria. Various methods have been employed to enhance solar water splitting devices, such as applying functional cocatalysts on the surface of the electrode to provide reaction sites and catalyze the HER and OER, as shown in Fig. 3. Furthermore, the use of cocatalysts can reduce photocorrosion and improve the chemical stability of the semiconductor-based photoelectrode.21,110–112
3. Role of LDHs
LDHs represent a class of anionic clay materials with a brucite-like layered structure, wherein various metal cations can occupy within the octahedral sites of the layers.113,114 Due to their structural properties, LDHs have been extensively explored in electrochemistry, photochemistry, adsorption, catalysis, and photocatalysis.115–117 Developing visible light active photocatalyst LDHs is essential for both research and practical application. Transition metal-based oxides, hydroxides, and LDHs especially those with Fe, Co, Ni, Zn, and Mn are promising due to their affordability, ease of preparation, and high activity, offering optimal adsorption energies and enhanced catalytic performance.118,119,120,121a,b,c,d The unique structure, tunable composition, and remarkable physicochemical properties of LDHs, along with their ability to integrate into sophisticated molecular assemblies, enhance semiconductor photoanode performance in PEC water splitting.122,123 Specifically, LDHs have received significant attention for their role in enhancing the performance of BiVO4 photoanodes. Studies have highlighted several key functions of LDHs when combined with BiVO4 in PEC water oxidation reactions, as presented in Fig. 4.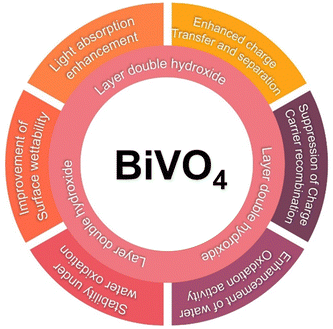 | ||
| Fig. 4 Schematic illustration of the role of LDHs in enhancing the PEC performance of BiVO4 photoanodes. | ||
3.1. Light absorption enhancement
Efficient light absorption and charge carrier generation at the photoanode/OEC interface are crucial for enhancing PEC water oxidation activity. LDHs, which can be considered as “doped semiconductors,” play a significant role in promoting the visible light absorption and utilization.124 By adjusting the ratio of metal cations in LDHs, it is possible to fine-tune their band gap and light absorption properties, thereby altering the range of light absorption and the oxidation–reduction potential.125Interfacial tuning of LDHs, such as modifying the ratio of metal cations, can effectively adjust the band gap and enhance PEC performance. For example, Yang et al. developed a three-dimensional (3D) BiVO4/Fe-based (Ni1−xFex and Co1−xFex) LDH core/shell heterostructure film.126 Their study revealed that the BiVO4/Ni0.5Fe0.5-LDH photoanode exhibited a photocurrent density that was four times higher than that of bare BiVO4 at 1.23 V vs. the reversible hydrogen electrode (RHE). Additionally, there was a cathodic shift in the onset potential of 320 mV, and the incident photon-to-current conversion efficiency (IPCE) for PEC water oxidation showed an approximate four-fold improvement compared to the bare BiVO4 mainly due to the optimized light absorption by band-gap engineering.
Moreover, LDHs have been found to possess a small band gap, considerably enhancing their light-harvesting capacities.127 For example, He et al. prepared a BiVO4@CoAl-LDH composite.128 The dual light-absorbing characteristics of this composite photoanode enhance its ability to capture irradiated light. Specifically, the lower bandgap CoAl-LDH (2.2 eV) absorbs longer wavelengths of visible light, while the broader bandgap BiVO4 (2.4 eV) absorbs shorter ones, as confirmed by UV-vis absorption and IPCE analysis. By integrating materials with distinct bandgaps, this design maximizes solar energy efficiency. Consequently, the BiVO4@CoAl-LDH photoanode exhibited improved PEC performance, achieving an IPCE at 400 nm that is double that of pure BiVO4. This substantial improvement is due to the synergistic effect of the dual light-absorbing properties and efficient charge separation facilitated by the LDHs.
3.2. Enhanced charge transfer and separation
Loading OEC on BiVO4 enhances overall PEC properties by increasing charge transfer of photogenerated holes from the semiconductor's VB to the catalyst, thereby increasing charge separation and allowing more holes to be involved in surface reactions.129,130 LDHs, with their variable valence states of metal cations, play a critical role in this process. When photogenerated holes are produced in the semiconductor, low-valence metal cations in LDHs are oxidized to higher valence states by photogenerated holes, enhancing charge carrier transfer and separation. For instance, Guo et al. synthesized the Mo-BiVO4/NiFe-LDH heterostructure through electrodeposition.131 The photogenerated holes in BiVO4 predominantly facilitate the oxidation of low valence Ni2+ to higher valence of Ni3+/Ni4+ within NiFe-LDH, subsequently contributing to the OER, thereby enhancing the PEC performance for water decomposition. Zhong and his collaborators developed a self-healing photoanode using ultrathin (u)-CoAl-LDH on Mo:BiVO4.132 In this photoanode, u-CoAl-LDH acts as an efficient OEC. By rapidly extracting photogenerated holes through the conversion of Co2+ to Co3+ species, it significantly accelerates the proton transfer process and redirects the rate-determining step of the Mo:BiVO4 photoanodes, which utilize a non-proton transfer mechanism (Fig. 5). Consequently, the charge separation efficiency is enhanced to nearly 100% on the photoanode surface and facilitates the overall PEC performance. Moreover, Huang et al. reported BiVO4/NiFe-LDH photoanodes, where the incorporation of nanosheet NiFe-LDH significantly shortens the transfer distance of photogenerated holes at the BiVO4/OER catalyst interface, thereby enhancing charge separation and photocurrent density.133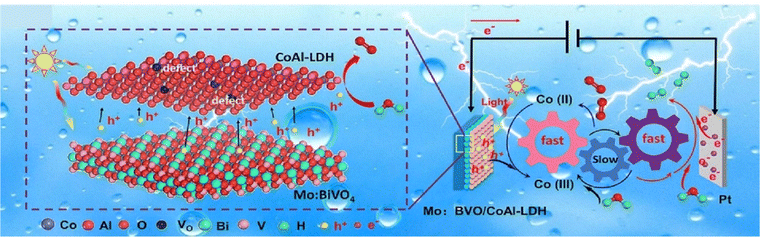 | ||
| Fig. 5 Schematic representation of PEC water splitting utilizing Mo:BiVO4/u-COAl-LDH. Reproduced with permission from ref. 132, Copyright 2023, Elsevier. | ||
3.3. Suppression of charge carrier recombination
Due to lattice defects or impurities in semiconductors, materials generate surface trapping states that facilitate rapid charge carrier recombination. This leads to a significant decrease in photocurrent density of pristine semiconductor photoanodes compared to their theoretical values, thereby hindering the overall performance of PEC water splitting.134a,b Deposition of the electrocatalyst, such as LDHs on the surface of a BiVO4 can reduce the excessive surface trapping states by facilitating interfacial hole transfer, and therefore suppress the recombination of photogenerated electron–hole pairs.134c In this respect, Meng and co-workers reported the deposition of NiFeV-LDH on B-BiVO4. The author suggested that the main factor restricting the photocurrent density of BiVO4 materials is severe surface charge recombination, as demonstrated using intensity modulated photocurrent spectroscopy (IMPS).135 They found that after decorating BiVO4 photoanodes with a NiFeV-LDH, the PEC performance of the BiVO4 photoanodes was significantly enhanced due to the efficient suppression of charge recombination.Moreover, Mane et al. developed a surface oxygen vacancy-incorporated (Ovac):BiVO4/NiFe-LDH photoanode,136a in which the NiFe-LDH transport layer extracts holes from the valence band of BiVO4:Ovac. This configuration enhances charge transport to the electrolyte, reduces surface trapping states, and promotes the participation of more energetic holes in the water-oxidation reaction. During this process, high-valence Ni species (Ni3+/Ni4+) are reduced to Ni2+, which minimizes charge recombination and completes the catalytic cycle (Fig. 6). Consequently, the excellent charge transfer ability of the Ovac:BiVO4/NiFe-LDH photoanode enhances PEC performance for water oxidation. Furthermore, clarifying the function of cocatalysts in suppressing surface charge recombination and enhancing catalytic activity contributes to the observed photocurrent improvements in semiconductor/electrocatalyst processes. Lin and Boettcher highlighted the importance of adaptive junctions at interfaces of these materials, where permeable electrocatalysts allow electrolyte access.111,136b The junctions modify the barrier height and band bending according to the cocatalyst's oxidation state and the applied bias, leading to increased photovoltage and enhanced charge transfer dynamics. This concept applies specifically to the deposition of catalysts on the photoanode surface, where the junction type is significantly influenced by the deposition method. In this regard, Chhetri et al. effectively utilized pulse plating to electrodeposit amorphous CoLa(OH)x mixed double hydroxide onto nanoporous BiVO4, achieving precise controlled layer of thickness and enhanced adhesion, resulting in a favorable BiVO4/MDH interface.136c This adaptive interface effectively suppresses photogenerated charge recombination and improves charge transport through grain size regulation, leading to a significant enhancement in PEC performance.
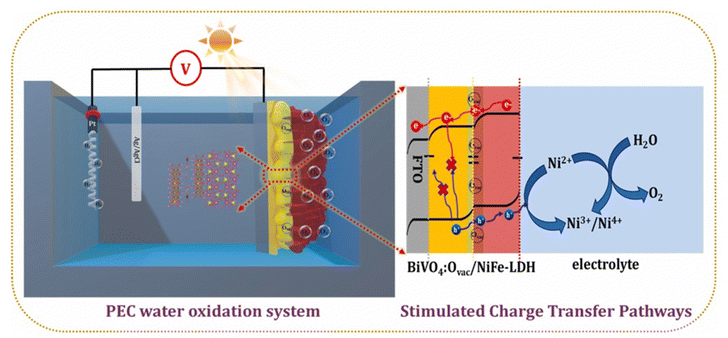 | ||
| Fig. 6 A schematic illustration of the process for the enhanced charge transport pathway in the Ovac:BiVO4/NiFe-LDH photoanode for PEC water oxidation. Reproduced with permission from ref. 136a, Copyright 2024, Elsevier. | ||
3.4. Enhancement of water oxidation activity
Due to the high overpotential and sluggish water oxidation kinetics, a large number of photogenerated charge carriers tend to recombine on the BiVO4 photoanode surface. This recombination significantly reduces the photocurrent density and lowers the reactivity of the photoanode.137–139 Loading OECs such as LDHs is an effective strategy to enhance the reactivity of the photoanode. For instance, Zhao et al. prepared a BiVO4/CoMn photoanode for efficient PEC water splitting.140 After loading the CoMn-LDH cocatalyst, the photocurrent density increased sharply, reaching 2.45 times that of the pristine BiVO4 photoanode. This considerable improvement suggests that decorating CoMn-LDH nanoflakes provides high-valence state active sites [Co3+δ/Mn3+δ (0 < δ < 1)] for water oxidation, by oxidizing Co2+/Mn2+ in the LDH nanoflakes through holes. Subsequently, the Co3+δ/Mn3+δ ions can be reversibly reduced to their original Co2+/Mn2+ state, as shown in Fig. 7a. This continuous cyclic activity enhances the hole transport and lowers the overpotential, thereby boosting the PEC water oxidation reaction.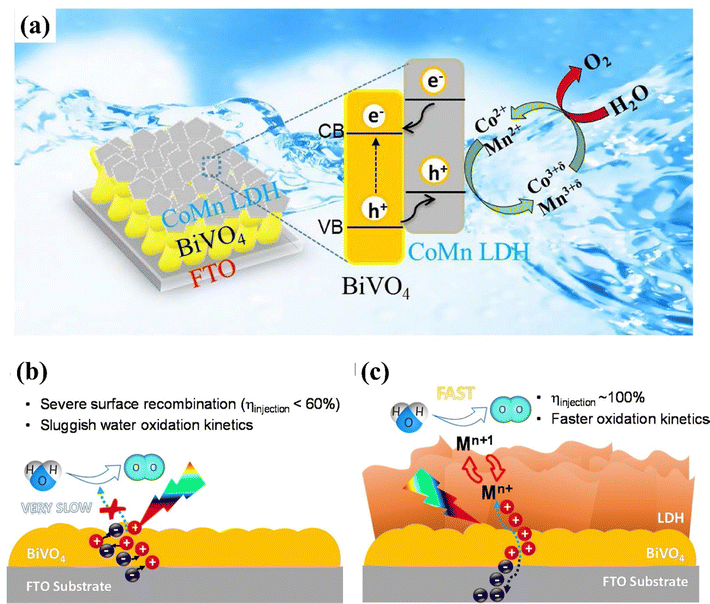 | ||
| Fig. 7 (a) Schematic representation of PEC water splitting utilizing the BiVO4/CoMn-LDH photoanode. Reproduced with permission from ref. 140, Copyright 2020, Elsevier; (b) and (c) Illustrative depiction of charge carrier dynamics during water oxidation on BiVO4 and BiVO4/CMZ-LDH photoelectrode. Reproduced with permission from ref. 141, Copyright 2019 Elsevier. | ||
Moreover, Vo et al. reported a BiVO4/CoMnZn photoanode prepared via an electrodeposition method.141 The primary cause of the catalytic activity in the OER is generally due to the presence of Co and Mn, whilst Zn plays a role in providing structural support and enabling cooperative effects. When the ternary CoMnZn-LDH is coupled with BiVO4, the M2+(Co, Mn) ions accept photogenerated holes from the VB of BiVO4 and undergo oxidation to form high-valence active sites (M3+ or M4+) due to their lower formation energy barrier. Subsequently, these ions with high valence levels undergo oxidation of H2O to produce O2 while reverting back to their initial valence state (Fig. 7b and c). This process enhances the rate at which interfacial holes are extracted from the surface of the photoelectrode, hence improving the overall water splitting efficiency.
3.5. Enhancements of stability under water oxidation
One of the most critical factors for the practical application of photoanodes in PEC is stability. However, the photostability of BiVO4-based photoanodes remains a significant challenge to achieving substantial PEC water splitting due to the tendency of V5+ ions in BiVO4 to dissolve in harsh electrolyte environments under solar radiation.142 Loading a cocatalyst layer to BiVO4 effectively inhibits the dissolution of V5+ from the BiVO4 lattice, enhances charge transfer rate, and inhibits photocorrosion.143–145 However, the ultra-thin cocatalyst layer is not consistently stable due to changes in the structure during operation and the loss of catalyst materials resulting from agitation and dissolution.146a,b The deposition of catalysts on photoanodes via spray pyrolysis is now being considered a simpler and more scalable method, facilitating precise control over layer structure and ensuring strong adhesion to substrates to address this issue.146c Meanwhile, LDHs manufactured through the hydrothermal approach demonstrate high particle purity, favorable distribution, ideal crystal shape, and excellent controllability.146dFor instance, Wang et al. prepared an oxygen vacancy-incorporated BiVO4/(Ovac)-NiFe-LDH photoanode via a hydrothermal method,146e demonstrating that the LDH acts as a hole-shuttling mediator for oxygen evolution. Loading Ovac-NiFe-LDH on BiVO4 greatly improves the photoanodes’ charge separation and injection efficiency. As a result, the BiVO4/(Ovac)-NiFe-LDH photoanode showed exceptional stability for 80 h in a V5+ ion saturated borate buffer electrolyte, as shown in Fig. 8a. He and his coworkers reported the synthesis of a NiFeY-LDH cocatalyst on BiVO4,147 where the inclusion of Y altered the chemical surroundings of Ni, resulting in a decrease in surface charge recombination. This modification led to a highly functional BiVO4/NiFeY-LDH photoanode that exhibited a substantial PEC performance and outstanding stability of approximately 25 h (Fig. 8b).
