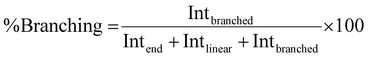Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Poly(malic acid) copolymers as degradable rheology modifiers in aqueous formulations†
Christina A. R.
Picken
a,
Orla
Buensoz
a,
Christopher
Fidge
b,
Paul
Price
b and
Michael P.
Shaver
 *a
*a
aSustainable Materials Innovation Hub, Royce Institute, University of Manchester, Oxford Road, M13 9PL, UK. E-mail: michael.shaver@manchester.ac.uk
bUnilever R&D, Port Sunlight Laboratory, Quarry Road East, Bebington, Wirral, UK
First published on 10th February 2025
Abstract
Polymeric rheological modifiers help tailor formulations to specific applications but many current technologies are poorly bio- or hydrolytically-degradable. This paper investigates the polycondensation of malic acid as an acid-rich branching monomer with lactic and glycolic acids to synthesise hydrophilic branched polyesters as potential formulation modifiers. The branching of the copolymers was characterised using quantitative 2D NMR spectroscopy and Mark–Houwink plots measured by gel permeation chromatography. The viscoelastic properties of the copolymers in solution were assessed within a shampoo formulation and showed increased viscosity and elastic behaviour compared to control samples. The formulations are hydrolytically degradable, with the performance of the shampoo formulations deteriorating over time.
Introduction
From toothpastes and shampoos to paints and lubricants to laundry detergents and foods, modern society relies upon water-based formulations. To perform their given applications, these formulations contain polymeric rheological modifiers which tune the viscosity and viscoelastic responses to stress to perform a particular function. Production of polymers for liquid formulations (PLFs) is estimated to be 29–36 million tonnes annually, despite being less than 10% of the formulation by weight.1 Most PLFs are fossil-derived and are typically poorly biodegradable. The resultant uncertainty in the end-of-life fate of polymers in wastewater systems has motivated and mobilised academia, industry and governing bodies to target more sustainable formulation polymers.2–4 We recently published an in-depth perspective on the challenges and opportunities for PLFs in sustainable formulations, highlighting the need for renewable feedstocks, controllable degradation, and sustainable manufacturing.5Carbomers are the most commonly used polymer for shampoo formulations (Fig. 1).1,6 These carboxylic acid-rich polymers are crosslinked to form networks that support swellable and pH responsive gels in aqueous solutions.7,8 When incorporated in formulated products such as shampoos, carbomers act to stabilise other ingredients and provide viscoelastic properties that allow products to flow and be applied to hands or hair. Unfortunately, carbomers are typically derived from fossil fuels and have no confirmed biodegradation mechanism in the environment,9 motivating the search for degradable and renewable alternatives.
 | ||
| Fig. 1 Carbomer structures are currently used as formulation polymers. Utilisation of malic acid to form branched polymeric materials for use in shampoo formulations which will degrade over time. | ||
Polyesters are a promising class of polymers which can be bio-derived and are hydrolytically or enzymatically degradable under specific conditions. Poly(lactic acid) and poly(3-hydroxybutyrate) are polyesters that have received significant attention as potential packaging plastic alternatives.10–13 Polyesters are typically synthesised by ring opening polymerisations of cyclic monomers or polycondensation of carboxylic acids and hydroxyls.14,15
In designing a suitable carbomer alternative, the polymer will likely require chemical functionalities in the form of carboxylic acid groups and a branched or crosslinked topology. Malic acid is an α-hydroxy acid, possessing two carboxylic acid functionalities and one hydroxyl group. Under condensation reaction conditions, the stoichiometric imbalance of reactive groups facilitates formation of an acid-rich polyester structure, which can further esterify to create trifunctional branch points.16,17 Branched copolymers formed from the copolymerisation of lactic and malic acid have been reported previously as drug delivery systems.18–20 Moreover, malic acid has been investigated previously as a monomer in homopolymerisations,17,21,22 co-polymerisations,18–20,23,24 and in block polycopolmerisations with macro initiators.19 In most instances, stannous(II) chloride was employed as catalyst (0.1–0.5%), with reaction temperatures between 110–140 °C. In this work, malic acid is used as a comonomer in polycondensations to access branched, degradable polyesters of variable topology. The resultant polymers are assessed on their ability to modify rheological performance, particularly of shampoo formulations. Formulation of the malic acid copolymers into shampoo and assessing the rheological profiles as a function of time allows for the functional properties of the poly(malic acid) polymers to be assessed. Polyester based fomulation polymers may have the potential to improve the degradability of both traditional and concentrated shampoo formulations, with the latter being hydratable and dilutable in domestic settings.