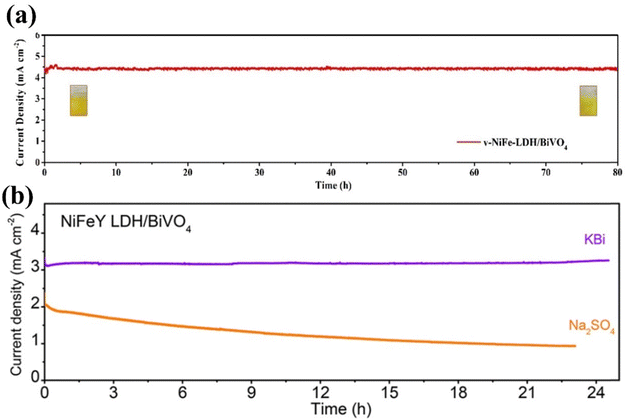 | ||
| Fig. 8 (a) Current vs. time curve of BiVO4/v-NiFe-LDH in KB + V at 1.23 VRHE over 80 h. Reproduced with permission from ref. 146e, Copyright 2023, American Chemical Society. (b) Current–time profiles of BiVO4/NiFeY-LDH in 1 M KBi (pH = 9) and 0.2 M Na2SO4 (pH = 7) at 0.8 VRHE for around 24 h. Reproduced with permission from ref. 147, Copyright 2020, American Chemical Society. | ||
3.6. Improvement of surface wettability
Wettability pertains to the extent of compatibility and affinity between a heterogeneous catalyst and liquid reactants. Surface roughness and micro-morphology have a significant impact on the surface wettability of semiconductor materials.148,149 A photoanode with a hydrophilic surface enhances water molecule adsorption and facilitates gas separation, hence enhancing the overall efficiency of PEC water splitting.150 For example, Yue et al. prepared a super-hydrophilic BiVO4/CoAl-LDH photoanode.151 This photoanode exhibited an impressive photocurrent density of 3.5 mA cm−2 at 1.23 vs. RHE, about 3.2 times higher than that of the bare BiVO4 photoanode, with a negative shift in onset potential of 0.30 V. The improved water oxidation capability is ascribed to the well-matched energy level between LDH and BiVO4, along with the engineered surface wettability. As illustrated in Fig. 9(a–i), the surface morphology of the BiVO4/CoAl-LDH photoanode is characterized by nanosheets. After plasma treatment, the thickness of BiVO4/H-CoAl-LDH is drastically reduced while its morphology remains unchanged. Moreover, BiVO4 and BiVO4/CoAl-LDH materials exhibit hydrophilic properties with contact angles of 27.75° and 46.84°, respectively. In contrast, H-CoAl-LDH/BiVO4 demonstrates exceptional superhydrophilicity, with a contact angle of 2.87°. The superhydrophilic surface of H-CoAl-LDH/BiVO4 accelerates the adsorption of water molecules on surface active sites, leading to outstanding surface reaction activity and enhanced PEC water oxidation efficiency.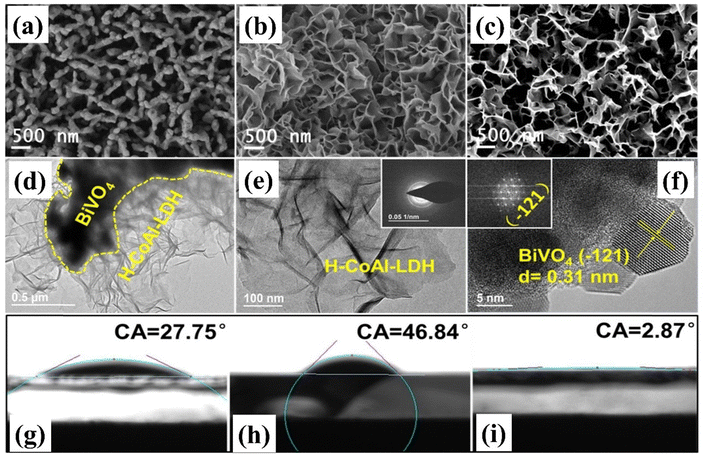 | ||
| Fig. 9 SEM images of (a) BiVO4, (b) BiVO4/CoAl-LDH, and (c) BiVO4/H-CoAl-LDH, and TEM and HTEM images of BiVO4/H-CoAl-LDH (d)–(f), inset in (e): diffraction ring, inset in (f): diffraction pattern; contact angle BiVO4 (g), BiVO4/CoAl-LDH (h) and BiVO4/H-CoAl-LDH (i) electrodes. Reproduced with permission from ref. 151, Copyright 2021, Elsevier. | ||
4. Recent advancement of BiVO4 and LDH for PEC water oxidation
In this chapter, BiVO4/bimetallic-LDH, BiVO4/trimetallic-LDH, BiVO4 composites/LDH, and BiVO4/LDH composites are reviewed and discussed to understand the relationship between their structure, properties, and PEC water splitting performance. Relevant research from 2015 to 2024 on BiVO4/LDH photoanodes for PEC water splitting is listed in Table 1.| Photoanode | Synthesis | Morphology | Electrolyte | Light source | Applied bias | Photocurrent (mA cm−2) | Stability (h) | Ref. | Publication year |
|---|---|---|---|---|---|---|---|---|---|
| BiVO4/Bimetallic LDH | |||||||||
| BiVO4/NiFe-LDH | Hydrothermal | Nanoparticles | 0.5 M Na2SO4 | Light emitting diode lamp (CEL-LED100) | 0.6 V vs. Ag/AgCl | 2.49 | 3 | 152 | 2018 |
| BiVO4/NiFe LDH | Hydrothermal | Nanosheet | 0.1 M KHCO3 (pH = 8.6) | AM 1.5 solar simulator | 1.23 V vs. RHE | 4.45 | 30 | 133 | 2017 |
| BiVO4/NiFe-LDH | Electrodeposition | — | 0.5 M Na2SO4 | AM 1.5 (100 mW cm−2) illuminator | 1.23 V vs. RHE | 1.21 | 12 | 126 | 2017 |
| BiVO4/NiCo-LDH | Electrodeposition | Nanoparticle | 0.5 M Na2SO4 (pH = 7.3) | AM 1.5 solar simulator | 1.23 V vs. RHE | 3.4 | 2 | 73 | 2020 |
| Nd: BiVO4/NiCo-LDH | Hydrothermal | Nanosheet | 0.5 M Na2SO4 (pH = 6.8) | AM 1.5 solar simulator (100 mW cm−2) | 1.23 V vs. RHE | 4.1 | 3 | 153 | 2024 |
| BiVO4/NiMn-LDH | Hydrothermal | Nanosheet | 0.5 M Na2SO4 | 500 W xenon lamp | 1.23 V vs. RHE | 0.83 | 0.5 | 154 | 2019 |
| BiVO4@CoAl-LDH | Hydrothermal | Nanosheets | 0.1 M PBS (pH = 7), 0.1 M H2O2 | 300 W xenon lamp | 1.23 V vs. RHE | 1.0 | — | 128 | 2015 |
| BiVO4/CoMn-LDH | Electrodeposition | Nanoflake | 0.5 M Kpi | AM 1.5 solar simulator | 1.23 V vs. RHE | 2.69 | 2.7 | 140 | 2020 |
| BiVO4/CoFe-LDH | Chemical bath deposition | Nanoparticle | 0.5 M KBi (pH = 9.2) | 100 mW cm−2 simulated sun light | 1.23 V vs. RHE | 4.3 | 1 | 155 | 2022 |
| BiVO4·Ovac/NiFe-LDH | Electrodeposition | Nanosheet | 0.5 M Na2SO4 | 300 W xenon arc lamp | 1.23 V vs. RHE | 2.92 | 20 | 136 | 2024 |
| Mo: BiVO4/NiFe-LDH | Electrodeposition | Nanosheet | 0.1 M Na2SO4 (pH = 7) | AM 1.5 solar simulator (100 mW cm−2) | 1.23 V vs. RHE | 1.58 | 2.2 | 131 | 2019 |
| Fe-BiVO4/CoFe-LDH | Electrodeposition | Nanoparticle | 0.3 M K2SO4, 0.2 M PBS | 500 W xenon lamp | 1.23 V vs. RHE | 2.56 | 5 | 156 | 2024 |
| BiVO4/CE-NiFe-LDH | Cation exchange | 0.5 M Na2SO4 | 150 W xenon lamp | 1.23 V vs. RHE | 4.03 | 10 | 157 | 2021 | |
| BiVO4/Trimetallic LDH | |||||||||
| BiVO4/ZnCoV-LDH | Electrodeposition | Nanosheet | 0.1 M NaBi (pH = 9.4) | 500 W xenon arc lamp | 1.23 V vs. RHE | 2.55 | 2 | 158 | 2020 |
| BiVO4/CoMnZn-LDH | Electrodeposition | Nanosheet | 0.1 M NaBi (pH = 9.4) | AM 1.5 solar simulator | 1.23 V vs. RHE | 1.06 | 141 | 2019 | |
| BiVO4/ZnCoFe-LDH | Electrodeposition | Nanolayer | 1 M Na2SO3 | AM 1.5 solar simulator | 1.23 V vs. RHE | 3.43 | 2.7 | 159 | 2021 |
| BiVO4/NiCoV-LDH | Electrodeposition | Nanosheet | 0.1 M KPi (pH = 7) | AM 1.5 xenon lamp (100 mW cm−2) | 1.23 V vs. RHE | 3.32 | 160 | 2024 | |
| B-BiVO4/NiFeV-LDH | Hydrothermal, drop casting | Nanosheet | 1 M KBi (pH = 9.3), 0.2 M Na2SO3 | 100 W xenon arc lamp | 1.23 V vs. RHE | 4.6 | 24 | 135 | 2021 |
| BiVO4/Y-NiFe-LDH | Hydrothermal | Nanosheet | 1 M KBi (pH = 9.5), 0.2 M Na2SO4 | AM 1.5 xenon lamp (100 mW cm−2) | 1.23 V vs. RHE | 5.2 | 25 | 147 | 2020 |
| BiVO4 Composites/LDH | |||||||||
| WO3/BiVO4/NiCo-LDH | Hydrothermal | Nanosheet | 0.5 M Na2SO4 (pH = 6.9) | 150 W xenon lamp | 1.23 V vs. RHE | 3.2 | 2 | 161 | 2024 |
| Fe2O3/BiVO4/NiFe-LDH | Electrodeposition | Nanosheets | 1 M NaOH (pH = 13.16) | 300 W xenon lamp | 1.8 V vs. RHE | 1.7 | — | 162 | 2018 |
| WO3/BiVO4/NiFe-LDH | Electrodeposition | Nanoparticle | 0.5 M Na2SO4 (pH = 7.0) | 300 W xenon lamp | 1.23 V vs. RHE | 1.78 | 24 | 163 | 2023 |
| TiO2/BiVO4/NiFe-LDH | Hydrothermal | Nanoparticle | 0.05 M Na2SO4 | 500 W xenon lamp | 0.8 V vs. RHE | 0.017 | 0.55 | 164 | 2019 |
| WO3-BiVO4-NiFeCr-LDH | Electrodeposition | Nanosheet | 0.1 M PBS (pH = 6.9) | AM 1.5G solar lamp | 1.23 V vs. RHE | 4.9 | 6 | 165 | 2021 |
| MoO3/BiVO4/CoMnZn-LDH | Electrodeposition | Nanosheet | 0.1 M Na2SO4 | 500 W xenon lamp | 1.23 V vs. RHE | 1.24 | 1 | 166 | 2020 |
| BiVO4/CdS/NiCo-LDH | Electrodeposition | Nanosheets | 0.5 M Na2SO3 (pH = 7) | — | 1.23 V vs. RHE | 2.72 | 1 | 167 | 2019 |
| BiVO4/CdS/NiFe-LDH | Electrodeposition | Nanosheet | 0.5 M Na2SO4 | 300 W xenon lamp | 1.23 V vs. RHE | 3.1 | 3 | 168 | 2024 |
| BiVO4/MoOx/NiFe-LDH | Electrodeposition | Nanosheet | 0.1 M KBi (pH = 9) | 100 mW cm−2 | 1.23 V vs. RHE | 2.7 | 0.069 | 169 | 2022 |
| BiVO4/MoO3/CoMn-LDH | Electrodeposition | Nanosheet | 1 M KBi (pH = 9) | 300 W xenon lamp, AM 1.5 solar light (100 mW cm−2) | 1.23 V vs. RHE | 3.78 | 2 | 170 | 2023 |
| BiVO4/CuFeO2/NiFe-LDH | Electrodeposition | Nanosheet | 1 M Na2SO4 | 300 W xenon arc lamp | 1.23 V vs. RHE | 4.34 | 5 | 171 | 2022 |
| BiVO4/CuCoO2/NiCo3-LDH | Electrodeposition | Nanosheet | 0.5 M Na2SO4 | xenon lamp (100 mW cm−2) | 1.8 V vs. RHE | 6.95 | 2 | 172 | 2023 |
| BiVO4/BNNPs/CoCr-LDH | Hydrothermal | — | 0.1 M Na2SO4 (pH = 6) | 1.5 G filter (100 mW cm−2) | 1.23 V vs. RHE | 3.8 | 3 | 173 | 2022 |
| BiVO4/FeOOH/ZnFe-LDH | Electrodeposition | Thin plates | 1 M Na2SO4 (pH = 7) | 300 W xenon arc lamp | 1.23 V vs. RHE | 4.92 | 1.66 | 174 | 2022 |
| BiVO4/CuPc/NiCo-LDH | Hydrothermal | Nanosheet | 0.5 M KBi (pH = 9.5) | 300 W solar simulator (AM 1.5 solar light) | 1.23 V vs. RHE | 4.03 | 15 | 175 | 2022 |
| BiVO4/P3HT-CuPc/NiCo-LDH | Hydrothermal | Nanosheet | 0.5 M KBi (pH = 9.5) | 300 W solar simulator (AM 1.5 solar light) | 1.23 V vs. RHE | 4.25 | 8 | 176 | 2022 |
| BiVO4/rGO/NiFe-LDH | Electrodeposition | Nanoarrays | 0.5 M Na2SO4 (pH = 6.9) | 150 W xenon lamp | 1.23 V vs. RHE | 1.30 | 10 | 177 | 2018 |
| BiVO4/rGO/NiFe-LDH | Electrodeposition | Nanosheets | Kpi (pH = 7) | 300 W xenon lamp | 1.23 V vs. RHE | 1.13 | 178 | 2019 | |
| BiVO4/GQDs/CoSn-LDH | Hydrothermal | Thin layer | 0.1 M KBi (pH = 11) | 300 W Tungsten halogen lamp | 1.23 V vs. RHE | 4.15 | 4 | 179 | 2021 |
| BiVO4/rGO/NiFe-LDH | electrodeposition | Nanoplates | 1 M KBi (pH = 9.33) | 150 W xenon lamp, AM 1.5 solar light | 1.23 V vs. RHE | 3.26 | 1 | 180 | 2020 |
| BiVO4/Au@SiO2/CoAl-LDH | Hydrothermal | Nanosheet | 0.1 M PBS (pH = 7) | 300 W xenon lamp | 1.23 V vs. RHE | 1.92 | 2 | 181 | 2018 |
| OH-BiVO4@C@NiFe-LDH | Electrodeposition | — | 0.5 M KBi (pH = 9.3) | AM 1.5 (100 mW cm−2) | 1.23 V vs. RHE | 5.31 | 20 | 182 | 2024 |
| BiVO4/AgAuNCs/CoNi-LDH | Electrodeposition | Nanosheets | 0.5 M Na2SO4 (pH = 7) | AM 1.5 (100 mW cm−2) | 1.23 V vs. RHE | 3.75 | 2.77 | 183 | 2024 |
| BiVO4/LDH Composites | |||||||||
| BiVO4/CoAl-LDH/Graphene | Hydrothermal | Nanosheet | Kpi (pH = 7) | 300 W xenon arc lamp | 1.23 V vs. RHE | 2.13 | — | 75 | 2017 |
| BiVO4/NiFe-LDH/CQD | Electrodeposition | Nanosheets | 0.5 M PBS (pH = 7) | 300 W xenon arc lamp | 1.23 V vs. RHE | 2.84 | 3 | 184 | 2018 |
| BiVO4/CoAl/CdTeQD | Hydrothermal | Nanosheet | 0.1 M PBS (pH = 7) | 300 W xenon lamp, AM 1.5 solar light (100 mW cm−2) | 1.23 V vs. RHE | 2.23 | 4 | 185 | 2016 |
| BiVO4@NiFe-LDHs/Ru | Electrodeposition | Nanosheet | 0.5 M KBi, 0.2 M Na2SO3 | 300 W xenon lamp | 1.23 V vs. RHE | 4.65 | 5 | 186 | 2024 |
| BiVO4/CoFe-Ac LDH/SAsPt | Hydrothermal | — | 1 M KBi | AM 1.5 xenon lamp (100 mW cm−2) | 1.23 V vs. RHE | 5.14 | 20 | 187 | 2023 |
| BiVO4/H-CoAl-LDH | Hydrothermal, plasma etching | Nanosheet | 0.5 M Na2SO4 | AM 1.5 solar light | 1.23 V vs. RHE | 3.5 | 3 | 151 | 2021 |
| Mo: BiVO4/u-CoAl-LDH | Coprecipitation, dipping coating | Nanosheet | KBi (pH = 9.5) | AM 1.5 xenon lamp (100 mW cm−2) | 1.23 V vs. RHE | 5.8 | 1.38 | 132 | 2023 |
| BiVO4/OvacNiFe-LDH | Hydrothermal | — | 0.5 M K3BO3 (pH= 9.5) | AM 1.5 xenon lamp (100 mW cm−2) | 1.23 V vs. RHE | 5.81 | 80 | 146 | 2023 |
| BiVO4/HD-NiCo-LDH | Drop casting | Nanosheet | 0.5 M Na2SO4 (pH = 7) | xenon lamp, AM 1.5 (100 mW cm−2) | 1.23 V vs. RHE | 4.54 | — | 188 | 2024 |
4.1. BiVO4/bimetallic LDHs
LDHs are multi-metal anionic clays having metal cations embedded into the octahedral sites of their layers.113,114,189 LDHs containing transition metals such as Co, Fe, Mn, and Ni have recently been recognized as efficient PEC water oxidation catalysts.190 Incorporating other transition metal elements into monometallic hydroxides such as Ni(OH)2 and Co(OH)2 is a versatile approach to enhance their intrinsic electrocatalytic activity. This bimetallic incorporation increases the number of active sites and modifies the electronic structure of the original metal hydroxides.191–194 Due to their efficient oxygen evolution activity, loading bimetallic LDHs onto BiVO4 is an effective approach to increase its PEC water splitting performance. For instance, Wang et al. studied Ni-based bimetallic NiFe-LDH on BiVO4 using a one-step hydrothermal process.152 HRTEM images of the NiFe-LDH/BiVO4 photoanode (Fig. 10a) revealed interplanar distances of approximately 0.363 and 0.318 nm, corresponding to the (511) and (421) planes, respectively, of the NiFe-LDH nanoparticles. The results demonstrated that NiFe-LDH nanoparticles enhance the visible light absorption through reflection and scattering, leading to a substantial enhancement in the PEC characteristics of BiVO4. Consequently, the BiVO4/NiFe-LDH photoanode achieved the highest photocurrent density of 2.49 mA cm−2 at 0.6 V vs. Ag/AgCl, indicating an increase of 217% and a factor of 2.5 compared to bare BiVO4. Furthermore, it exceeds by 3.07 and 1.25 times, of BiVO4/Ni(OH)2 (0.81 mA cm−2) and BiVO4/Fe(OH)2 (1.98 mA cm−2) photoanodes (Fig. 10b) and demonstrated superior onset potentials and stability.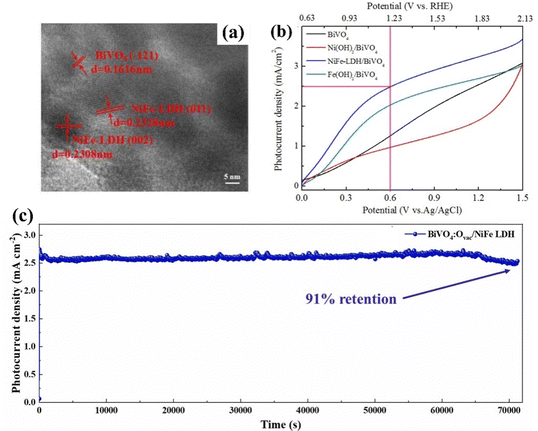 | ||
| Fig. 10 (a) HRTEM of the BiVO4/NiFe- LDH photoanode, (b) current–voltage curves of BiVO4, BiVO4/Ni(OH)2, BiVO4/Fe(OH)2, and BiVO4-LDH/BiVO4 photoanodes under visible light illumination. Reproduced with permission from ref. 152, Copyright 2020, Elsevier. (c) Stability test for the Ovac:BiVO4/NiFe-LDH photoanode. Reproduced with permission from ref. 136, Copyright 2024, Elsevier. | ||
Similarly, Huang and his coworkers fabricated a BiVO4/NiFe-LDH photoanode, incorporating NiFe-LDH nanosheet arrays as the OEC to facilitate charge separation and enhance surface reaction kinetics.133 This design resulted in a photocurrent density of 4.02 mA cm−2 at 1.23 V vs. RHE, representing a 2.8-fold increase compared to that of pristine BiVO4. The photoanode demonstrated an impressive, applied bias photon-to-current efficiency (ABPE) of 1.07% at 0.75 V vs. RHE and showcased a notable O2 evolution rate of 29.6 μmol h−1 cm−2, maintaining high activity for more than 30 h in a weak KHCO3 alkaline electrolyte.