Results and discussion
Polymer synthesis
Malic acid (co)-polymers were synthesised by polycondensation reactions modified from those previously reported.21 At elevated temperatures malic acid can undergo an intramolecular condensation reaction to form fumaric acid which when incorporated into the polymer results in inactive chain ends.21 To identify suitable reaction conditions, polymerisation temperature was varied from 100 to 140 °C, with and without an exaggerated amount of catalyst, and 1H NMR spectroscopic analysis revealed 100 and 120 °C as optimal temperatures to promote polymerisation and impede fumaric acid formation (Fig. S1 and S2†). The presence of 1% tin(II) chloride catalyst did not affect the formation of fumaric acid at the lower temperatures and was thus employed as catalyst at the previously reported loading of 0.36 wt%.21,25 Copolymerisations of malic acid (MA) with molar ratios of glycolic (GA) or lactic acid (LA) were conducted following previous procedures21,22 under vacuum using tin(II) chloride (0.36 wt%) as a catalyst, using either standard or forcing conditions (Table S1†) (Fig. 2). For standard conditions (denoted S), malic acid and comonomer were heated to 110 °C for 24 hours to allow esters to form by equilibration. After which, vacuum was applied for 6 hours. Forcing conditions (denoted F) used the same equilibration step however the vacuum was applied for 24 hours with an additional final 6 hours at 120 °C. The homopolymerisation of malic acid was conducted using the same times as the standard conditions but required a temperature of 120 °C to melt. To understand the effect that reaction conditions have on topology, the polycondensation conditions (standard and forcing conditions), type of comonomer (lactic and glycolic), molar ratios of comonomer (0, 10 and 40%) and the addition of a tetra-functional alcohol crosslinker, pentaerythritol (PE), were investigated (Table 1).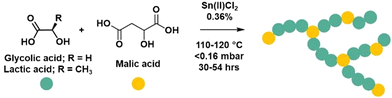 | ||
| Fig. 2 Schematic of the polymerisation of malic acid and comonomers to form polymers in which the malic acid can act as branching agent as well as linear and end groups. | ||
| Entry | Sample | T g, °C | T d5%, °C | M n, g mol−1 | M w, g mol−1 | Đ | MA branched, % | MA linear % | MA end group % |
|---|---|---|---|---|---|---|---|---|---|
| T g = glass transition temperature; Td5% = 5% degradation temperature; Mn = number average molecular weight; Mw = weight average molecular weight; Mn, Mw and Đ calculated by GPC using pH 6 aqueous eluent.a Calculated using THF GPC. Branching, linear and end group analyses calculated by 1H NMR spectroscopy. | |||||||||
| 1 | PMA | 5.61, 35.08 | 203.9 | 80 | 1430 | 17.7 | 7.4 | 77.4 | 15.2 |
| 2 | PMLA40:60-S | 8.92 | 211.6 | 360 | 3300 | 9.2 | 4.6 | 57.6 | 37.8 |
| 3 | PMLA40:60-S | −0.39 | 214.6 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
2.0 | 7.1 | 57.8 | 35.1 |
| 4 | PMLA40:60-F | 35.04 | 219.3 | 2800 | 2850 | 1.02 | 7.6 | 76.6 | 15.8 |
| 5 | PMLA40:60-F | 45.91 | 221.1 | 1930 | 5540 | 2.9 | 5.0 | 78.9 | 16.0 |
| 6 | PMLA40:60-F | 48.90 | 211.6 | 700 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 810 |
21.1 | 6.2 | 80.5 | 13.3 |
| 7 | PE-PMLA40:60-F | 18.96 | 196.4 | 2840 | 8570 | 3.0 | 8.3 | 61.7 | 30.0 |
| 8 | PGMA40:60-F | 45.71 | 233.8 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740a 740a |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540a 540a |
1.2a | 4.4 | 91.0 | 4.7 |
| 9 | PE-PMGA40:60-F | 32.57 | 229.7 | 110 | 430 | 3.9 | 5.0 | 80.7 | 14.3 |
| 10 | PMLA10:90-F | 10.13, 33.12 | 216.8 | 3120a | 3120a | 1.0a | 3.5 | 60.2 | 36.4 |
Polymer characterisation
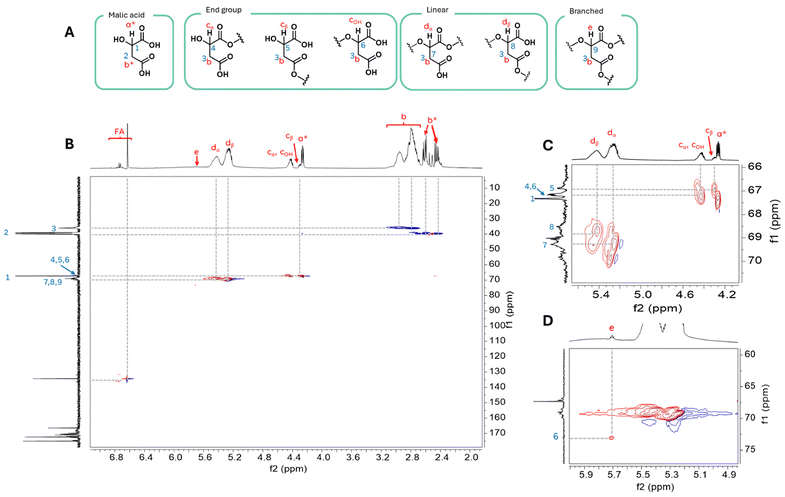
Different topologies of the same polymer backbone can alter the properties and behaviour of the polymeric material. The topology of branched polymers can be elucidated by quantifying the number of branched points and by qualitatively assessing the relative solvation properties. To understand the branching of the polymer, NMR spectroscopy, gel-permeation chromatography (GPC) and thermal analysis (TGA/DSC) were employed. GPC compares the solvation of polymers and quantifies molecular weight (MW) and intrinsic viscosity (IV). Analysis of the synthesised PMA homopolymer by GPC showed that it possessed low molecular weight with high dispersity (Mw 1400 g mol−1, Đ 17.7), suggesting the presence of wide range of oligomer and polymer chain lengths. The DSC thermogram for PMA displayed the presence of two glass transitions (Tg = 5.6 and 35.1 °C), suggesting the presence of two chemically distinct polymeric species.Careful analysis of NMR spectra differentiates malic acid environments within the homopolymer (i.e. chain end, linear or branched units), as shown in Fig. 3. 2D NMR spectroscopy was used to elucidate the structures, with the presence of monomeric MA in the 1H NMR spectrum at 2.38–2.67 ppm (b*; methylene protons) and 4.26 (a*; methine protons) (Fig. 3B) confirmed an incomplete reaction, despite the elevated temperature required to melt the monomer. Resonances observed at 2.67–3.13 ppm were identified as methylene protons from PMA units (b). MA can incorporate into the polymer as either a linear monomer, an end group monomer or as a fully esterified branching monomer (Fig. 3A). Whilst MA can degrade into fumaric acid (FA) forming unreactive end groups, only 4.1% was observed, which given the number of reactive sites was considered a limited amount. Furthermore, MA can esterify through the acid α to the methine proton or through the β-acid. Both contribute to the splitting of the peaks into roughly overlapping peaks of similar size. The two methylene proton environments in the PMA units could not be distinguished from one another by NMR spectroscopy due to similar coupling patterns in 2D NMR spectra. However, the methine protons of MA (a*; 4.26 ppm) and PMA units are readily distinguishable and reveal a corresponding downfield methine resonance shift in the 1H NMR spectra upon esterification of the acid and alcohol groups. Chain end malic acids display methine proton resonance between 4.29–4.48 ppm (COH), coupling to both acid (4 and iv; Fig. S3†). The methine proton of linear segments are observed further downfield as two distinct resonances: the α-MA at 5.36–5.54 ppm (Cα) and β-MA at 5.19–5.36 ppm (Cβ).
The methine proton of the branched group (e; 5.63 ppm) coupled via HSQC to a methine carbon (6; 73.0 ppm), indicative of a more electron withdrawn environment (Fig. S4†). Furthermore, this proton coupled via HMBC to carbonyl ester carbons (7; 170.6 ppm); this lower chemical shift than the carbonyl acid (172.8 ppm) provides further evidence for the assignment. The ratios of 1H resonances identified were used to determine the relative amounts of each monomer environment and thus the degree of branching in the homopolymer. A majority of MA units were linear (77.4%, 7.4% MA branch points, Table 1, entry 1).
The assignments made from homopolymer NMR spectra were correlated to those for copolymers to define the degree of branching (Fig. S5–S9†). Where resonances overlapped, peak deconvolution was used to obtain accurate integrations. Conversions of monomers and the formations were calculated to show a high degree of monomer conversion with less than 6% fumaric acid found within the polymer products (Table S2†). The addition of non-branching comonomers (60% GA or LA) did not significantly alter branching ratios (3.5–8.3%; Table 1).
The polycondensation of MA and LA using standard conditions (6 hours under vacuum at 110 °C) formed PMLA40:60-S (entries 2 and 3, Table 1). 1H NMR spectroscopic analysis revealed the presence of a large proportion of unreacted monomers and the GPC showed a heterogenous polymer population, which explains the large discrepancy in molecular size calculations. Employing more forceful conditions (total time under vacuum of 30 hours at temperatures 110–120 °C) produced PMLA40:60-F with minimal unreacted monomer, lower dispersities and higher Tg values, indicative of a more homogenous polymer product (Fig. S11†). To investigate the reproducibility of this method, the reaction was conducted in triplicate. Variations were observed in Tg and molecular weight measurements which highlights the challenges in scale up of polycondensations. As polycondensations are driven by the removal of byproduct using vacuum, variations in measured pressure varied inside the identical apparatus, which may explain the observed variations. Using forceful conditions gave a consistently larger Mw over PMLA40:60-S and branching analysis suggests 7.1 ± 0.7% of the malic groups were branched.