Mane et al. incorporated surficial oxygen vacancies onto BiVO4 and developed the Ovac:BiVO4/NiFe-LDH photoanode.136 The introduction of oxygen vacancies effectively adapts band energetics, improves light absorption, and elevates carrier density. The 2D-NiFe-LDH nanosheets act as a proficient hole transport layer at the electrode/electrolyte interface and provide photocorrosion protection. The Ovac:BiVO4/NiFe-LDH photoanode exhibited a photocurrent density of 2.92 mA cm−2, reflecting a 3-fold improvement over the pristine BiVO4. The extensive stability testing demonstrated that the combined impact of these efficacious strategies lead to a 91% retention of the photocurrent density of the Ovac: BiVO4/NiFe-LDH photoanode following 20 h of irradiation, as depicted in Fig. 10c.
Guo et al. prepared a Mo:BiVO4/NiFe-LDH photoanode for PEC water splitting.131 The incorporation of Mo into the BiVO4 lattice enhances its visible light capabilities and elevates carrier concentration. In this design, the Ni component effectively captures photo-generated holes to facilitate the oxidation of low-valence Ni2+ into high-valence Ni3+ and Ni4+ within the potential range of 0.6 to 1.6 V vs. RHE (Fig. 11a). The BiV0.97Mo0.03O4/NiFe-LDH photoanode exhibited a 64%incident photon-to-current efficiency (Fig. 11b), surpassing the 10% IPCE of undoped BiVO4, along with a significant shift in the onset potential and enhanced stability. The photocurrent density BiV0.97Mo0.03O4/NiFe-LDH photoanode showed 1.58 mA cm−2 at 1.23 V vs. RHE, which is 1.25 times and 3.16 times higher than that of BiV0.97Mo0.03O4 and pure BiVO4.
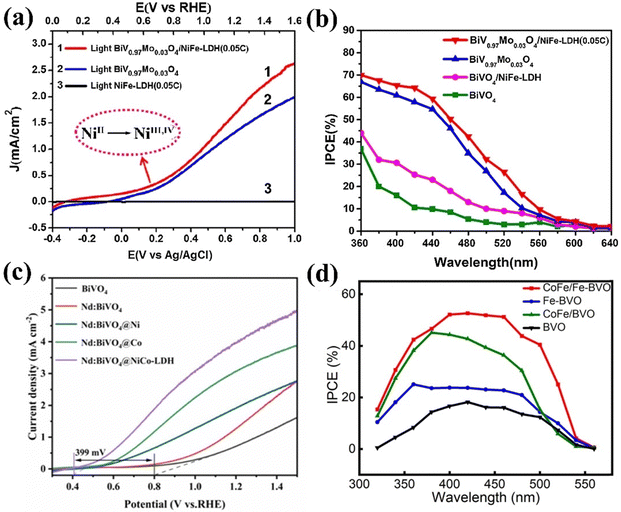 | ||
| Fig. 11 (a) Photocurrent density of the BiV0.97Mo0.03O4/NiFe-LDH [0.05C(Coulomb)] photoanode, (b) IPCE of BiVO4, BiV0.97Mo0.03O4 and BiV0.97Mo0.03O4/NiFe-LDH (0.05C) photoanodes. Reproduced with permission from ref. 131, Copyright 2019, Elsevier. (c) Linear sweep voltammetry (LSV) curves for the Nd:BiVO4@NiCo-LDH photoanode. Reproduced with permission from ref. 153, Copyright 2024, Elsevier. (d) Photoconversion efficiency of monochromatic incident light based on wavelength. Reproduced with permission from ref. 156, Copyright 2024, American Chemical Society. | ||
Moreover, She et al. constructed a BiVO4/NiCo-LDH using electrodeposition.73 This heterojunction structure significantly improved light absorption and electron–hole separation, resulting in a photocurrent of 3.4 mA cm−2 at 1.23 V vs. RHE, surpassing that of the bare BiVO4 (1.1 mA cm−2).
Wang et al. developed a photoanode consisting of Nd:BiVO4 and NiCo-LDH cocatalyst.153 The inclusion of Nd greatly increases the carrier density and lowers the charge transfer resistance, which improves the BiVO4 photoanode's electrical conductivity. The NiCo-LDH catalyst works as a bifunctional OER co-catalyst, with Co improving hole extraction and Ni increasing active sites, thus enhancing PEC water oxidation activity. Consequently, the Nd: BiVO4/NiCo-LDH photoanode showed a photocurrent density of 4.1 mA cm−2 at 1.23 V vs. RHE (Fig. 11c), indicating an enhancement of approximately 5.1 times relative to the unmodified BiVO4, with a negative shift in onset potential of 399 mV.
Zhang and colleagues designed a pyramidal-shaped BiVO4/NiMn-LDH composite photoanode using a one-step hydrothermal method.154 The compatibility of the energy level between BiVO4 and NiMn-LDH facilitates hole transport from BiVO4's valence band to NiMn-LDH. Therefore, NiMn-LDH captured h+ through M2+/M3+ to generate M3+/M4+ (M = Ni, Mn). By promoting hole collection and injections, this process reduces charge carrier recombination in the BiVO4/NiMn-LDH composite photoanode relative to bare BiVO4, leading to enhanced OER efficiency. Consequently, the BiVO4/NiMn-LDH photoanode showed an optimal photocurrent of 0.83 mA cm−2 at 1.23 V vs. RHE, which is two times higher than that of bare BiVO4. Moreover, the onset potential of the BiVO4/NiMn-LDH photoanode showed a cathodic shift around 480 mV.
Wei et al. investigated the role of CoFe-LDH as an oxygen cocatalyst for PEC-water splitting on BiVO4.155 They reported two types of LDHs: crystalline CoFe-LDH (C), which was deposited on BiVO4 using a low-temperature chemical bath method, and amorphous CoFe-LDH (E) coatings that were achieved through electrodeposition. The amorphous CoFe-LDH (E) layer primarily acted as a “shunt channel,” instead of participating in the water oxidation reaction. In contrast, the crystalline CoFe-LDH effectively disrupted the “shunt channel” between the cocatalyst and the FTO substrate, thereby inhibiting the recombination of photogenerated charges. Moreover, the interface between the crystalline CoFe-LDH (C) and BiVO4 exhibited fewer surface trap states than the amorphous layer. The BiVO4/CoFe-LDH (C) photoanodes achieved a photocurrent of 4.3 mA cm−2 at 1.23 V vs. RHE, with an ABPE% of 1.2% at 1.01 V, which is 1.5 times higher than that of the amorphous BiVO4/CoFe-LDH (E) and four times higher than that of bare BiVO4.
He et al. synthesized a BiVO4/CoAl-LDH photoanode using a hydrothermal method for water oxidation.128 The investigation found that the CoAl-LDH nanosheet formed interconnected nanowalls with a thickness of 20 nm, resulting in more active sites and enhanced carrier-transporting capabilities, allowing holes to diffuse to the photoanode's surface. Consequently, the BiVO4/CoAl-LDH photoanode exhibited a 540 mV cathodic shift in onset potential and a photocurrent density of 1.0 mA cm−2 at 1.23 V.
Recently, Fe-BiVO4/CoFe-LDH was designed by Chen et al. for PEC water splitting.156 In this study, CoFe-LDH promotes carrier utilization and suppresses electron–hole recombination, whereas iron doping facilitates charge separation and transfer. The CoFe/Fe-BiVO4 photoanode displayed a photocurrent of 2.56 mA cm−2 at 1.23 V vs. RHE and an IPCE of 52.1% at 400 nm, representing a 270% increase in photocurrent and a 2.2-fold enhancement in IPCE over bare BiVO4 (Fig. 11d). Furthermore, the surface charge transport efficiency increased from 16.8% to 62.5% at 1.23 V vs. RHE.
4.2. BiVO4/trimetallic LDH
Due to the high tunability in LDH composition, various active centers can be readily integrated into their structure, enabling precise regulation of active species and the electronic configuration of LDH.195–197 Consequently, many bimetallic LDHs have exhibited considerable catalytic activity towards the OER. Nevertheless, many of the bimetallic LDHs still suffer from low electrical conductivity.198–202 This limitation can be addressed through third-metal doping, which regulates the electronic configurations of pristine LDHs and enhances the synergistic interactions between doped and host metals, resulting in trimetallic LDHs with superior electrocatalytic performance.203 Despite the difficulty in selection of elemental combination mainly based on the ionic radii, recent research has revealed that LDHs comprising three different transition metals demonstrate higher charge-carrier transportation and improved electronic conductivity. For example, Vo and coworkers designed a novel ZnCoV-LDH and decorated it on a BiVO4 photoanode using electrodeposition.158 Fig. 12(a–d) demonstrate that the introduction of V5+ ions caused the binding energy of Co 2p to shift by 1.2 eV to a higher level, indicating that Co is in an electron-deficient state. Consequently, the Co3+/Co2+ ratio is higher in ZnCoV-LDH compared to ZnCo-LDH. Additionally, it was indicated that the partial electron transfer from Co to V is facilitated by oxygen bridges. Furthermore, the binding energy of the surface hydroxyl (OH−) groups shifts to the left, suggesting a stronger adsorption affinity of OH− to the metal sites. These observations suggest that ZnCoV-LDH facilitates the rapid movement of photogenerated holes from BiVO4 to the ZnCoV-LDH layer on the outside. As a result, the ZnCoV-LDH/BiVO4 photoanode demonstrated a 370 mV cathodic shift in onset potential and achieved a photocurrent density of 2.7 mA cm−2 at 1.23 V vs. RHE, representing a fourfold increase compared to unmodified BiVO4.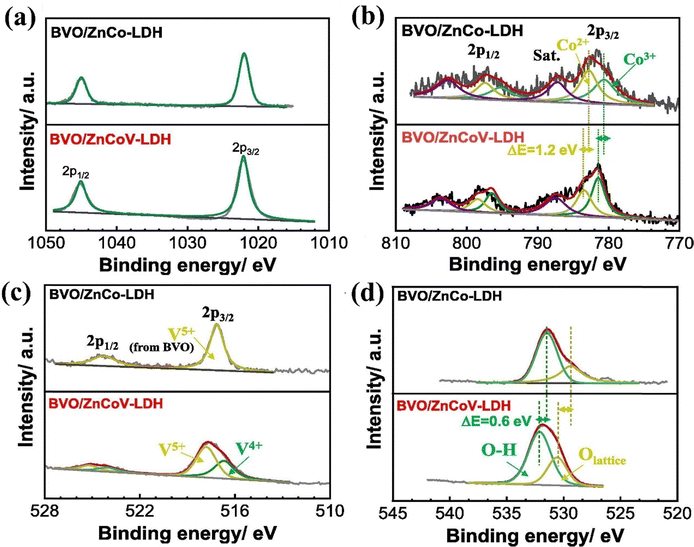 | ||
| Fig. 12 High resolution XPS analysis of (a) Zn 2p, (b) Co 2p, (c) V 2p, and (d) O 1s regions of ZnCo-LDH and ZnCoV-LDH on BiVO4/ZnCoV-LDH. Reproduced with permission from ref. 158, Copyright 2020, Elsevier. | ||
The same authors fabricated BiVO4/CoMnZn LDH by the electrodeposition method as well.141 The modification of CMZ-LDH on BiVO4 is confirmed by XRD through the observation of a weak (003) reflection. Six Raman peaks were observed at approximately 127, 212, 329, 368, 709, and 827 cm−1, corresponding to the distinctive vibrational modes of scheelite monoclinic BiVO4. Additional peaks observed at around 475 and 520 cm−1 were ascribed to the bending mode of O–M–O (where M denotes Co, Mn, and Zn), as illustrated in Fig. 13(a and b). In this study, CMZ-LDH acts as a cocatalyst, lowering the onset potential, enhancing the photocurrent, and serving as a protective layer for BiVO4. Consequently, the composite photoanode exhibited a photocurrent density of 1.06 mA cm−2, which is 1.7 times higher than that of the bare BiVO4. Moreover, the deposition of CMZ-LDH on the BiVO4 photoanode induced a significant cathodic shift of 280 mV in the onset potential for PEC water oxidation, suggesting effective band bending. This is evident from the change in the open circuit photovoltage (OCP). As shown in Fig. 13c, the BVO4/CMZ-LDH photoanode exhibits a higher OCP in comparison to the bare BiVO4 photoanode, indicating better charge separation due to larger band bending and hence improved PEC performance.