Increasing the ratio of LA from 60 to 90% was hypothesised to increase the degree of polymerisation, owing to the reduced discrepancy between hydroxyl and carboxyl groups, as per step growth stipulations. The reaction product, PMLA10:90-F, was insoluble in aqueous solutions with marginally larger molecular weight (Mw 3100 g mol−1, Đ 1.0, THF eluent) while not directly comparable to polymers measured using aqueous GPC. Like the homopolymer, a diphasic Tg profile was observed indicating two chemically distinct components (Tg = 10.1, 33.1 °C). Notably, the degree of malic acid branching, as determined by NMR spectroscopy, was lower than that of PMLA40:60-F and PMA. Using GA as comonomer produced PMGA40:60-F with reduced solubility in pH 6 buffer compared to PMLA copolymers, and thus GPC analysis was conducted in THF. MW data for PMGA40:60-F (Mw 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 g mol−1, Đ 1.2) was considerably larger than for other PMA copolymers, which may be attributed to poor solvent interactions in THF. The poor solubility of PMGA40:60-F is consistent with literature reports of GA derived polymers possessing limited solubility.26,27 The relative level of branching between polymers of the same chemical structure can be deduced by decreased Tg with increased branching, owing to the greater free volume of the end groups.
540 g mol−1, Đ 1.2) was considerably larger than for other PMA copolymers, which may be attributed to poor solvent interactions in THF. The poor solubility of PMGA40:60-F is consistent with literature reports of GA derived polymers possessing limited solubility.26,27 The relative level of branching between polymers of the same chemical structure can be deduced by decreased Tg with increased branching, owing to the greater free volume of the end groups.
The plot of log IV as a function of log MW, known as the Mark–Houwink plot,28–30 gives an indication of the density of the polymer structure; a lower intrinsic viscosity for the same molecular weight indicates a more dense and therefore more branched structure. Intrinsic viscosity can also be used to determine degrees of branching, as a decreased IV for a similar MW polymer is expected owing to the more densely packed structure. The addition of tetra-alcohol pentaerythritol (PE) to the copolymerisations was expected to increase crosslinks and branching through condensation of PE alcohols and acid terminating groups. Additionally, the inclusion of PE increases the ratio of hydroxyl to acid groups which is expected to drive the reaction closer to completion and achieve larger molecular weights.31,32 PE-PMLA40:60-F showed larger MW (Mw 8600 g mol−1, Đ 3.0) as well as decreased Tg (19.0 °C) and IV compared to PMLA40:60-F, indicating a more densely branched structure (Fig. 4). Addition of PE to PMGA caused an unexpected decrease in MW (Mw 430 g mol−1, Đ 3.9), a decrease in Tg (32.6 °C) and a product soluble in pH 6 buffer. Such a decrease in MW suggests that transesterification of the ester bonds occurred preferentially to the condensation reactions causing a breakdown of the polymer backbone to form smaller and more soluble products.
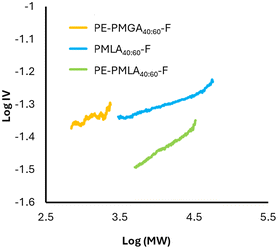 | ||
| Fig. 4 Mark–Houwink plot of aqueous soluble polyesters. IV = intrinsic viscosity; MW = molecular weight. | ||
Rheology
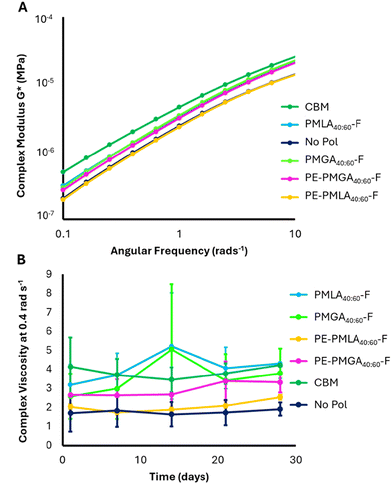
Viscosity and complex modulus were measured as a function of angular frequency. Low angular frequencies in a frequency sweep could be considered representative of bulk behaviour when formulation is stationary in the bottle. The viscoelasticity of the formulations is characterised by the storage modulus (G′, “elastic-like”) and the loss modulus (G′′, “liquid-like”). Complex modulus (G*) describes the entire viscoelastic behaviour of the sample and indicates the resistance to deformation of the sample, a greater G* indicates greater structural resistance to stress.Using carbomer as a model rheological modifier, the formulation method was optimised to standardise the sodium chloride (NaCl) concentration, which affects the self-assembly of the surfactant micelles (Fig. S12†).33,34 Furthermore, it was observed that freshly made formulations contained small air bubbles which artificially elevated the measured viscosity. After settling for 24 hours the viscosity lowered and remained constant for up to 6 days (Fig. S13†). From these studies, 0.65% NaCl was added to all shampoo solutions which were analysed 24 hours after formulation. The synthesised MA co-polyesters achieved initial viscosities between 2.3 and 3.1 Pa s at 0.4 rad s−1 with greater differences at lower angular frequencies (Fig. 5). All polyesters possessed lower initial viscosities than carbomer (4.0 Pa s at 0.4 rad s−1) and viscosity fluctuations, particularly for PMLA40:60-F and PMGA40:60-F (Fig. 5B). Fluctuations are thought to be a result of interactions of the polymer degradation products with micelles (as discussed in later sections) and significant differences between polyesters do not appear to be present. Initial frequency sweep data also showed that all polyesters possessed lower complex moduli than carbomer (Fig. 5A), which apart from PE-PMLA40:60-F, all showed increased structural response in comparison to the no polymer control. No substantial changes in complex modulus were observed over time (Fig. S14†). The increased branching of PE-PMLA40:60-F did not result in higher viscosity or greater structured formulations than PMLA40:60-F. In fact, the viscosity of PE-PMLA40:60-F matched that of the no polymer control, indicating that the increased branching had a deleterious effect on the formulation structure. The crosslinked nature of PE offers the opportunity for quaternary crosslinks in addition to the tertiary branching imbued by the branched malic acid units. These crosslinks may result in a greater density of polymer chains which could limit their ability to swell or entangle, particularly in low concentrations formulations. Therefore, much like the degree of branching, the density of branching can drive the rheological behaviour of these polyesters.
Surprisingly, PMGA40:60-F, which showed poor solubility in aqueous buffers, was able to incorporate with the surfactant mixture (sodium lauryl ether sulfate (SLES) and cocamidopropyl betaine (CAPB)) during the formulation process, as evidenced by the increased viscosity compared to no polymer control, with a comparable complex modulus to more soluble polyesters. The more water-soluble PE-PMGA40:60-F, expected to have greater branching than PMGA40:60-F demonstrated the same trend in decreased viscosity and complex modulus as observed for PMLA40:60-F and PE-PMLA40:60-F. Therefore, the water solubility of the polymer may not correlate with increased shampoo structuring ability.
Polymer stability
Degradable formulation polymers represent an opportunity for improved end-of-life fate. However, in designing degradable aqueous formulation polymers, premature degradation must be avoided to ensure a consistent product. As an ester bond is hydrolysed, a corresponding carboxylic acid is formed, which with enough degradation events will eventually cause a measurable pH decrease. Whilst a decrease in pH has potential negative implications on performance and biocompatibility, it provided a means to quantify potential polyester degradation. To assess the stability of the polymer within the formulations, the pH was tracked over time. The pH of polyester-formulated shampoos decreased over 28 days, with the largest decrease observed in the first 7 days (Fig. 6A). Over the 28-day study period, lactic acid-based copolymers showed greater pH stability (δpH = −0.36 and −0.29 for PMLA40:60-F and PE-PMLA40:60-F, respectively) than the glycolic acid copolymers (δpH = −0.50 and −0.49 for PMGA40:60-F and PE-PMGA40:60-F, respectively). The pH of both carbomer and no polymer controls remained stable which confirms that any pH decrease is a result of the polymer degradation. To confirm the polymer stability, PMLA40:60-F was used as a model to track changes in molecular weight profile when dissolved in an aqueous buffer at pH 4 (Fig. 6B). The pH-dependant degradation of PMLA40:60-F was confirmed by GPC which showed a gradual shift of the lower retention, high molecular weight species, to longer retention times considered to be monomeric species which corresponded to lower calculated peak molecular weights over a period of 7 days. Whilst the pH indicates degradation of the polymer, the rheological frequency experiments do not appear to show changes over time. The viscosity of each polymer showed fluctuations (Fig. 5B) over the 28-day study period, which may be a result of micellar changes in response to the polymer degradation and subsequent changes in pH (Fig. 6A).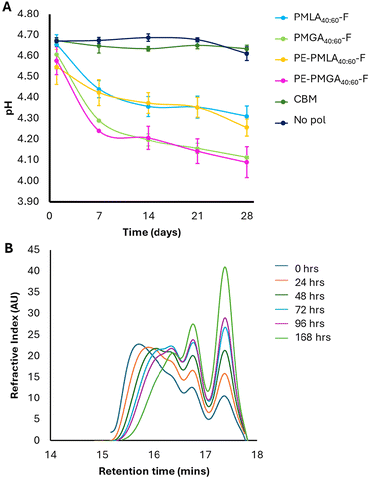 | ||
| Fig. 6 Polymer degradation. (A) pH changes in shampoo formulations as a function of time. (B) Gel permeation chromatograms of PMLA40:60-F when incubated in pH 4 buffer as a function of time. | ||
Most shampoo formulations contain pigment, such as mica, for aesthetic purposes and one role of carbomers is to suspend the pigment in solution. The addition of mica into the polyester formulations yielded a highly reflective and glossy looking finish to the shampoos, whereas incorporation into the carbomer formulation was visibly more granular. Over time, however, the no polymer control and polyesters were less able to suspend the pigment and a layer of precipitate formed at the bottom of the bottle (Fig. S15†). Whilst PMLA40:60-F formulations demonstrated a slower sedimentation process, the stability did not compare to the carbomer formulations. The lack of polyester stability shown by the sedimentation of pigment provides evidence to the rheology data, notably that the polyesters do not have as much resistance or elasticity as the carbomer. Of the polyesters, PMLA appears to possess the greatest structuring ability as measured by rheology, which is verified by the slower sedimentation of the pigment.