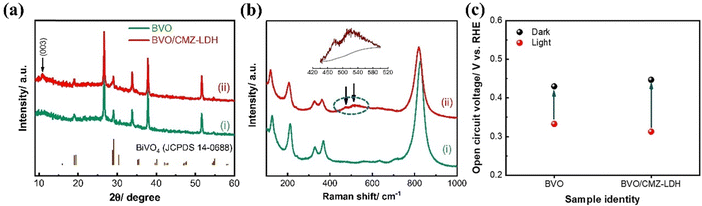 | ||
| Fig. 13 (a) XRD patterns, (b) Raman spectra, and (c) open circuit configurations of the BiVO4/CMZ-LDH photoanode. Reproduced with permission from ref. 141, Copyright 2019, Elsevier. | ||
Meng et al. prepared BiVO4/NiFeV LDH via a hydrothermal method, where NiFeV LDH served as both a water oxidation electrocatalyst and a hole reservoir, thereby reducing surface charge recombination and improving interfacial charge transfer.135 Consequently, the NiFeV/B-BiVO4 photoanode showcased an outstanding photostability, a minimal onset potential of around 0.2 V vs. RHE, and a photocurrent density of 4.6 mA cm−2 at 1.23 V vs. RHE.
He et al. designed BiVO4/NiFeY LDH using a hydrothermal method.147 The XPS and density functional theory simulation demonstrated that the incorporation of Y into Ni sites promotes efficient charge transfer between Ni and Fe by tuning the local electronic environment of the NiFe LDH, as depicted in Fig. 14(a and b). This significantly increases the transfer rate of electrons and holes at the interface of the photocatalyst. Hence, NiFeY LDH reduces interface charge recombination between BiVO4 and the cocatalyst, preventing the photocorrosion of BiVO4. The optimized BiVO4/NiFeYLDH, as depicted in Fig. 14c, exhibited an onset potential of 0.31 VRHE and a photocurrent density of 5.2 mA cm−2 at 1.23 VRHE, surpassing the performance of BiVO4/NiFeLDH (3.2 mA cm−2).
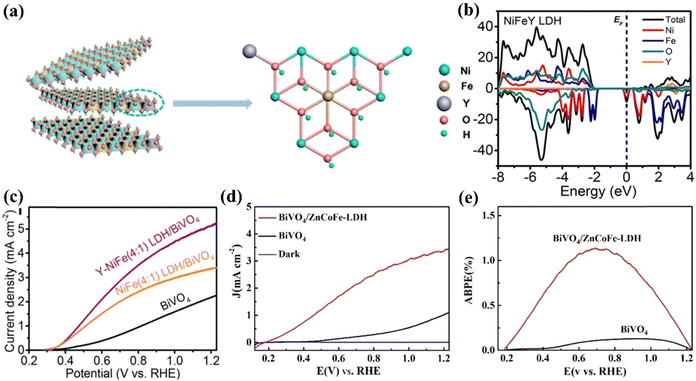 | ||
| Fig. 14 (a) Structure model and (b) DFT-based DOS spectra of NiFeY LDH and (c) LSV curves of the BiVO4/NiFeY photoelectrode under illumination. Reproduced with permission from ref. 147, Copyright 2020, American Chemical Society. (d) J–V curves of the BiVO4/ZnCoFe-LDH photoanode (e) ABPE curves of the bare BiVO4 and BiVO4/ZnCoFe-LDH photoanodes. Reproduced with permission from ref. 159, Copyright 2021, Wiley-VCH GmbH. | ||
Wen et al. constructed a BiVO4/ZnCoFe-LDH photoanode by conformally decorating a ZnCoFe-LDH on a porous BiVO4 photoanode using an electrodeposition method.159 The p–n heterojunction formed between ZnCoFe-LDH and BiVO4 enhances the transfer and separation of photogenerated charges, extends light absorption capabilities, and facilitates the kinetics of surface water oxidation. Consequently, the BiVO4/ZnCoFe-LDH photoanode exhibited a photocurrent density of 3.43 mA cm−2 at 1.23 V vs. RHE, representing a threefold increase compared to the bare BiVO4 photoanode. (Fig. 14d). It also demonstrated a notable negative shift in the onset potential from 0.51 V to 0.21 V vs. RHE (Fig. 14e), suggesting improved cocatalytic performance and a higher rate of surface OER.
Recently Huang et al. developed a ternary NiCoV-LDH nanosheet on a BiVO4 photoanode using a low-bias voltage electrodeposition process.160 The SEM images revealed that LDH deposition at −0.1 V resulted in unique crystalline and amorphous structures, providing more active sites. The XRD peaks of BiVO4 correspond to monoclinic bismuth vanadate (JCPDS No. 14–0688), and the peaks at 13.0° and 23.9° conform to the lattice planes of NiCoV-LDH, as depicted in Fig. 15(a–c). In this study, the introduction of vanadium (V5+) elements created strong electronic interactions with Ni and Co, facilitating the transformation of Co2+ into Co3+ with enhanced catalytic activity. Simultaneously, V5+ was reduced to V3+, increasing the sample's conductivity (Fig. 15d). Thus, BiVO4/NiCoV-LDH nanosheets effectively facilitated carrier transfer at the semiconductor/electrolyte interface. Consequently, the BiVO4/NiCoV-LDH photoanode demonstrated a significant increase in photocurrent density, reaching 3.32 mA cm−2 at 1.23 V vs. RHE, a 2.84-fold increase compared to pristine BiVO4. It was also 1.59 and 1.25 times greater than those of the BiVO4/NiCo-LDH photoanodes prepared at −0.1 V and −0.7 V without the introduction of V elements, respectively. Additionally, the surface efficiency of BiVO4/NiCoV-LDH reached 71%, representing a 2.45-fold improvement over the bare BiVO4.
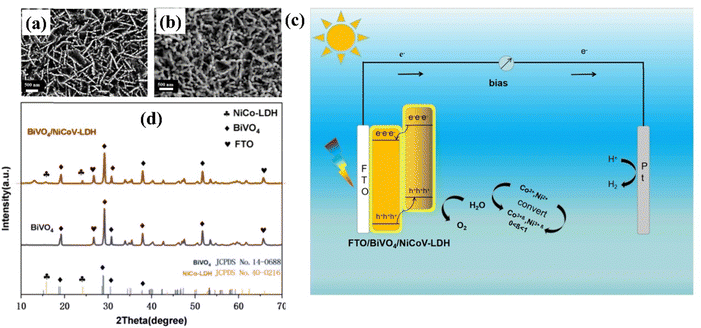 | ||
| Fig. 15 (a) and (b) SEM images and (d) XRD patterns of BiVO4/NiCoV-LDH. (c) Water splitting mechanism of the BiVO4/NiCoV-LDH photoelectrode. Reproduced with permission from ref. 160, Copyright 2024, American Chemical Society. | ||
4.3. BiVO4 composites/LDHs
The N-type BiVO4 semiconductor is regarded as a potential photoanode material for water splitting because of its appropriate band alignment and high theoretical conversion efficiency of up to 9.1%. However, it suffers from severe charge carrier recombination due to its low carrier mobility (0.044 cm V−1 s−1) and insufficient water oxidation kinetics.204,205 Several approaches have been employed to improve its PEC properties, including integrating BiVO4 with different semiconductor metal oxides or sulfides, or decorating BiVO4 with rGO, HTL, and QD to create p–n or n–n heterojunction composites.206–210 Nevertheless, the impact of semiconductor heterojunctions is restricted by the saturation of the built-in electric field, which results from charge accumulation during the water oxidation process.211 Therefore, an efficient and cost-effective cocatalyst is required to expedite the water oxidation reaction, since the OER at the anode remains a significant hurdle for efficient solar PEC water splitting. LDHs have emerged as a promising class of OECs because of their affordability and compatibility with semiconductor materials. LDHs containing elements such as Ni, Mn, Co, and Fe have been proven to be proficient electrochemical catalysts.119,120,121b,c,d In the past few years, researchers have devoted significant efforts to the development of BiVO4 heterojunction composites/LDHs to improve the overall PEC water splitting performance of the BiVO4 photoanode.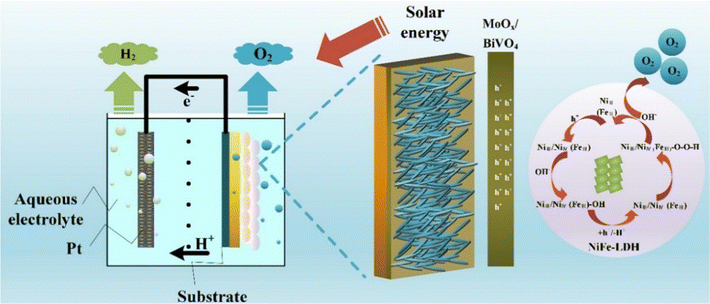 | ||
| Fig. 16 Water splitting mechanism of the optimized BiVO4/MoOx/NiFe photoelectrode. Reproduced with permission from ref. 169, Copyright 2022, Elsevier. | ||
Li et al. constructed a BiVO4/MoO3/CoMn-LDH photoanode using electrodeposition and calcination.170 The MoO3/BiVO4 heterojunction facilitates efficient carrier transfer and inhibits charge recombination, while CoMn-LDH boosts OER kinetics and visible light absorption. Their combined effect greatly increases charge separation and light harvesting in the BiVO4/MoO3/CoMn-LDH photoanode, reached a photocurrent density of 3.78 mA cm−2 at 1.23 V vs. RHE. This value is 1.26 and 3.78 times greater than those of BiVO4/MoO3 and pure BiVO4, respectively.
LDHs and p-type semiconductor metal oxides were deposited on an n-type BiVO4 photoanode surface, resulting in enhanced surface catalytic efficiency and improved separation of charge carriers. For example, Yin et al. created a BiVO4/CuFeO2/NiFe-LDH photoanode by incorporating CuFeO2 and NiFe-LDH onto BiVO4 via electrodeposition.171 The ultra-thin p-type CuFeO2 layer was introduced at the interface of BiVO4 to create a p–n heterojunction that promotes charge transfer, while NiFe-LDH served as an OEC to extract holes and convert OH− from the electrolyte into O2, providing active sites for water oxidation. Hence, the efficiency of the PEC water splitting of the BiVO4 photoanode was improved by the synergistic effects of CuFeO2 and NiFe-LDH, which effectively prevented charge recombination, as shown in Fig. 17. The BiVO4/CuFeO2/NiFe-LDH photoanode demonstrated a photocurrent density of 4.34 mA cm−2 at 1.23 V vs. RHE, which is 3.78-fold superior to that of bare BiVO4. Additionally, ABPE was 2.48%, representing an increase of 5.28 times compared to that of pristine BiVO4.
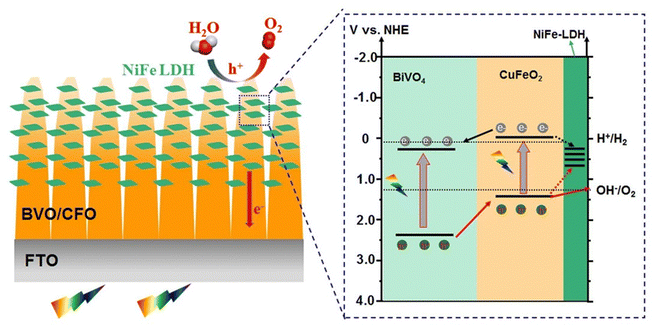 | ||
| Fig. 17 The energy diagram of the BiVO4/CFO/NiFe-LDH photoanode on PEC water oxidation. Reprinted with permission from ref. 171, Copyright 2022, Elsevier. | ||
Zhang et al. prepared BiVO4/CuCoO2/NiCo3-LDH for PEC water splitting, where NiCo3-LDH was incorporated into BiVO4/CuCoO2 via electrodeposition to act as an OEC.172 Consequently, the photocurrent density of BiVO4/CuCoO2/NiCo3-LDH reached 6.95 mA cm−2 at 1.8 V vs. RHE, representing an increase of around 24.8 times compared to BiVO4 and 5.5 times greater than that of BiVO4/CuCoO2. Additionally, it demonstrated good stability, maintaining 70% of its performance after 2 h of operation.
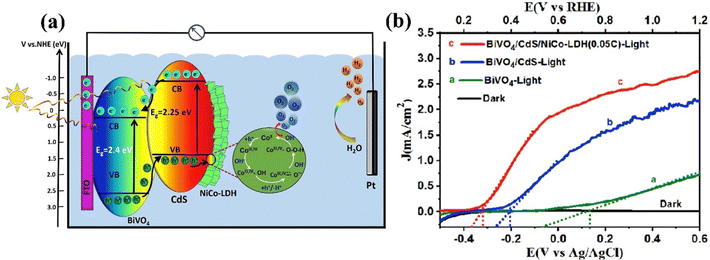 | ||
| Fig. 18 (a) Schematic diagram of PEC water splitting for BiVO4/CdS/NiCo-LDH. (b) Onset potentials of BiVO4/MoOx/NiFe photoanode. Reprinted with permission from ref. 167, Copyright 2022, Elsevier. | ||
Recently, Dong et al. fabricated a triadic BiVO4/CdS/NiFe-LDH photoanode, by sequentially depositing CdS nanoparticles and NiFe-LDH nanosheets onto BiVO4.168 The n–n heterojunction formed between CdS and BiVO4 greatly enhances charge separation and transfer by reducing recombination of charge carrier, while NiFe-LDH facilities faster transfer of holes from the CdS/BiVO4 junction. This combined effect markedly improves the PEC properties. The BiVO4/CdS/NiFe-LDH photoanode exhibited a photocurrent density of 3.1 mA cm−2 at 1.23 V vs. RHE, which is 5.8 times superior to that of the bare BiVO4 photoanode and 4.9 and 4.3 times higher than those of BiVO4/CdS and BiVO4/NiFe-LDH photoanodes, respectively. Additionally, it exhibited a cathodic shift of 540 mV and maintains a stable photocurrent density over 3 h of irradiation.
 | ||
| Fig. 19 (a) Schematic mechanism of PEC water splitting on Fe2O3/BiVO4/NiFe-LDH. Reproduced with permission from ref. 162, Copyright 2018, Elsevier. (b) TOF-SIMS profile of WO3/BiVO4/NiFe-LDH, (c) Stability test of the WO3/BiVO4/NiFe-LDH photoanode. Reprinted with permission from ref. 163, Copyright 2023, Elsevier. | ||
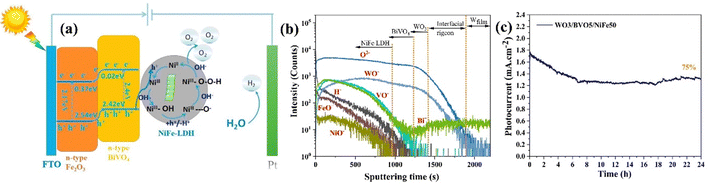
Kolaei et al. developed a heterojunction photoanode consisting of WO3/BiVO4/NiFe-LDH.163 The time-of-flight secondary ion mass spectrometry (TOF-SIMS) profile (Fig. 19b) validated the structure of the WO3/BiVO4/NiFe photoelectrode, where NiFe-LDH acts as an efficient co-catalyst. It efficiently extracts photogenerated holes from the WO3/BiVO4 photoanode surface, enabling their participation in the OER at the interface of electrode–electrolyte. Consequently, the WO3/BiVO4/NiFe photoanode was able to sustain approximately 75% of its original photocurrent over 24 h (Fig. 19c), reaching a peak photocurrent density of around 1.78 mA cm−2 at 1.23 V vs. RHE.
Singh et al. constructed a WO3/BiVO4-NiFeCr photoanode.165 The study revealed that the utilization of a sputtered WO3 underlayer in the type-II WO3/BiVO4 configuration resulted in enhanced electron–hole separation. The inclusion of Cr to NiFe-LDH enhanced its electrical conductivity. Thus, electrodeposited trimetallic NiFeCr-LDH on the WO3/BiVO4 heterojunction enhances the kinetics of water oxidation, resulting in consistent PEC water splitting performance in neutral electrolyte solution. Consequently, the WO3/BiVO4-NiFeCr photoanode achieved a photocurrent density of 4.9 mA cm−2 at 1.23 V vs. RHE, representing a 1.6-fold enhancement relative to WO3/BiVO4 (2.9 mA cm−2) and a 1.2-fold increase compared to WO3/BiVO4-NiFe (3.8 mA cm−2).
Zhou et al. developed a photoanode consisting of TiO2/BiVO4/NiFe-LDH.164 The NiFe-LDH co-catalyst deposited on TiO2/BiVO4 effectively removed accumulated photogenerated holes from the surface of the photoanode. Consequently, the TiO2/BiVO4/NiFe-LDH photoanode exhibited a notable enhancement in photocurrent, achieving 17.6 μA cm−2, exceeding those of the BiVO4 and TiO2/BiVO4 photoanodes by factors of 3.6 and 2.5, respectively.
Bai and colleagues synthesized a new triadic photoanode consisting of MoO3/BiVO4/CoMnZn-LDH by combining metal–organic decomposition and electrodeposition techniques.166 In this design, the MoO3/BiVO4 heterojunction effectively suppressed electron–hole recombination, whereas CoMnZn-LDH facilitated efficient hole transfer from BiVO4 to the electrolyte, reducing photocorrosion and enhancing PEC water splitting efficiency. Consequently, the MoO3/BiVO4/CoMnZn-LDH photoanode demonstrated a photocurrent density of 1.24 mA cm−2 at 1.23 V vs. RHE, surpassing that of the pure BiVO4 photoanode by a factor of 2.21 and 1.51-fold enhancement compared to that of MoO3/BiVO4 photoanode.