We have shown that the structure of PMA copolymers can be varied by reaction conditions, monomer type, monomer molar ratios and addition of a crosslinker. The resultant polymers have variable solubility, molecular weight and levels of branching. The imbalance of carboxylic and hydroxyl groups in PMA homopolymers gave low molecular weight, soluble products with low Tg and 7.4% branching. Forcing conditions in PMLA synthesis afforded variable, but higher, molecular weights with consistent branching (7.1 ± 0.7%). Addition of pentaerythritol increased branching to 8.3%; the polymers had a lower intrinsic viscosity indicating a more dense, branched structure. Switching the comonomer to glycolic acid or reducing the malic acid content reduced aqueous solubility of the polymers. Interestingly, poor solubility correlated with lower levels of branching, highlighting the importance of end group concentration in controlling solubility.
This work also highlights the need to assess formulation stability in developing sustainable polymeric alternatives, with both shelf-life and hydrolytic stability important metrics. While formulations could capably suspend mica, none of the polyesters maintained stable pH or suspended mica throughout the 28-day testing window. Lactic acid copolymers exhibited reduced hydrolytic degradation, although in aqueous buffer degradation could be accelerated.
Rheological assessment of formulations, while showing an increase in viscosity above the no polymer control, showed few links between structure and performance. Polymers with lower molecular weights and higher degree of branching behave more like star polymers in this respect.35 Polymer solubility also had little effect on the formulation rheology, suggesting the shampoo formulations can function across a broader range of polymer hydrophilicities than first hypothesised.
Finally, despite rheological testing being sensitive to small changes in salt concentrations, no difference between fresh and degraded formulations were detected, with surprising stability in rheological performance even through evidenced hydrolytic degradation The key performance indicator for rheological stability appears to be the complex modulus at day 1. Together, this work highlights the challenges of consistencies in polycondensations and the balance between performance and stability in polyesters for aqueous formulations.
Conclusions
In summary, malic acid was used as a branching and acid-enriching monomer in copolymerisations with lactic and glycolic acids. To the best of our knowledge, this is the first report of PMGA copolymers. The branching nature and density of the copolymers produced was confirmed using 1H and 13C 2D NMR, which combined with Mark–Houwink plots to characterise relative topologies. The rheological behaviours of poly(malic acid) copolymers formulated into shampoos in general showed increased structuring ability compared to the no-polymer control, suggesting that the polymer structures contribute to increased viscoelastic behaviour. Polycondensation with an uneven ratio of active groups limited the molecular weight, contributing to the inferior structuring compared to carbomer formulations. While the polyesters studied were initially able to suspend pigments, the stability of these formulations was low, correlating with a decrease in pH over time. Interestingly, no rheological changes were observed over the study period. A significant portion of degradation of the polyesters in aqueous solutions was shown to occur within 7 days which suggests that such polymers may be useful in rehydrating formulations which are solubilised in the domestic setting. This work highlights the challenges of incorporating acid functionalities into acid-labile polymers such as polyesters and can be used to inform the future design of more sustainable PLFs. For formulations which require stability over longer time periods, bonds with greater hydrolytic stability are necessary and is a focus of our ongoing work.Experimental
Malic acid thermal degradation to fumaric acid
DL-Malic acid (1 g, 7.5 mmol) with and without stannous chloride (1%) was added to four vials and each vial heated to 100, 120, 140 °C for 24 hours. The total contents of the vial were dissolved in DMSO-δ6 and analysed by 1H NMR spectroscopy. Ratio of degradation was determined by integration of the fumaric and malic acid resonances and calculation using the following equation.Poly(malic acid) synthesis
DL-Malic acid (40.23 g, 300 mmol) and stannous dichloride (145 mg, 0.36 wt%) were charged in a 3-neck flask fitted with PTFE coated stirrer rod. The reaction vessel was then equipped with a distillation bridge, a vacuum adapter, a valve for nitrogen flow, and a 250 mL collection flask and connected to a Schlenk line. Under a steady stream of nitrogen, the reaction flask was heated to 110 °C on a heating block and stirred at a constant rate of 200 rpm. After 24 hours, the reaction was subjected to vacuum slowly applied over 20 minutes and held. Over the course of the reaction, the water by-product was distilled off into the liquid nitrogen-cooled collection flask. After 6 hours, the reaction was terminated, and the molten polymer was poured out of the reaction vessel and stored in a desiccator.Method for synthesis of PMA copolymers using poly(malic40-lactic acid60) as standard