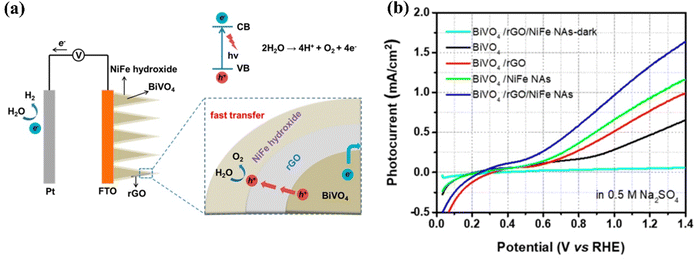 | ||
| Fig. 20 (a) Schematic representation of PEC water oxidation on BiVO4/rGO/NiFe (NAas) nanoarray photoanode. (b) Polarization curves of PEC response on BiVO4/rGO/NiFe-LDH photoanodes. Reprinted with permission from ref.177, Copyright 2018, American Chemical Society. | ||
Sun et al. developed a BiVO4/rGO/NiFe-LDH photoanode by sequentially loading rGO nanosheets onto BiVO4 and then depositing NiFe-LDH at the BiVO4/rGO interface using simple electrodeposition techniques.178 The rGO nanosheets improve charge separation and transfer at the interface of BiVO4, while NiFe-LDH acts as a cocatalyst to enhance the kinetics of water oxidation. Consequently, the photoanode achieved a photocurrent density of 1.13 mA cm−2 at 1.23 V vs. RHE, surpassing that of BiVO4/rGO and pure BiVO4 by factors of 1.45 and 2.17, respectively. Moreover, it showed a 124 mV cathodic shift in the onset potential when exposed to visible light.
Chen et al. developed a BiVO4/rGO/NiFe-LDH photoanode, where NiFe-LDH serves as a catalyst for water oxidation, facilitating the transport of photo-generated holes from the BiVO4 photoelectrode to the electrolyte.180 Meanwhile, rGO nanosheets decrease electron–hole recombination by acting as an effective mediator of electron shuttling. As a result, the photocurrent density of the BiVO4/rGO/NiFe-LDH photoanode increases to 3.26 mA cm−2 at 1.23 V vs. RHE, representing an increase of 2.85 times compared to bare BiVO4 (1.14 mA cm−2).
Liu et al. developed BiVO4/FeOOH/ZnFe-LDH photoelectrodes to improve the PEC performance of BiVO4.174 In this design, FeOOH acts as a hole-extraction bridge between BiVO4 and the ZnFe-LDH interface. The combined effects of FeOOH and ZnFe-LDH lowered the overpotential for water oxidation, enhancing the photogenerated carrier transfer and improving the kinetics of oxidation reaction. Consequently, the BVO/FeOOH/ZnFe-LDH photoelectrodes achieved a photocurrent density of 4.92 mA cm−2 at 1.23 V vs. RHE, indicating a 3.25-fold enhancement relative to bare BiVO4 and a 1.4-fold increase compared to the BiVO4/FeOOH electrode.
Mohanta et al. used a thin layer of two-dimensional boron nitride nanoplatelets (BNNPs) as an ultra-rapid hole extractor and developed a BiVO4/BNNPs/CoCr-LDH photoanode for PEC water splitting,175 where CoCr-LDH enhances reaction kinetics (Fig. 21a). Therefore, the BiVO4/BNNPs/CoCr-LDH photoanode showed a cathodic shift of about 360 mV and a photocurrent density of 3.8 mA cm−2 at 1.23 V vs. RHE (Fig. 21b), 3.2-fold greater than that of bare BiVO4.
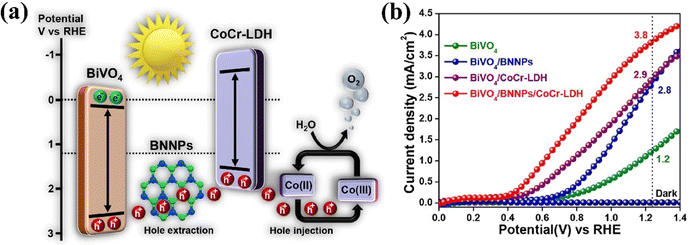 | ||
| Fig. 21 (a) PEC mechanism of the BiVO4/BNNPs/CoCr-LDHs photoanode (b) LSV of the BiVO4/BNNPs/CoCr-LDH photoanode under 1 sun illumination. Reproduced with permission from ref. 175, Copyright 2022, Elsevier. | ||
Alam et al. employed graphene quantum dots (GQDs) as conductive linkers to extract holes from the BiVO4 surface and developed a BiVO4/GQDs/CoSn-LDH photoanode, where CoSn-LDH provides surface-active centers.179 As a result, the photocurrent density of the BiVO4/GQDs/CoSn-LDH photoanode attains 4.15 mA cm−2, which is threefold greater than that of bare BiVO4, due to the synergistic impact of GQDs and CoSn-LDH in facilitating hole extraction and transport to the surface reaction site. Furthermore, the photoanode exhibited improved stability, maintaining 90% of its initial performance following 4 h of continuous exposure to light.
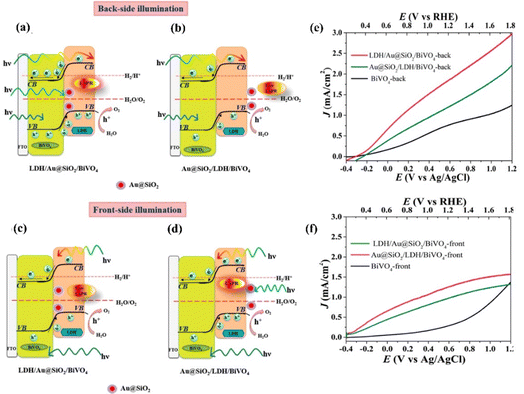 | ||
| Fig. 22 Energy levels and photogenerated charge-transfer process in composite photoanodes of BiVO4/SiO2@Au/LDH(Left) and BiVO4/LDH/SiO2@Au (Right) under irradiation from (a) and (b) back-side and (c) and (d) front-side. The respective LSV curves for (e) back-side and (f) front-side illumination. Reprinted with permission from ref. 181, Copyright 2018, American Chemical Society. | ||
Recently, Bai et al. designed an OH-BiVO4@C/NiFe-LDH composite photoanode for PEC water splitting.182 The study found that the addition of a carbon layer improved electronic conductivity and significantly polarized the electronic field due to hydroxylation, facilitating both interfacial charge transfer and bulk charge separation. Consequently, the optimized photoanode achieved an impressive photocurrent density of 5.31 mA cm−2 at 1.23 V vs. RHE. Furthermore, the stability of the OH-BiVO4@C@NiFe-LDH photoanode was significantly enhanced by the protective carbon layer and the improved redeposition of Fe active sites in NiFe-LDH catalysts, which was induced by increased adsorption of Fe(III) onto Ni sites via hydroxylation. After 20 h of irradiation, the material maintained over 87.5% of its initial photocurrent density, owing to a self-healing mechanism (Fig. 23).
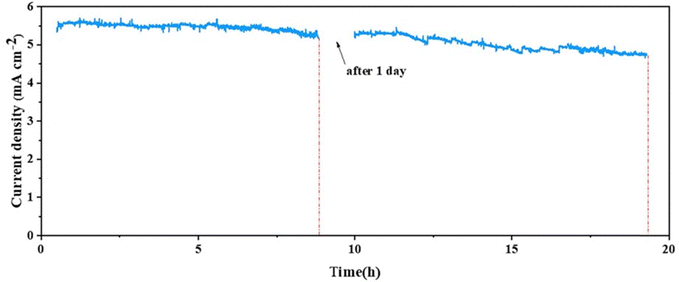 | ||
| Fig. 23 Long-term stability test for the OH-BiVO4@C@NiFe-LDH photoanode [KBi + 100 μM Fe(III)], ref.182, Copyright 2024, Elsevier. | ||
4.4. BiVO4/LDH composites
Exfoliating the lamellar structure of LDHs or coupling them with carbon-based materials, QDs, and single atom catalysts is critical for the OER due to the ability of LDH composites to generate numerous catalytic active sites, enhance electrical conductivity, and facilitate the movement of electrolyte ions. Therefore, constructing LDH composites with BiVO4 is an exceptionally efficient technique for enhancing the PEC performance of bare BiVO4. For example, graphene is known as a 2D layered carbon material, exhibiting exceptional electrical conductivity and metal-like properties. Utilizing the advantages of graphene, Zhang et al. developed the BiVO4@CoAl-LDH@graphen triadic photoanode through a one-step hydrothermal method.75 In this design, CoAl-LDH expedites the kinetics of water oxidation, whereas graphene promotes the transfer of photo-generated charges, thus extending the distance of carrier transfer within the charge transfer channels, efficiently inhibiting carrier recombination, and enhancing water oxidation efficiency, as depicted in Fig. 24a. Consequently, BiVO4@CoAl-LDH@graphen photoanode demonstrated a photocurrent density of 2.13 mA cm−2 at 1.23 V vs. RHE, which is fourfold greater than that of pure BiVO4. Moreover, the oxidation efficiency remained impressive at 80% under low voltage (Fig. 24b). Additionally, IPCE of BiVO4@CoAl-LDH@graphen was 52% at 400 nm.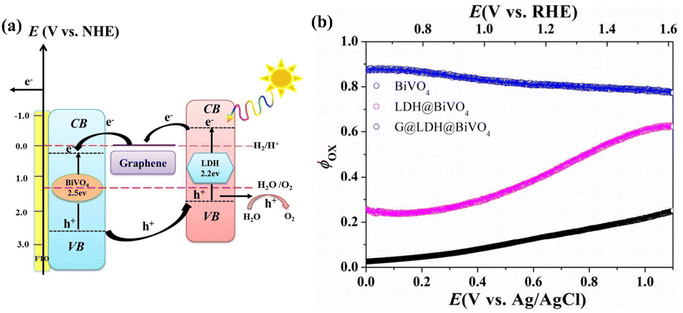 | ||
| Fig. 24 (a) Charge transfer mechanism and (b) oxidation efficiency (Φox) of BiVO4@LDH graphene photoanodes. Reprinted with permission from ref. 75, Copyright 2017, American Chemical Society. | ||
The inclusion of CDs into NiFe-LDH enhances its electrical conductivity and electrocatalytic activity, leading to improved overall PEC performance in water splitting. By sequentially incorporating NiFe-LDH and CDs onto the BiVO4 photoanode, Lv et al. developed a ternary BiVO4/NiFe-LDH/CDs photoanode for PEC water splitting.184 In this design, NiFe-LDH effectively enhances the kinetics of oxygen evolution. Additionally, the incorporation of CDs reduces overpotential and charge transfer resistance during the oxygen evolution process. Consequently, the resulting ternary BiVO4/NiFe-LDH/CDs photoanode exhibited a notable increase in photocurrent, reaching 2.84 mA cm−2 at 1.23 V vs. RHE.
CdTe QDs possess outstanding light-harvesting and charge-transfer properties, thus can be used as charge transfer promoters or photo sensitizers in PEC photoelectrodes. Tang et al. created a triadic BiVO4@CoAl-LDH@CdTeQD photoanode by sequentially depositing red- and green-emission CdTe QDs from an aqueous solution onto the CoAl-LDH/BiVO4 photoanode, as shown in Fig. 25a.185 The successive Type-II band alignments of CdTeQD@CoAl-LDH and BiVO4@CoAl-LDH enhance movements of electron to the counter electrode and facilitate hole migration to the surface throughout water oxidation. Moreover, the isolated anchoring and uniform distribution of CdTe QDs on 2D CoAl-LDH nanosheets improve light harvesting, charge separation, and hole extraction, potentially via the transfer of hot electrons or holes (Fig. 25b). Therefore, the BiVO4@CoAl-LDH@CdTeQD photoanode exhibited a photocurrent density of 2.23 mA cm−2 at 1.23 V vs. RHE, which is twice as compared to that of dyadic BiVO4@CoAl-LDH. Furthermore, the efficiency of water oxidation surpasses 90% at a low potential of 0.5 V vs. RHE (Fig. 25c).
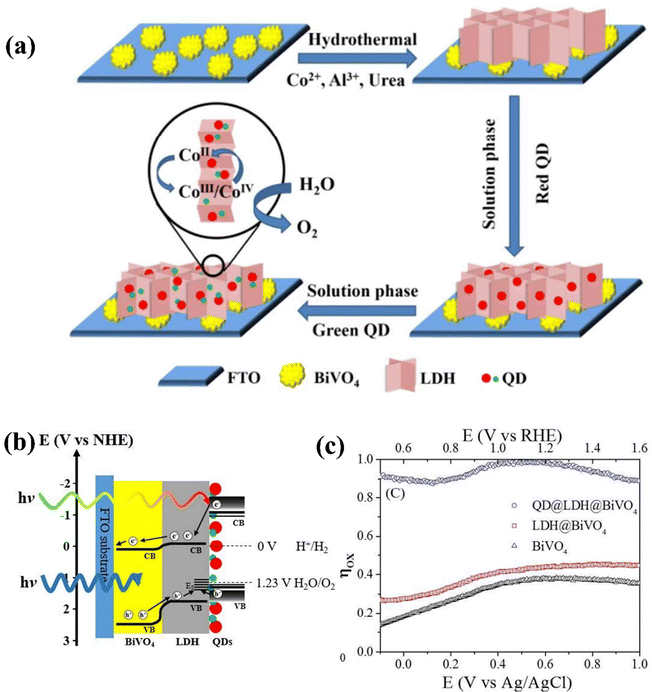 | ||
| Fig. 25 (a) Synthesis process, (b) charge transfer pathways, and (c) oxidation efficiency (Φox) of BiVO4@LDH@QD photoanodes. Reprinted with permission from ref. 185, Copyright 2016, American Chemical Society. | ||
Moreover, LDHs can be easily exfoliated into monolayers through chemical or mechanical exfoliation techniques. The obtained ultrathin layers exhibit a substantial number of exposed active sites and a vast surface area, leading to high intrinsic catalytic activity.89,221 Loading ultrathin nanosheet LDHs onto BiVO4 photoanodes is an effective approach to improve the overall PEC performance of BiVO4. For example, Zhong et al. fabricated a Mo:BiVO4/CoAl-LDH photoanode for PEC water splitting.132 The authors studied two different LDHs, bulk (b) CoAl-LDH and ultrathin (u)-CoAl-LDH, on Mo:BiVO4 using a dip coating method. Among these, the u-CoAl-LDH on Mo:BiVO4 demonstrated superior photoexcited charge separation, as confirmed by LSV measurements and open-circuit potential investigation. As shown in Fig. 26a, under illumination, the current density of Mo: BiVO4/u-CoAl-LDH achieves 5.8 mA cm−2 at 1.23 V vs. RHE, representing an increase of 1.5 and 3.62 times compared to Mo:BiVO4 and BiVO4, respectively. This suggests that the ultrathin structure enhances the photogenerated charge carrier movement because of its shorter charge transport path, whereas the bulk one impedes hole transit. Furthermore, compared to Mo:BiVO4, the Mo:BiVO4/u-CoAl-LDH photoanode demonstrates a notably higher photovoltage of 0.38 V, suggesting an improved driving force for effective charge separation (Fig. 26b). Additionally, the Mo:BiVO4/u-CoAl-LDH photoanode demonstrated self-healing capabilities due to the facile repair of oxygen defects in u-CoAl-LDH via the Co3+/Co2+ cycle, allowing the PEC performance to naturally restore in the air (Fig. 26c).