DL-Malic acid (16.1 g, 120 mmol, 4 eq.), L-lactic acid (16.6 g, 184 mmol, 6 eq.) and stannous dichloride (117 mg, 0.36 wt%) were charged in a 3-neck flask fitted with PTFE coated stirrer rod. The reaction vessel was then equipped with a distillation bridge, a vacuum adapter, a valve for nitrogen flow, and a 250 mL collection flask and connected to a Schlenk line. Under a steady stream of nitrogen, the reaction flask was heated to 110 °C on a heating block and stirred at a constant rate of 200 rpm. After 24 hours, the reaction was subjected to vacuum (<0.16 mbar) which was slowly applied over 20 minutes and held over the course of the reaction, the water by-product was distilled off into the liquid nitrogen-cooled collection flask. For standard (S) polycondensation: the reaction was left under vacuum at 110 °C for 6 hours. For forced (F) polycondensation: The reaction was left under vacuum at 110 °C for 24 hours, then 120 °C for 6 hours. Conversion was monitored using 1H NMR spectroscopy, comparing the relative integrals of monomer and monomer units and summarised in Table S2.† At the end of the reaction, the polymer melt was poured onto a dish and stored in a desiccator.Branching analysis by NMR
Using quantitative 1H NMR the branching analysis was conducted by comparison of the branching integral at 5.66–5.77 ppm as a percentage of all PMA methine-derived resonance integrals; diacid-, α- and β-chain end groups at 4.49–4.26 ppm as well as α- and β-linear groups 5.14–5.55 ppm. Peak deconvolution was conducted where overlaps occurred.Branching analysis by Mark–Houwink plots
Gel permeation chromatography was used to analyse the polymer samples, as described in the Method section. Not all polymers were soluble in aqueous pH 6 buffer and thus such polymers were analysed in a more suitable eluent system, namely THF or pH9 aqueous buffer. Universal calibration allows for the molecular weight of random coil polymers with good solvent interactions to be determined, regardless of the chemical differences between polymer samples and calibration standards. η = KMα Mark–Houwink equation, where η = intrinsic viscosity; M = molecular weight; α and K are constants. Using the relationship between plots of log(η) as a function of log(M) creates Mark–Houwink plots which can be compared between polymers. For polymers with similar chemistries and molecular weights, the intrinsic viscosities can be compared qualitatively; with lower intrinsic viscosities indicating a smaller hydrodynamic volume and thus more branching. As different polymers were soluble in different eluents, and polymer–solvent interactions are key to calculations, comparisons of polymers were conducted between those analysed on the same GPC system.Shampoo formulations
Shampoos were made on a 50 g scale, with 0.2–1.0% polymer concentration. For carbomer control, a 4% polymer slurry was used and diluted in water. For all test polymers, the solid polymer (0.2 g) was directly added to water. The solution was stirred with an overhead stirrer 100 rpm overnight before surfactants sodium lauryl ether sulfate (SLES) and cocamidopropyl betaine (CAPB) were added and stirred until integrated. A solution of sodium EDTA (0.025 g) and sodium benzoate (0.225 g) in 2 mL was added followed by a solution of mica (0.073 g). The solution was made up to 49.67 g with water, pH adjusted to 4.69 by addition of citric acid (50 wt% solution) and NaCl added (0.33 g). The formulation was stirred for 15 min, bottled and left to sit for 16–24 hours before testing.Shampoo degradation
At each rheology testing time point, the pH of the shampoo samples remaining were measured. In addition, the visual appearance of the pigment incorporation and suspension within the shampoo was assessed over the study time.Polymer degradation
PMLA was dissolved in GPC buffers solutions at pH 4.0, 6.0, and 8.2 at a concentration of 10 mg mL−1. At the specified time points, an aliquot (500 μL) was removed, diluted with pH6 GPC buffer (500 μL) to a final concentration of 5 mg mL−1 and analysed using pH 6 GPC to determine MW. Chromatograms were analysed as described above and the MWs plotted as a function of time for polymers in each of the buffers.Author contributions
Christina Picken: conceptualization, writing – original draft, editing. Orla Buensoz: writing – review & editing. Paul Price: funding acquisition, writing – review & editing. Christopher Fidge: funding acquisition, writing – review & editing. Michael P. Shaver: conceptualization, funding acquisition, supervision, writing – review & editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
Unilever provided sponsorship for this project as part of their efforts to improve product and process sustainability.Acknowledgements
The authors would like to thank Unilever and the University of Manchester for their financial support. This work was also supported by the Sustainable Materials Innovation Hub, funded through the European Regional Development Fund OC15R19P and the Henry Royce Institute for Advanced Materials, funded through EPSRC grants EP/R00661X/1, EP/S019367/1, EP/P025021/1, EP/P025498/1.References
- RSC, Royal Society of Chemistry Polymers in liquid formulations Technical report, RSC, 2021.
- RSC, Royal Society of Chemistry Polymers in liquid formulations Summary report, 2021.
- S. Agrawal, P. Gurjar, S. Devadiga, P. Pingale and A. Rajput, in Handbook of Biodegradable Polymers, Jenny Stanford Publishing, 2024 Search PubMed.
- S. Gupta, S. Sharma, A. K. Nadda, M. S. B. Husain and A. Gupta, Mater. Today: Proc., 2022, 68, 873–879 CAS.
- C. A. R. Picken, O. Buensoz, P. D. Price, C. Fidge, L. Points and M. P. Shaver, Chem. Sci., 2023, 14, 12926–12940 RSC.
- C. J. Thompson, N. Ainger, P. Starck, O. O. Mykhaylyk and A. J. Ryan, Macromol. Chem. Phys., 2023, 224, 2200420 CrossRef CAS.