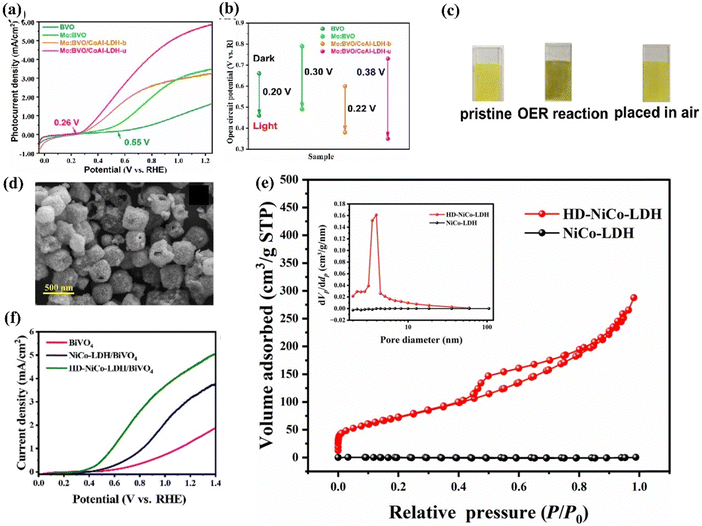 | ||
| Fig. 26 (a) Photocurrent, (b) open circuit potential, and (c) photographs of Mo:BiVO4/u-CoAL-LDH photoanode films before and after reaction, and being placed in the air for one day. Reprinted with permission from ref. 132, Copyright 2023, Elsevier. (d) SEM image, (e) N2 adsorption isotherm and pore size distribution curves, and (f) LSV curves of BiVO4/HD-NiCo-LDH photoanodes. Reprinted from ref. 188, CC. BY 4.0. | ||
Feng et al. synthesized hollow dodecahedral NiCo-LDH (HD-NiCo-LDH) and lamellar NiCo-LDH (NiCo-LDH), which were loaded onto the BiVO4 photoanode for PEC water splitting.188 As illustrated in Fig. 26(d and e), the stacking of ultra-thin nanosheets in HD-NiCo-LDH produces a higher specific surface area and pore size compared to NiCo-LDH. This leads to an abundance of active catalytic sites and promotes the effective extraction and separation of photogenerated holes. Consequently, the BiVO4/HD-NiCo-LDH photoanode exhibited a photocurrent density of 4.54 mA cm−2 at 1.23 V vs. RHE (Fig. 26f), representing increases of 1.39 and 3.46 times compared to the BiVO4/NiCo-LDH and bare BiVO4 photoanodes, respectively.
Coupling single atom noble metals such as Pt or Ru to LDHs may result in electronic interaction between the LDHs’ support and single atoms, resulting in increased catalytic activity, selectivity and stability of the catalyst. Incorporating NiFe-LDH-supported single Ru atoms on to BiVO4, Sun et al. developed a BiVO4@NiFe-LDHs/Ru photoanode for PEC water splitting.186 The Cs coated STEM (Fig. 27a and b) and EXAFS (Fig. 27c) provided strong evidence that the Ru atoms are anchored to NiFe-LDHs through oxygen coordination, resulting in the formation of Ru–O–M bonds. These bonds facilitate electron rearrangement, leading to enhanced charge carrier separation and injection. Consequently, BiVO4@NiFe-LDHs/Ru achieved a high photocurrent density of 4.65 mA cm−2 at 1.23 V vs. RHE. Moreover, the XPS spectra showed that the presence of Ru atoms causes the V(5−x)+ ions to form, which helps stabilize the V atoms in the lattice of BiVO4 and prevents V5+ dissolution during the process of PEC water oxidation. It maintained 94.8% of its original photocurrent density value over a period of 5 h. The DFT calculations indicated that Ru single atoms anchored to BiVO4@NiFe-LDHs decrease the reaction energy barrier of the rate-limiting step (*O → *OOH), thus facilitating the OER process (Fig. 27d).
 | ||
| Fig. 27 (a) and (b) Cs-corrected STEM images, (c) WT-EXAFS signals of the BiVO4@NiFe-LDH/Ru photoanode. (d) Free energy diagram of the OER process for BiVO4@NiFe-LDHs and BiVO4@NiFe-LDHs/Ru photoanodes. Reprinted with permission from ref. 186, Copyright 2023, Elsevier. | ||
Gao et al. developed a BiVO4/AC-CoFe/Pt photoanode by incorporating an amorphous/crystalline (A/C) CoFe LDH heterostructure with a single atomic Pt supported on BiVO4 photoanodes.187 In this design, the single atom (SA) Pt/AC-CoFe catalyst exhibited a much lower overpotential of 230 mV at 10 mA cm−2 compared to the AC-CoFe catalyst, as shown in Fig. 28a. In addition to enhancing the OER process, the SAs Pt anchored on the AC-CoFe catalyst also improves charge separation and transport within the BiVO4 photoelectrode. The SAs Pt/AC-CoFe/BiVO4 exhibited an outstanding photocurrent density of 5.14 mA cm−2 at 1.23 V vs. RHE (Fig. 28b), which is 3.0 and 1.6 times greater than that of bare BiVO4 and AC-CoFe/BiVO4, respectively, and it also demonstrated a stability for 20 h (Fig. 28c).
 | ||
| Fig. 28 (a) LSV curves of RuO2, AC-CoFe, and SAs pt/AC-CoFe in 1 M KOH and (b) BiVO4/AC-CoFe/SAsPt photoanodes under AM 1.5G illumination. (c) Stability of the BiVO4/AC-CoFe/SAsPt photoanode in 1 M KBi. Reprinted with permission from ref. 187, Copyright 2023, Elsevier. | ||
5. Conclusion and outlook
BiVO4 is a prominent visible light semiconductor photoanode for PEC water splitting. Combining LDHs with BiVO4 photoanodes provides a significant breakthrough in PEC water splitting due to their synergistic properties. LDHs improve charge separation and decrease recombination, addressing key limitations of BiVO4, including sluggish reaction kinetics and inadequate charge transport. This integration lowers the reaction overpotential and boosts the OER kinetics, leading to enhanced overall performance of the PEC system. This review explored various composite structures, including BiVO4 combined with bimetallic and trimetallic LDHs, as well as other BiVO4-based composites like BiVO4/metal oxides, metal sulfides, and various charge transport layers integrated with LDHs. It also covered LDH composites incorporating materials such as graphene, CDs, QDs, and single-atom catalysts, along with techniques for surface engineering and LDH exfoliation with BiVO4. The main intention of the integration of LDHs with BiVO4 demonstrated significant improvements in photocurrent density, onset potential, and overall stability, making these composites attractive for sustainable hydrogen production. However, despite these advancements, challenges remain in optimizing the interface, improving stability, and enhancing overall efficiency. In accordance with the discussions in this review, the following obstacles and prospects are identified as critical to the advancement of the development of high-performance BiVO4/LDH composites for PEC water splitting.(i) Despite significant progress in coupling transition metal based LDHs with BiVO4 photoanodes for PEC water splitting, challenges concerning stability and efficiency still hinder their widespread implementation in practical applications. The interfacial charge recombination between LDHs and BiVO4 is a significant obstacle that affects the charge transfer efficiency. To address this issue, future research ought to focus on interface engineering to further improve charge separation and reduce recombination. By employing various methods like interlayers such as hole storage, electron transport layer, and doping, advanced heterojunctions, it is possible to effectively eliminate the interfacial energy barriers. This facilitates the rapid transfer of holes through the chemical bonding of LDHs and BiVO4, ultimately resulting in enhanced effectiveness of the PEC process. Moreover, Since LDHs remain stable in alkaline electrolytes, future studies should focus on ensuring their stability under harsh solution conditions and solar irradiation. Developing self-healing materials, protective coatings, and degradation prevention strategies will be critical for ensuring the long-term functioning of BiVO4/LDH photoanodes. Furthermore, due to the low conductivity and limited specific surface area of traditional LDHs, research into new LDH compositions such as multi-metal LDHs, QD-incorporated LDHs, and single-atom catalysts could lead to the discovery of materials with superior catalytic properties, specifically tailored for improved performance in BiVO4/LDH composites.
(ii) Due to their tunable compositional and structural features, LDHs serve as a versatile catalytic structure capable of addressing kinetically challenging reactions. Optimizing the loading conditions of LDHs onto BiVO4 photoanodes is critical for improving PEC efficiency. Excessive loading can potentially hinder the efficient light absorption by BiVO4, whereas inadequate loading may result in a lack of sufficient catalytic sites. To achieve the desired balance, it is necessary to have precise control over the deposition process as well as a thorough understanding of the interaction between LDHs and BiVO4. Advanced characterization techniques could play an important role in optimizing the loading process to achieve outstanding performance. Moreover, the role of specific intercalated anions within LDHs on the PEC water splitting performance of BiVO4/LDHs composites remains yet to be explored, offering a promising direction for future research.
(iii) Understanding the intrinsic mechanism of PEC water oxidation is critical for selection and modification of photoanodes. In addition to the extensive studies on morphology, elemental composition, and valence states, the advancement of in situ characterization techniques like X-ray absorption spectroscopy (XAS), transient absorption spectroscopy (TAS), and operando spectroscopic studies is crucial for understanding surface charge transfer, identifying active sites, and elucidating reaction kinetics in the PEC water splitting process.38,222,223 Moreover, direct visualization of electron transfer between atoms is made possible by in situ irradiation X-ray photoelectron spectroscopy (SIXPS), thereby rendering it easier to investigate the movement and separation of charges during atom activation.224 Furthermore, computational techniques, such as density functional theory (DFT), and machine learning approaches hold significant potential for predicting and selecting materials, identifying adsorption sites, and extrapolating reaction pathways.225–227 Therefore, the integration of experimental techniques with theoretical calculations is essential for unraveling the intricate mechanisms in LDH modified BiVO4 systems.
(iv) Since theoretical efficiency of the BiVO4 photoanode is close to 9.1%, it stands as a potentially effective material for achieving high PEC water splitting efficiency through unbiased photoanode-PV and photoanode-photocathode configurations.228,229 The BiVO4-photocathode tandem is regarded as more desirable for practical applications due to its lower cost and simpler electrical connection compared to the BiVO4-PV configuration.230,231 Therefore, developing efficient photocathodes is another big challenge to improve the STH efficiency of BiVO4 photoanodes in unbiased PEC water splitting systems. The coupling of BiVO4 with improved photocathodes and LDHs holds great promise for boosting STH efficiency, advancing PEC water splitting technology toward practical applications. We expect that the utilization of BiVO4 photoanodes and LDHs will lead to significant progress in the coming years.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI) Grant Number JP23K26377.References
- J. Deng, Y. Su, D. Liu, P. Yang, B. Liu and C. Liu, Chem. Rev., 2019, 119, 9221–9259 CrossRef CAS PubMed.
- J. Barber, Chem. Soc. Rev., 2009, 38, 185–196 RSC.
- M. M. Abouelela, G. Kawamura, W. K. Tan and A. Matsuda, J. Colloid Interface Sci., 2023, 629, 958–970 CrossRef CAS PubMed.
- C. Liu, N. P. Dasgupta and P. Yang, Chem. Mater., 2014, 26, 415–422 CrossRef CAS.
- F. Yilmaz, M. T. Balta and R. Selbaş, Renewable Sustainable Energy Rev., 2016, 56, 171–178 CrossRef CAS.
- U. Asghar, S. Rafiq, A. Anwar, T. Iqbal, A. Ahmed, F. Jamil, M. S. Khurram, M. M. Akbar, A. Farooq, N. S. Shah and Y.-K. Park, J. Environ. Chem. Eng., 2021, 9, 106064 CrossRef CAS.
- M. Ali, E. Pervaiz, T. Noor, O. Rabi, R. Zahra and M. Yang, Int. J. Energy Res., 2021, 45, 1190–1226 CrossRef CAS.
- P. A. Koyale and S. D. Delekar, Int. J. Hydrogen Energy, 2024, 51, 515–530 CrossRef CAS.
- C. V. Reddy, K. R. Reddy, N. P. Shetti, J. Shim, T. M. Aminabhavi and D. D. Dionysiou, Int. J. Hydrogen Energy, 2020, 45, 18331–18347 CrossRef CAS.
- M. A. Gaikwad, U. P. Suryawanshi, U. V. Ghorpade, J. S. Jang, M. P. Suryawanshi and J. H. Kim, Small, 2022, 18, 2105084 CrossRef CAS PubMed.
- C. Jiang, S. J. A. Moniz, A. Wang, T. Zhang and J. Tang, Chem. Soc. Rev., 2017, 46, 4645–4660 RSC.
- S. Wang, X. Wang, B. Liu, Z. Guo, K. (Ken) Ostrikov, L. Wang and W. Huang, Nanoscale, 2021, 13, 17989–18009 RSC.
- Y. Qiu, Z. Pan, H. Chen, D. Ye, L. Guo, Z. Fan and S. Yang, Sci. Bull., 2019, 64, 1348–1380 CrossRef CAS PubMed.
- K. Wang, D. Huang, X. Li, K. Feng, M. Shao, J. Yi, W. He and L. Qiao, Electron, 2023, 1, e4 CrossRef.
- A. Raveendran, M. Chandran and R. Dhanusuraman, RSC Adv., 2023, 13, 3843–3876 RSC.
- I. R. Hamdani and A. N. Bhaskarwar, Renewable Sustainable Energy Rev., 2021, 138, 10503 CrossRef.
- L. Fu, Z. Li and X. Shang, Int. J. Hydrogen Energy, 2024, 55, 611–624 CrossRef CAS.
- S. Chen, T. Takata and K. Domen, Nat. Rev. Mater., 2017, 2, 17050 CrossRef CAS.
- C. Li, W. Fan, S. Chen and F. Zhang, Chem. – Eur. J., 2022, 28, e202201812 CrossRef CAS PubMed.
- L. Wang, Y. Zhang, W. Li and L. Wang, Mater. Rep.: Energy, 2023, 3, 100232 CAS.
- W. Wang, M. Xu, X. Xu, W. Zhou and Z. Shao, Angew. Chem., Int. Ed., 2020, 59, 136–152 CrossRef CAS PubMed.
- A. Vilanova, P. Dias, T. Lopes and A. Mendes, Chem. Soc. Rev., 2024, 53, 2388–2434 RSC.
- M. M. Abouelela, G. Kawamura and A. Matsuda, J. Cleaner Prod., 2021, 294, 126200 CrossRef CAS.
- P. P. Edwards, V. L. Kuznetsov, W. I. F. David and N. P. Brandon, Energy Policy, 2008, 36, 4356–4362 CrossRef.
- B. S. Kalanoor, H. Seo and S. S. Kalanur, Mater. Sci. Energy Technol., 2018, 1, 49–62 Search PubMed.
- M. Lee, S. Haas, V. Smirnov, T. Merdzhanova and U. Rau, ChemElectroChem, 2022, 9, e202200838 CrossRef CAS.
- L. Ding, Y. Zhang, T. Wang, P. Li and K. Chang, Catal. Sci. Technol., 2024, 14, 1730–1755 RSC.
- J. H. Kim, D. Hansora, P. Sharma, J.-W. Jang and J. S. Lee, Chem. Soc. Rev., 2019, 48, 1908–1971 RSC.
- S. Li, W. Xu, L. Meng, W. Tian and L. Li, Small Sci., 2022, 2, 2100112 CrossRef CAS.
- L. Clarizia, M. N. Nadagouda and D. D. Dionysiou, Curr. Opin. Green Sustainable Chem., 2023, 41, 100825 CrossRef CAS PubMed.
- S. Tembhurne, F. Nandjou and S. Haussener, Nat. Energy, 2019, 4, 399–407 CrossRef CAS.
- R. Lin, H. Lei, D. Ruan, K. Jiang, X. Yu, Z. Wang, W. Mai and H. Yan, Nano Energy, 2019, 56, 82–91 CrossRef CAS.
- M. Joseph, M. Kumar, S. Haridas, C. Subrahmanyam and S. N. Remello, Energy Adv., 2024, 3, 30–59 RSC.
- M. Mohamed Abouelela, G. Kawamura and A. Matsuda, J. Energy Chem., 2022, 73, 189–213 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- A. G. Tamirat, J. Rick, A. A. Dubale, W.-N. Su and B.-J. Hwang, Nanoscale Horiz., 2016, 1, 243–267 RSC.
- Y. Wang, W. Tian, C. Chen, W. Xu and L. Li, Adv. Funct. Mater., 2019, 29, 1809036 CrossRef.
- G. Dong, L. Yan and Y. Bi, J. Mater. Chem. A, 2023, 11, 3888–3903 RSC.
- H. Sun, J. Dai, W. Zhou and Z. Shao, Energy Fuel, 2020, 34, 10547–10567 CrossRef CAS.
- P. Mane, I. V. Bagal, H. Bae, A. N. Kadam, V. Burungale, J. Heo, S.-W. Ryu and J.-S. Ha, Int. J. Hydrogen Energy, 2022, 47, 39796–39828 CrossRef CAS.
- D. Cui, L. Wang, K. Xu, L. Ren, L. Wang, Y. Yu, Y. Du and W. Hao, J. Mater. Chem. A, 2018, 6, 2193–2199 RSC.
- R. Siavash Moakhar, S. M. Hosseini-Hosseinabad, S. Masudy-Panah, A. Seza, M. Jalali, H. Fallah-Arani, F. Dabir, S. Gholipour, Y. Abdi, M. Bagheri-Hariri, N. Riahi-Noori, Y. Lim, A. Hagfeldt and M. Saliba, Adv. Mater., 2021, 33, 2007285 CrossRef CAS PubMed.
- W. Jiang, C. Ni, L. Zhang, M. Shi, J. Qu, H. Zhou, C. Zhang, R. Chen, X. Wang, C. Li and R. Li, Angew. Chem., 2022, 134, e202207161 CrossRef.