- J. Brady, T. Dürig, P. I. Lee and J.-X. Li, in Developing Solid Oral Dosage Forms, ed. Y. Qiu, Y. Chen, G. G. Z. Zhang, L. Yu and R. V. Mantri, Academic Press, Boston, 2nd edn, 2017, pp. 181–223 Search PubMed.
- F. K. Oppong, L. Rubatat, B. J. Frisken, A. E. Bailey and J. R. de Bruyn, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 041405 CrossRef PubMed.
- T. W. Schwarz and G. Levy, J. Am. Pharm. Assoc., Sci. Ed., 1958, 47, 442–443 CrossRef CAS PubMed.
- N. Sharma, Acad. J. Polym. Sci., 2019, 2, 60–62 Search PubMed.
- S. M. Satti and A. A. Shah, Lett. Appl. Microbiol., 2020, 70, 413–430 CrossRef CAS PubMed.
- N.-A. A. B. Taib, M. R. Rahman, D. Huda, K. K. Kuok, S. Hamdan, M. K. B. Bakri, M. R. M. B. Julaihi and A. Khan, Polym. Bull., 2023, 80, 1179–1213 CrossRef CAS.
- Y. Wang, R.-J. van Putten, A. Tietema, J. R. Parsons and G.-J. M. Gruter, Green Chem., 2024, 26, 3698–3716 RSC.
- M. G. McKee, S. Unal, G. L. Wilkes and T. E. Long, Prog. Polym. Sci., 2005, 30, 507–539 CrossRef CAS.
- G. X. De Hoe, T. Şucu and M. P. Shaver, Acc. Chem. Res., 2022, 55, 1514–1523 CrossRef CAS PubMed.
- Y. Li, L. Huang, S. Zhou, J. Li, C. Qi and H. Tao, Polymer, 2024, 293, 126634 CrossRef CAS.
- T. Su, X. Peng, J. Cao, J. Chang, R. Liu, Z. Gu and B. He, RSC Adv., 2015, 5, 13157–13165 RSC.
- L. Wang, K.-G. Neoh, E.-T. Kang, B. Shuter and S.-C. Wang, Biomaterials, 2010, 31, 3502–3511 CrossRef CAS PubMed.
- Y. Zhang, C. Ni, G. Shi, J. Wang, M. Zhang and W. Li, Med. Chem. Res., 2015, 24, 1189–1195 CrossRef CAS.
- J. Wang, C. Ni, Y. Zhang, M. Zhang, W. Li, B. Yao and L. Zhang, Colloids Surf., B, 2014, 115, 275–279 CrossRef CAS PubMed.
- T. Kajiyama, T. Taguchi, H. Kobayashi, K. Kataoka and J. Tanaka, Polym. Degrad. Stab., 2003, 81, 525–530 CrossRef CAS.
- T. Kajiyama, H. Kobayashi, K. Morisaku, T. Taguchi, K. Kataoka and J. Tanaka, Polym. Degrad. Stab., 2004, 84, 151–157 CrossRef CAS.
- J. Telegdi, L. Trif, E. Nagy, J. Mihály and N. Molnár, J. Therm. Anal. Calorim., 2017, 129, 991–1000 CrossRef CAS.
- M. Nagata, Y. Kono, W. Sakai and N. Tsutsumi, Macromolecules, 1999, 32, 7762–7767 CrossRef CAS.
- T. Kajiyama, H. Kobayashi, K. Morisaku, T. Taguchi, K. Kataoka and J. Tanaka, Polym. Degrad. Stab., 2004, 84, 151–157 CrossRef CAS.
- D. K. Gilding and A. M. Reed, Polymer, 1979, 20, 1459–1464 CrossRef CAS.
- S. Tang, R. Zhang, F. Liu and X. Liu, Eur. Polym. J., 2015, 72, 83–88 CrossRef CAS.
- J. Lesec and M. Millequant, Int. J. Polym. Anal. Charact., 1996, 2, 305–321 CrossRef CAS.
- G. Cleaver, Branching Analysis of Polyethylenes by GPC and Agilent Cirrus Multi Detector Software, Agilent Techologies Inc., 2015 Search PubMed.
- K. McEwan, R. K. Randev, D. M. Haddleton and B. MacCreath, Analysis of Star Polymers Using the Agilent 1260 Infinity Multi-Detector GPC/SEC System, Agilent Technologies Inc., 2016 Search PubMed.
- W. H. Carothers, Trans. Faraday Soc., 1936, 32, 39–49 RSC.
- L. De Keer, P. H. M. Van Steenberge, M.-F. Reyniers and D. R. D'hooge, Polymers, 2021, 13, 2410 CrossRef CAS PubMed.
- T. Majeed, T. I. Sølling and M. S. Kamal, J. Pet. Sci. Eng., 2020, 187, 106871 CrossRef CAS.
- C. A. Paternina, A. K. Londoño, M. Rondon, R. Mercado and S. Muñoz, J. Pet. Sci. Eng., 2019, 182, 106300 CrossRef CAS.
- D. J. A. Cameron and M. P. Shaver, Chem. Soc. Rev., 2011, 40, 1761–1776 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py01382d |
| This journal is © The Royal Society of Chemistry 2025 |