- R. L. Spray and K.-S. Choi, Chem. Mater., 2009, 21, 3701–3709 CrossRef CAS.
- G. Wang, Y. Ling, H. Wang, X. Yang, C. Wang, J. Z. Zhang and Y. Li, Energy Environ. Sci., 2012, 5, 6180 RSC.
- A. U. Pawar, C. W. Kim, M. J. Kang and Y. S. Kang, Nano Energy, 2016, 20, 156–167 CrossRef CAS.
- I. Chauhan, K. K. Patra, H. Bajpai, N. B. Mhamane, K. N. Salgaonkar and C. S. Gopinath, Dalton Trans., 2023, 52, 2051–2061 RSC.
- M. Zhong, J. Shi, F. Xiong, W. Zhang and C. Li, Sol. Energy, 2012, 86, 756–763 CrossRef CAS.
- A. D. DeAngelis, K. C. Kemp, N. Gaillard and K. S. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 8445–8451 CrossRef CAS PubMed.
- B. R. Lee, S. Choi, W. S. Cheon, J. W. Yang, M. G. Lee, S. H. Park and H. W. Jang, Electron. Mater. Lett., 2022, 18, 391–399 CrossRef CAS.
- G. Lv, L. Long, X. Wu, Y. Qian, G. Zhou, F. Pan, Z. Li and D. Wang, Appl. Surf. Sci., 2023, 609, 155335 CrossRef CAS.
- J. Fu, Z. Fan, M. Nakabayashi, H. Ju, N. Pastukhova, Y. Xiao, C. Feng, N. Shibata, K. Domen and Y. Li, Nat. Commun., 2022, 13, 729 CrossRef CAS PubMed.
- X. Shen, T. Zhao, H. Su, M. Yang, J. Chen, Y. Liu, R. Yanagi, D. Solanki and S. Hu, Adv. Energy Mater., 2022, 12, 2201314 CrossRef CAS.
- H. Huang, L. Liu, Y. Zhang and N. Tian, RSC Adv., 2015, 5, 1161–1167 RSC.
- S. Mary, C. Murugan and A. Pandikumar, J. Colloid Interface Sci., 2022, 608, 2482–2492 CrossRef PubMed.
- M. N. Shaddad, P. Arunachalam, M. Hezam, N. M. BinSaeedan, S. Gimenez, J. Bisquert and A. M. Al-Mayouf, J. Catal., 2023, 418, 51–63 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- P. Subramanyam, T. Khan, G. Neeraja Sinha, D. Suryakala and C. Subrahmanyam, Int. J. Hydrogen Energy, 2020, 45, 7779–7787 CrossRef CAS.
- S. Alam, T. K. Sahu and M. Qureshi, ACS Sustainable Chem. Eng., 2021, 9, 5155–5165 CrossRef CAS.
- Y. Qiu, W. Liu, W. Chen, W. Chen, G. Zhou, P.-C. Hsu, R. Zhang, Z. Liang, S. Fan, Y. Zhang and Y. Cui, Sci. Adv., 2016, 2, e1501764 CrossRef PubMed.
- Y. Kuang, Q. Jia, H. Nishiyama, T. Yamada, A. Kudo and K. Domen, Adv. Energy Mater., 2016, 6, 1501645 CrossRef.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- W. J. Jo, J. Jang, K. Kong, H. J. Kang, J. Y. Kim, H. Jun, K. P. S. Parmar and J. S. Lee, Angew. Chem., 2012, 124, 3201–3205 CrossRef.
- M. Tayebi and B.-K. Lee, Catal. Today, 2021, 361, 183–190 CrossRef CAS.
- W. Luo, J. Wang, X. Zhao, Z. Zhao, Z. Li and Z. Zou, Phys. Chem. Chem. Phys., 2013, 15, 1006–1013 RSC.
- C. Zhou, S. Wang, Z. Zhao, Z. Shi, S. Yan and Z. Zou, Adv. Funct. Mater., 2018, 28, 1801214 CrossRef.
- H. S. Han, S. Shin, D. H. Kim, I. J. Park, J. S. Kim, P.-S. Huang, J.-K. Lee, I. S. Cho and X. Zheng, Energy Environ. Sci., 2018, 11, 1299–1306 RSC.
- P. M. Rao, L. Cai, C. Liu, I. S. Cho, C. H. Lee, J. M. Weisse, P. Yang and X. Zheng, Nano Lett., 2014, 14, 1099–1105 CrossRef CAS PubMed.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 CrossRef CAS PubMed.
- Y. Liu, B. R. Wygant, K. Kawashima, O. Mabayoje, T. E. Hong, S. G. Lee, J. Lin, J.-H. Kim, K. Yubuta, W. Li, J. Li and C. B. Mullins, Appl. Catal., B, 2019, 245, 227–239 CrossRef CAS.
- S. Wang, T. He, J. Yun, Y. Hu, M. Xiao, A. Du and L. Wang, Adv. Funct. Mater., 2018, 28, 1802685 CrossRef.
- H. Saada, R. Abdallah, B. Fabre, D. Floner, S. Fryars, A. Vacher, V. Dorcet, C. Meriadec, S. Ababou-Girard and G. Loget, ChemElectroChem, 2019, 6, 613–617 CrossRef CAS.
- H. She, P. Yue, X. Ma, J. Huang, L. Wang and Q. Wang, Appl. Catal., B, 2020, 263, 118280 CrossRef CAS.
- D. K. Zhong, S. Choi and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370–18377 CrossRef CAS PubMed.
- X. Zhang, R. Wang, F. Li, Z. An, M. Pu and X. Xiang, Ind. Eng. Chem. Res., 2017, 56, 10711–10719 CrossRef CAS.
- B. Zhang, X. Huang, Y. Zhang, G. Lu, L. Chou and Y. Bi, Angew. Chem., Int. Ed., 2020, 59, 18990–18995 CrossRef CAS PubMed.
- W. Zhang, Y. Zhang, H. Yuan, J. Li, L. Ding, S. Chu, L. Wang, W. Zhai and Z. Jiao, J. Colloid Interface Sci., 2022, 616, 631–640 CrossRef CAS PubMed.
- S. Wang, T. He, P. Chen, A. Du, K. (Ken) Ostrikov, W. Huang and L. Wang, Adv. Mater., 2020, 32, 2001385 CrossRef CAS PubMed.
- L. Cai, J. Zhao, H. Li, J. Park, I. S. Cho, H. S. Han and X. Zheng, ACS Energy Lett., 2016, 1, 624–632 CrossRef CAS.
- Z. Zhang, X. Huang, B. Zhang and Y. Bi, Energy Environ. Sci., 2022, 15, 2867–2873 RSC.
- Y. Xu, X. Wang, H. Chen, D. Kuang and C. Su, Adv. Funct. Mater., 2016, 26, 4414–4421 CrossRef CAS.
- R. Chen, L. Meng, W. Xu and L. Li, Small, 2024, 20, 2304807 CrossRef CAS PubMed.
- D. Tang, J. Liu, X. Wu, R. Liu, X. Han, Y. Han, H. Huang, Y. Liu and Z. Kang, ACS Appl. Mater. Interfaces, 2014, 6, 7918–7925 CrossRef CAS PubMed.
- Z. Gu, J. J. Atherton and Z. P. Xu, Chem. Commun., 2015, 51, 3024–3036 RSC.
- Z. Li, M. Shao, H. An, Z. Wang, S. Xu, M. Wei, D. G. Evans and X. Duan, Chem. Sci., 2015, 6, 6624–6631 RSC.
- Y. Wang, D. Yan, S. El Hankari, Y. Zou and S. Wang, Adv. Sci., 2018, 5, 1800064 CrossRef PubMed.
- D. P. Sahoo, K. K. Das, S. Mansingh, S. Sultana and K. Parida, Coord. Chem. Rev., 2022, 469, 214666 CrossRef CAS.
- L. Qian, Z. Lu, T. Xu, X. Wu, Y. Tian, Y. Li, Z. Huo, X. Sun and X. Duan, Adv. Energy Mater., 2015, 469, 1500245 CrossRef.
- L. Lv, Z. Yang, K. Chen, C. Wang and Y. Xiong, Adv. Energy Mater., 2019, 9, 1803358 CrossRef.
- P. Prabha Sarangi, D. Prava Sahoo, U. Aparajita Mohanty, S. Nayak and K. Parida, ChemCatChem, 2024, 16, e202301533 CrossRef CAS.
- S. Wang, D. Cui, W. Hao and Y. Du, Energy Fuel, 2022, 36, 11394–11403 CrossRef CAS.
- Y. Yang, S. Niu, D. Han, T. Liu, G. Wang and Y. Li, Adv. Energy Mater., 2017, 7, 1700555 CrossRef.
- J. G. Mavroides, D. I. Tchernev, J. A. Kafalas and D. F. Kolesar, Mater. Res. Bull., 1975, 10, 1023–1030 CrossRef CAS.
- C. Jiang, S. J. A. Moniz, A. Wang, T. Zhang and J. Tang, Chem. Soc. Rev., 2017, 46, 4645–4660 RSC.
- K. Sivula, J. Phys. Chem. Lett., 2013, 4, 1624–1633 CrossRef CAS PubMed.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC.
- M. Kumar, B. Meena, P. Subramanyam, D. Suryakala and C. Subrahmanyam, NPG Asia Mater., 2022, 14, 88 CrossRef CAS.
- Y. Li and J. Z. Zhang, Laser Photonics Rev., 2010, 4, 517–528 CrossRef CAS.
- Y. Lin, G. Yuan, R. Liu, S. Zhou, S. W. Sheehan and D. Wang, Chem. Phys. Lett., 2011, 507, 209–215 CrossRef CAS.
- M. Zhou, X. W. (David) Lou and Y. Xie, Nano Today, 2013, 8, 598–618 CrossRef CAS.
- Z. Zhang and J. T. Yates, Chem. Rev., 2012, 112, 5520–5551 CrossRef CAS PubMed.
- C. Ding, J. Shi, Z. Wang and C. Li, ACS Catal., 2017, 7, 675–688 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- H. M. Chen, C. K. Chen, R.-S. Liu, L. Zhang, J. Zhang and D. P. Wilkinson, Chem. Soc. Rev., 2012, 4, 5654 RSC.
- C. Ros, T. Andreu and J. R. Morante, J. Mater. Chem. A, 2020, 8, 10625–10669 RSC.
- X. Liu, J. Chi, B. Dong and Y. Sun, ChemElectroChem, 2019, 6, 2157–2166 CrossRef CAS.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179–209 CrossRef CAS.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- P. Ma and D. Wang, Nanometerials for Energy Conversion and Storage, ed. W. Dunwei, and C. Guozhong, World Scientific, Europe, 2018, pp. 1–61 Search PubMed.
- Y. Hou, X. Zhuang and X. Feng, Small Methods, 2017, 1, 1700090 CrossRef.
- M. R. Nellist, F. A. L. Laskowski, F. Lin, T. J. Mills and S. W. Boettcher, Acc. Chem. Res., 2016, 49, 733–740 CrossRef CAS PubMed.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900–1909 CrossRef CAS PubMed.
- Q. Wang and D. O. Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- F. Cavani, F. Trifirò and A. Vaccari, Catal. Today, 1991, 11, 173–301 CrossRef CAS.
- G. R. Williams and D. O. Hare, J. Mater. Chem., 2006, 16, 3065–3074 RSC.
- L. Mohapatra and K. Parida, J. Mater. Chem. A, 2016, 4, 10744–10766 RSC.
- M. Shao, R. Zhang, Z. Li, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2015, 51, 15880–15893 RSC.
- X. Zou, A. Goswami and T. Asefa, J. Am. Chem. Soc., 2013, 135, 17242–17245 CrossRef CAS PubMed.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed.
- F. Song and X. Hu, J. Am. Chem. Soc., 2014, 136, 16481–16484 CrossRef CAS PubMed.
- (a) M. Shao, F. Ning, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 580–586 CrossRef CAS; (b) R. A. Krivina, Y. Ou, Q. Xu, L. P. Twight, T. N. Stovall and S. W. Boettcher, Acc. Mater. Res., 2021, 2, 548–558 CrossRef CAS; (c) M. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng and S. W. Boettcher, J. Am. Chem. Soc., 2017, 139, 11361–11364 CrossRef CAS PubMed; (d) L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- M. Laipan, J. Yu, R. Zhu, J. Zhu, A. T. Smith, H. He, D. O’Hare and L. Sun, Mater. Horiz., 2019, 7, 715–745 RSC.
- R. Gao, J. Zhu and D. Yan, Nanoscale, 2021, 13, 13593–13603 RSC.
- C. G. Silva, Y. Bouizi, V. Fornés and H. García, J. Am. Chem. Soc., 2009, 131, 13833–13839 CrossRef PubMed.
- S.-M. Xu, T. Pan, Y.-B. Dou, H. Yan, S.-T. Zhang, F.-Y. Ning, W.-Y. Shi and M. Wei, J. Phys. Chem. C, 2015, 119, 18823–18834 CrossRef CAS.
- Y. Zhu, J. Ren, X. Yang, G. Chang, Y. Bu, G. Wei, W. Han and D. Yang, J. Mater. Chem. A, 2017, 5, 9952–9959 RSC.
- B. J. Trześniewski, O. Diaz-Morales, D. A. Vermaas, A. Longo, W. Bras, M. T. M. Koper and W. A. Smith, J. Am. Chem. Soc., 2015, 137, 15112–15121 CrossRef PubMed.
- W. He, R. Wang, L. Zhang, J. Zhu, X. Xiang and F. Li, J. Mater. Chem. A, 2015, 3, 17977–17982 RSC.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- Y. Xu, A. Li, T. Yao, C. Ma, X. Zhang, J. H. Shah and H. Han, ChemSusChem, 2017, 10, 4277–4305 CrossRef CAS PubMed.
- J. Guo, X. Yang, S. Bai, X. Xiang, R. Luo, J. He and A. Chen, J. Colloid Interface Sci., 2019, 540, 9–19 CrossRef CAS PubMed.
- Y. Zhong, C. Wu, X. Jia, S. Sun, D. Chen, W. Yao, H. Ding, J. Zhang and T. Ma, J. Chem. Eng., 2023, 465, 142893 CrossRef CAS.
- Y. Huang, Y. Yu, Y. Xin, N. Meng, Y. Yu and B. Zhang, Sci. China Mater., 2017, 60, 193–207 CrossRef.
- (a) Z. Zhang and J. T. Yates, Chem. Rev., 2012, 112, 5520–5551 CrossRef CAS PubMed; (b) H. L. Tan, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2017, 5, 16498–16521 RSC; (c) F. A. L. Laskowski, M. R. Nellist, J. Qiu and S. W. Boettcher, J. Am. Chem. Soc., 2019, 141, 1394–1405 CrossRef CAS PubMed.
- Q. Meng, B. Zhang, H. Yang, C. Liu, Y. Li, A. Kravchenko, X. Sheng, L. Fan, F. Li and L. Sun, Mater. Adv., 2021, 2, 4323–4332 RSC.
- (a) P. Mane, V. Burungale, H. Bae, C. Seong, J. Heo, S. H. Kang and J.-S. Ha, J. Power Sources, 2024, 591, 233832 CrossRef CAS; (b) F. Lin and S. W. Boettcher, Nat. Mater., 2014, 13, 81–86 CrossRef CAS PubMed; (c) M. Chhetri, S. Dey and C. N. R. Rao, ACS Energy Lett., 2017, 2, 1062–1069 Search PubMed.
- L. Shi, S. Zhuo, M. Abulikemu, G. Mettela, T. Palaniselvam, S. Rasul, B. Tang, B. Yan, N. B. Saleh and P. Wang, RSC Adv., 2018, 8, 29179–29188 Search PubMed.
- N. M. Shaddad, M. Hezam, P. Arunachalam, N. M. AL-Saeedan, S. Gimenez, J. Bisquert and A. M. Al-Mayouf, Mater. Lett., 2022, 325, 132799 CrossRef.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 CrossRef CAS PubMed.
- F. Zhao, N. Li, Y. Wu, X. Wen, Q. Zhao, G. Liu and J. Li, Int. J. Hydrogen Energy, 2020, 45, 31902–31912 CrossRef CAS.
- T.-G. Vo, Y. Tai and C.-Y. Chiang, J. Catal., 2019, 370, 1–10 CrossRef CAS.
- R. Lei, Y. Tang, W. Qiu, S. Yan, X. Tian, Q. Wang, Q. Chen, Z. Wang, W. Qian, Q. Xu, S. Yang and X. Wang, Nano Lett., 2023, 23, 11785–11792 CrossRef CAS PubMed.
- D. K. Lee and K.-S. Choi, Nat. Energy, 2017, 3, 53–60 CrossRef.
- R. Gao and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 23094–23099 CrossRef CAS PubMed.
- R.-T. Gao, N. T. Nguyen, T. Nakajima, J. He, X. Liu, X. Zhang, L. Wang and L. Wu, Sci. Adv., 2023, 9, eade4589 CrossRef PubMed.
- (a) Y. Kuang, Q. Jia, G. Ma, T. Hisatomi, T. Minegishi, H. Nishiyama, M. Nakabayashi, N. Shibata, T. Yamada, A. Kudo and K. Domen, Nat. Energy, 2016, 2, 16191 CrossRef; (b) R. Gao, D. He, L. Wu, K. Hu, X. Liu, Y. Su and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 6213–6218 CrossRef CAS PubMed; (c) N. An, H. Tian, Y. Zhou, Y. Zou, H. Xiu, Y. Cao, Y. Wang, J. Li, D. Liu and Y. Kuang, J. Energy Chem., 2022, 66, 657–665 CrossRef CAS; (d) S. Jiang, M. Zhang, C. Xu, G. Liu, K. Zhang, Z. Zhang, H.-Q. Peng, B. Liu and W. Zhang, ACS Nano, 2024, 18, 16413–16449 CrossRef CAS PubMed; (e) T. Wang, Y. Zhang, Y. Zang, Y. Yu, M. Zhuang, W. Zhang and X. Tao, Ind. Eng. Chem. Res., 2023, 62, 12538–12548 CrossRef CAS.
- D. He, R.-T. Gao, S. Liu, M. Sun, X. Liu, K. Hu, Y. Su and L. Wang, ACS Catal., 2020, 10, 10570–10576 CrossRef CAS.
- L. Wang and F. Xiao, ChemCatChem, 2014, 6, 3048–3052 CrossRef CAS.
- R. Xu, D. Zhu, K. Du, D. Cui, H. Feng, W. Hao, D. Tian and Y. Du, Mater. Today Energy, 2022, 25, 100961 CrossRef CAS.
- M. Yu, Z. Wang, J. Liu, F. Sun, P. Yang and J. Qiu, Nano Energy, 2019, 63, 103880 CrossRef CAS.
- P. Yue, H. She, L. Zhang, B. Niu, R. Lian, J. Huang, L. Wang and Q. Wang, Appl. Catal., B, 2021, 286, 119875 Search PubMed.
- Q. Wang, T. Niu, L. Wang, J. Huang and H. She, Chin. J. Catal., 2018, 39, 613–618 CrossRef CAS.
- M. Wang, L. Wu, L. Geng, L. Gao, J. Ge, H. Niu, H. Li and J. Jin, J. Alloys Compd., 2024, 987, 174183 CrossRef CAS.
- T. Zhang, Y. Lu, J. Wang, Z. Wang, W. Zhang, X. Wang, J. Su and L. Guo, Nanotechnology, 2020, 31, 115707 CrossRef CAS PubMed.
- P. Wei, Y. Wen, K. Lin and X. Li, Inorg. Chem. Front., 2022, 9, 4685–4694 RSC.
- M. Chen, X. Chang, Z. Ma, X. Gao and L. Jia, ACS Appl. Nano Mater., 2024, 7, 15255–15266 CrossRef CAS.
- D. A. Reddy, K. A. J. Reddy, M. Gopannagari, Y. Kim, A. P. Rangappa, D. P. Kumar and T. K. Kim, Appl. Surf. Sci., 2021, 570, 151134 CrossRef CAS.
- T.-G. Vo, K.-F. Chang and C.-Y. Chiang, J. Catal., 2020, 391, 336–345 CrossRef CAS.
- X. Wen, M. Fan, Q. Zhao, J. Li and G. Liu, Chem. – Asian J., 2021, 16, 4095–4102 CrossRef CAS PubMed.
- Z. Huang, X. Cheng, L. Xia, W. Yao, Y. Min, Q. Xu and Q. Wu, ACS Appl. Nano Mater., 2024, 7, 11655–11665 CrossRef CAS.
- D. Kim and S.-H. Baek, Ceram. Int., 2024, 50, 32706–32716 CrossRef CAS.
- S. Bai, H. Chu, X. Xiang, R. Luo, J. He and A. Chen, Chem. Eng. J., 2018, 350, 148–156 CrossRef CAS.
- M. Kolaei, B.-K. Lee and Z. Masoumi, J. Alloys Compd., 2023, 968, 172133 CrossRef CAS.
- W. Zhou, T. Jiang, Y. Zhao, C. Xu, C. Pei and H. Xue, J. Colloid Interface Sci., 2019, 549, 42–49 CrossRef CAS PubMed.
- A. Singh, S. Karmakar and S. Basu, Int. J. Hydrogen Energy, 2021, 46, 39868–39881 CrossRef CAS.
- S. Bai, J. Han, K. Zhang, J. Sun, J. Guo, R. Luo, D. Li and A. Chen, ACS Sustainable Chem. Eng., 2020, 8, 4076–4084 CrossRef CAS.
- J. Jiang, M. Wang, R. Li, L. Ma and L. Guo, Int. J. Hydrogen Energy, 2013, 38, 13069–13076 CrossRef CAS.
- G. Dong, T. Chen, F. Kou, F. Xie, C. Xiao, J. Liang, C. Lou, J. Zhuang and S. Du, Nanomaterials, 2024, 14, 1100 CrossRef CAS PubMed.
- B. Kang, M. Bilal Hussain, X. Cheng, C. Peng and Z. Wang, J. Colloid Interface Sci., 2022, 626, 146–155 CrossRef CAS PubMed.
- H. Li, M. Lyu, X. Cheng, Y. Lai and Z. Dong, J. Catal., 2023, 428, 115203 CrossRef CAS.
- G. Yin, C. Liu, T. Shi, D. Ji, Y. Yao and Z. Chen, J. Photochem. Photobiol., A, 2022, 426, 113742 CrossRef CAS.
- J. Zhang, L. Wang, M. Li, Z. Zhang, M. Yang, H. Wu, Q. Zhang and X. Xu, J. Alloys Compd., 2023, 965, 171508 CrossRef CAS.
- T. Wang, H. Pei, Y. Zhang, R. Li, J. Zhang and T. Peng, ACS Appl. Energy Mater., 2022, 5, 11271–11282 CrossRef CAS.
- C. Liu, Y. Zhang, G. Yin, T. Shi, Y. Zhang and Z. Chen, Inorg. Chem. Front., 2022, 9, 6431–6440 RSC.
- M. K. Mohanta, T. K. Sahu, S. Bhowmick and M. Qureshi, Electrochim. Acta, 2022, 415, 140269 CrossRef CAS.
- H. Pei, S. Xu, Y. Zhang, Y. Zhou, R. Li and T. Peng, Appl. Catal., B, 2022, 318, 121865 CrossRef CAS.
- X. Han, Y. Wei, J. Su and Y. Zhao, ACS Sustainable Chem. Eng., 2018, 6, 14695–14703 CrossRef CAS.
- L. Sun, J. Sun, X. Yang, S. Bai, Y. Feng, R. Luo, D. Li and A. Chen, Dalton Trans., 2019, 48, 16091–16098 RSC.
- S. Alam and M. Qureshi, J. Phys. Chem. Lett., 2021, 12, 8947–8955 CrossRef CAS PubMed.
- H. Chen, S. Wang, J. Wu, X. Zhang, J. Zhang, M. Lyu, B. Luo, G. Qian and L. Wang, J. Mater. Chem. A, 2020, 8, 13231–13240 RSC.
- R. Wang, L. Luo, X. Zhu, Y. Yan, B. Zhang, X. Xiang and J. He, ACS Appl. Energy Mater., 2018, 1, 3577–3586 CrossRef CAS.
- W. Bai, H. Li, Y. Hu, J. Wang, A. Li and P. François-Xavier Corvini, Chem. Eng. J., 2024, 479, 147713 CrossRef CAS.
- W. Chen, G. Jin, Y. Liu, Q. Wei and J. Tang, ACS Appl. Mater. Interfaces, 2024, 16, 23296–23304 Search PubMed.
- X. Lv, X. Xiao, M. Cao, Y. Bu, C. Wang and M. Wang, Appl. Surf. Sci., 2018, 439, 1065–1071 CrossRef CAS.
- Y. Tang, R. Wang, Y. Yang, D. Yan and X. Xiang, ACS Appl. Mater. Interfaces, 2016, 8, 19446–19455 Search PubMed.
- Y. Sun, H. Li, Y. Hu, J. Wang, A. Li and P. F.-X. Corvini, Appl. Catal., B, 2024, 340, 123269 Search PubMed.
- M. Gao, N. T. Nguyen, R.-T. Gao, X. Liu, X. Zhang and L. Wang, Appl. Catal., B, 2023, 336, 122920 CrossRef CAS.
- S. Feng, S. Fan, L. Li, Z. Sun, H. Tang, Y. Xu, L. Fang and C. Wang, Nano Res. Energy, 2024, 3, e9120117 CrossRef.
- M. Xu and M. Wei, Adv. Funct. Mater., 2018, 28, 1802943 CrossRef.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Nørskov, A. Nilsson and A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305–1313 CrossRef CAS PubMed.
- L. Chen, H. Zhang, L. Chen, X. Wei, J. Shi and M. He, J. Mater. Chem. A, 2017, 5, 22568–22575 RSC.
- L. Yu, J. F. Yang, B. Y. Guan, Y. Lu and X. W. (David) Lou, Angew. Chem., 2018, 130, 178–182 CrossRef.
- C. Zhang, M. Shao, L. Zhou, Z. Li, K. Xiao and M. Wei, ACS Appl. Mater. Interfaces, 2016, 8, 33697–33703 Search PubMed.
- G. Jia, Y. Hu, Q. Qian, Y. Yao, S. Zhang, Z. Li and Z. Zou, ACS Appl. Mater. Interfaces, 2016, 8, 14527–14534 CrossRef CAS PubMed.
- M. Guo, L. Zhou, Y. Li, Q. Zheng, F. Xie and D. Lin, J. Mater. Chem. A, 2019, 7, 13130–13141 Search PubMed.
- H. Liu, Y. Wang, X. Lu, Y. Hu, G. Zhu, R. Chen, L. Ma, H. Zhu, Z. Tie, J. Liu and Z. Jin, Nano Energy, 2017, 35, 350–357 CrossRef CAS.
- J. Bao, Z. Wang, J. Xie, L. Xu, F. Lei, M. Guan, Y. Zhao, Y. Huang and H. Li, Chem. Commun., 2019, 55, 3521–3524 RSC.
- R. A. Sayed, S. E. Abd El Hafiz, N. Gamal, Y. GadelHak and W. M. A. el Rouby, J. Alloys Compd., 2017, 728, 1171–1179 CrossRef CAS.
- R. Chong, B. Wang, C. Su, D. Li, L. Mao, Z. Chang and L. Zhang, J. Mater. Chem. A, 2017, 5, 8583–8590 RSC.
- R. Zhang, M. Shao, S. Xu, F. Ning, L. Zhou and M. Wei, Nano Energy, 2017, 33, 21–28 CrossRef CAS.
- G. Wang, B. Wang, C. Su, D. Li, L. Zhang, R. Chong and Z. Chang, J. Catal., 2018, 359, 287–295 CrossRef CAS.
- K. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo and L. Sun, Nat. Commun., 2016, 7, 11981 CrossRef CAS PubMed.
- X. Long, S. Xiao, Z. Wang, X. Zheng and S. Yang, Chem. Commun., 2015, 51, 1120–1123 RSC.
- X. Yang, J. Cui, L. Lin, A. Bian, J. Dai, W. Du, S. Guo, J. Hu and X. Xu, Adv. Sci., 2024, 11, 2305567 CrossRef CAS PubMed.
- H. Zhang, H. Li, Z. Wang, Z. Zheng, P. Wang, Y. Liu, X. Zhang, X. Qin, Y. Dai and B. Huang, Appl. Catal., B, 2018, 238, 586–591 CrossRef CAS.
- T. Soltani, A. Tayyebi and B.-K. Lee, Sol. Energy Mater. Sol. Cells, 2018, 185, 325–332 CrossRef CAS.
- J. Li, J. Bai, X. Niu, X. Li, S. Chen, J. Wang and B. Zhou, Int. J. Hydrogen Energy, 2018, 43, 18202–18210 CrossRef CAS.
- D. Chen, Z. Liu, Z. Guo, W. Yan and Y. Xin, J. Mater. Chem. A, 2018, 6, 20393–20401 RSC.
- X. Wu, J. Zhao, S. Guo, L. Wang, W. Shi, H. Huang, Y. Liu and Z. Kang, Nanoscale, 2016, 8, 17314–17321 RSC.
- P. Hemmatpour and A. Nezamzadeh-Ejhieh, Chemosphere, 2022, 307, 135925 CrossRef CAS PubMed.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 RSC.
- S. Bai, Q. Li, J. Han, X. Yang, X. Shu, J. Sun, L. Sun, R. Luo, D. Li and A. Chen, Int. J. Hydrogen Energy, 2019, 44, 24642–24652 CrossRef CAS.
- K. Sivula, F. Le Formal and M. Grätzel, ChemSusChem, 2011, 4, 432–449 CrossRef CAS PubMed.
- S. Wang, B. Liu, X. Wang, Y. Zhang and W. Huang, Nano Res., 2022, 15, 7026–7033 CrossRef CAS.
- M. B. Costa, M. A. de Araújo, M. V. de Tinoco, J. F. de Brito and L. H. Mascaro, J. Energy Chem., 2022, 73, 88–113 CrossRef CAS.
- D. Sharma, S. Upadhyay, A. Verma, V. R. Satsangi, R. Shrivastav and S. Dass, Thin Solid Films, 2015, 574, 125–131 CrossRef CAS.
- L. Banszerus, M. Schmitz, S. Engels, J. Dauber, M. Oellers, F. Haupt, K. Watanabe, T. Taniguchi, B. Beschoten and C. Stampfer, Sci. Adv., 2015, 1, e1500222 CrossRef PubMed.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- H. M. Chen, C. K. Chen, C.-J. Chen, L.-C. Cheng, P. C. Wu, B. H. Cheng, Y. Z. Ho, M. L. Tseng, Y.-Y. Hsu, T.-S. Chan, J.-F. Lee, R.-S. Liu and D. P. Tsai, ACS Nano, 2012, 6, 7362–7372 CrossRef CAS PubMed.
- S.-J. Lin, K.-C. Lee, J.-L. Wu and J.-Y. Wu, Sol. Energy, 2012, 86, 2600–2605 CrossRef CAS.
- Y. Sun, S. Gao and Y. Xie, Chem. Soc. Rev., 2014, 4, 530–546 RSC.
- B. J. Trześniewski, I. A. Digdaya, T. Nagaki, S. Ravishankar, I. Herraiz-Cardona, D. A. Vermaas, A. Longo, S. Gimenez and W. A. Smith, Energy Environ. Sci., 2017, 10, 1517–1529 RSC.
- J. Pina, P. Dias, C. Serpa, J. Azevedo, A. Mendes and J. Sérgio Seixas de Melo, J. Phys. Chem. C, 2021, 125, 8274–8284 CrossRef CAS.
- X. Liu, G. Dong, S. Li, G. Lu and Y. Bi, J. Am. Chem. Soc., 2016, 138, 2917–2920 CrossRef CAS PubMed.
- J. Hu, X. Zhao, W. Chen, H. Su and Z. Chen, J. Phys. Chem. C, 2017, 121, 18702–18709 CrossRef CAS.
- M. Huang, S. Wang and H. Zhu, J. Mater. Chem. A, 2023, 11, 21619–21627 RSC.
- Z. Wang, Y. Gu, L. Zheng, J. Hou, H. Zheng, S. Sun and L. Wang, Adv. Mater., 2022, 34, 2106776 CrossRef CAS PubMed.
- Y. Pihosh, I. Turkevych, K. Mawatari, J. Uemura, Y. Kazoe, S. Kosar, K. Makita, T. Sugaya, T. Matsui, D. Fujita, M. Tosa, M. Kondo and T. Kitamori, Sci. Rep., 2015, 5, 11141 CrossRef PubMed.
- J. H. Kim, Y. Jo, J. H. Kim, J. W. Jang, H. J. Kang, Y. H. Lee, D. S. Kim, Y. Jun and J. S. Lee, ACS Nano, 2015, 9, 11820–11829 CrossRef CAS PubMed.
- Y. Zhang, H. Lv, Z. Zhang, L. Wang, X. Wu and H. Xu, Adv. Mater., 2021, 33, 2008264 CrossRef CAS PubMed.
- S. Ye, W. Shi, Y. Liu, D. Li, H. Yin, H. Chi, Y. Luo, N. Ta, F. Fan, X. Wang and C. Li, J. Am. Chem. Soc., 2021, 143, 12499–12508 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |