 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insight into the atomic-level structure of γ-alumina using a multinuclear NMR crystallographic approach†
M. Bonifac
Legrady‡
,
Daniel M.
Dawson
 ,
Paul B.
Webb
,
Paul B.
Webb
 and
Sharon E.
Ashbrook
and
Sharon E.
Ashbrook
 *
*
School of Chemistry, EaStCHEM and Centre of Magnetic Resonance, University of St Andrews, North Haugh, St Andrews KY16 9ST, UK. E-mail: sema@st-andrews.ac.uk
First published on 12th March 2025
Abstract
The combination of multinuclear NMR spectroscopy with 17O isotopic enrichment and DFT calculations provided detailed insight into both the bulk and surface structure of γ-Al2O3. Comparison of experimental 17O NMR spectra to computational predictions confirmed that bulk γ-Al2O3 contains Al cations primarily in “spinel-like” sites, with roughly equal numbers of alternating AlVI and AlIV vacancies in disordered “chains”. The work showed that overlap of signals from OIV and OIII species complicates detailed spectral analysis and highlighted potential problems with previous work where structural conclusions are based on an unambiguous assignment (and quantification) of these signals. There was no evidence for the presence of H, or for any significant levels of O vacancies, in the bulk structure of γ-Al2O3. Computational predictions from structural models for different surfaces showed a wide variety of protonated and non-protonated O species occur. Assignment of signals for two types of protonated O species was achieved using variable temperature CP and TRAPDOR experiments, with the sharper and broader resonances attributed to more accessible surface sites that interact more strongly with water and less accessible aluminols, respectively. DFT-predicted 1H NMR parameters confirmed the 1H shift increases with denticity but is also dependent on the coordination number of the next nearest neighbour Al species. Spectral assignments were also supported by 1H–27Al RESPDOR experiments, which identified spectral components resulting from μ1, μ2 and μ3 aluminols. Combining these with 1H–27Al D-HMQC experiments showed that (i) μ1 aluminols are more likely to be bound to AlIV, (ii) μ2 aluminols are coordinated to all three types of Al, but with a higher proportion bound to similar types of Al and (iii) μ3 aluminols are most likely bound to higher coordinated Al species. 1H DQ MAS spectroscopy confirmed no aluminols exist exclusively in isolation but showed that the closest proximities are between bridging aluminols coordinated to AlIV and/or AlV species.
Introduction
Transition aluminas (Al2O3) are among the most widely used oxides in heterogeneous catalysis.1 Alumina is employed as a support material for processes including methanol production and Fischer–Tropsch synthesis, and can be used to catalyse transformations such as alcohol dehydration, skeletal isomerisation or the removal of sulfur from gases in the Claus process.2–6 The versatility of aluminas can be attributed primarily to a high mechanical and chemical stability, high surface area and porosity, and the Lewis-acidity of coordinatively unsaturated surface Al sites. Yet despite their industrial importance, the atomic-scale surface, sub surface and bulk structure of these materials is not well understood.There are many alumina polymorphs or “forms”,7 typically denoted by Greek letters, with the most thermodynamically stable being α-Al2O3. Aluminas can be categorised into two types based on the arrangement of oxygens in the structure, with a hexagonal close packed (hcp) array for the α, κ and χ forms and a face centred cubic close packed (fcc) array for the η, γ, δ and θ forms.4,7–11 The distinct forms are then further distinguished by different arrangements of the Al cations. γ-Al2O3 is one of the most studied transition aluminas, yet its precise structure is still the subject of considerable and ongoing debate, with several different models proposed in the literature.
γ-Al2O3 is often described as a non-stoichiometric or defect spinel,12,13 with a cubic unit cell that contains cation vacancies to maintain charge neutrality, although consensus on the preferential location of the vacancies (i.e., on tetrahedral or octahedral sites) and on any clustering or ordering has yet to be reached. A number of computational studies determined it was energetically most favourable to locate vacancies on the octahedral (AlVI) sites with maximum separation between them.11,14–20 However, other authors concluded from molecular dynamics (MD) simulations that cation vacancies are located at tetrahedral (AlIV) sites,21 while Lee et al. suggested vacancies were present on both types of sites (∼63% AlIV and ∼37% AlVI).22 Many experimental studies have also tried to resolve this controversy, with results from X-ray diffraction (XRD), electron microscopy and 27Al NMR spectroscopy suggesting vacancies were located exclusively on AlVI sites,23–28 while other electron diffraction and NMR studies have been interpreted as evidence for vacancies occurring at AlIV sites.29–33 Some diffraction-based studies have also reported tetragonal distortions of the cubic unit cell.13,29,34,35 It has also been suggested from diffraction data that Al3+ cations could occupy non-spinel interstitial positions (although there is also some debate over whether these are AlIV or AlVI sites),36,37 while work from Paglia et al. (using primarily diffraction, microscopy and computation) proposed a tetragonal model for bulk γ-Al2O3, with over 40% of the Al3+ cations occupying AlVI/AlIV non-spinel sites.35 A different non-spinel model, derived from MD simulations and exhibiting a monoclinic unit cell was described by Krokidis.38
Surprisingly few detailed NMR studies have been carried out on γ-Al2O3, likely reflecting the challenges associated with highly disordered materials.39 The majority that have been performed have largely focussed on 27Al NMR, drawing conclusions from the relative intensities of the AlIV and AlVI signals in magic-angle spinning (MAS) NMR spectra.38,40 Although the 27Al chemical shift depends strongly on coordination number, the broadening arising from the quadrupolar interaction, and as a result of disorder, often limits the extraction of precise parameters, particularly at the lower B0 field strengths used in earlier work.39 It has also been shown that the 27Al MAS NMR spectrum can vary with the methods used for sample preparation, the particle size, surface area and hydration state.38,40 However, the nature of the cation site (i.e., spinel or non-spinel) is not easily determined from the 27Al NMR spectrum (as the immediate local environments in these two are very similar), with cation vacancies occurring only in the second coordination sphere, resulting in a more minor variation in the NMR parameters through small changes in the positions of the neighbouring O2− anions. In contrast, the local geometry around the O2− anions changes significantly with neighbouring cation vacancies, and the arrangement of the surrounding Al3+ cations depends crucially upon whether they occupy spinel or non-spinel positions in the structure. Given the low natural abundance of the NMR-active 17O isotope (0.037%), and the cost and practical challenges associated with isotopic enrichment,4117O NMR spectroscopy of alumina has been much less common,42–47 although early work was performed by Walter and Oldfield (as discussed later).42 Perhaps of most note, is recent high-field NMR work using double-quantum NMR spectroscopy to identify different types of surface and sub surface species, which indicated a non-random distribution of O species, but used the earlier assignment of the signals proposed by Walter and Oldfield.43
In this work we combine multinuclear (1H, 17O and 27Al) and multidimensional NMR spectroscopy of γ-Al2O3 with periodic planewave density functional theory (DFT) calculations of potential structural models of the bulk and surface structure to gain insight into the atomic-scale structure of this important, yet poorly understood, material. This NMR crystallographic approach is vital for interpreting the spectroscopic signals observed and showing that these can often result from an overlap of resonances from different crystallographic environments, which has hindered spectral assignment in previous work. We show that 17O NMR spectroscopy is a sensitive probe of the detailed local structure, and of the surface structure in particular, providing, when combined with computation, one of the most successful approaches to date for structural characterisation of this industrially important material.
Methodology
Synthesis and isotopic enrichment
Commercial boehmite-derived γ-Al2O3 was obtained from Sasol UK Ltd (brand name Puralox) and used either as received, after dehydration or after 17O enrichment. Samples were dehydrated at 150–300 °C by heating 150 mg in a quartz vial in a sand bath on a hotplate. For dehydration at higher temperatures 100–200 mg of sample was transferred into a quartz vial and heated under vacuum at 500 or 700 °C for 12–24 h in a tube furnace. After dehydration, samples were packed into commercial ZrO2 rotors in a glovebox under an inert N2 atmosphere. Isotopic enrichment was carried using post-synthetic exchange with 70% 17O2 (g). Approximately 150 mg of sample was transferred into a quartz vial and immersed in liquid N2, before exposure to 17O2 (g), which then condensed in the pores. The vial was then returned to room temperature, placed in a tube furnace and heated at a temperature between 500 and 950 °C for 12–72 h. Samples isotopically enriched at X °C will be referred to as γ-Al217O3(X °C) throughout. Although the absolute level of enrichment was not measured directly, this is estimated to be ∼25–30% by comparison to materials with known enrichment levels.NMR spectroscopy
Solid-state NMR spectra were acquired either in house using Bruker Avance III instruments equipped with 9.4 or 14.1 T wide-bore magnets, at the Scottish High-field NMR facility using a Bruker Avance NEO spectrometer equipped with a standard-bore 18.8 T magnet, or at the UK High-Field NMR facility using a Bruker Avance III spectrometer equipped with a 20.0 T wide-bore magnet. Powdered samples were packed into ZrO2 rotors and rotated at MAS rates of 5–40 kHz or using conventional double- or triple-resonance probes. NMR spectra are shown referenced relative to H2O(l) for 17O, 1 M Al(NO3)3 (aq) for 27Al (determined using a secondary reference of aluminium acetylacetonate (δiso = 0 ppm)) and TMS for 1H (determined using a secondary reference of L-alanine (δ(NH3+) = 8.5 ppm)). Spectra were acquired at ambient temperature unless otherwise stated. High-resolution NMR spectra were acquired using MQMAS48 (27Al) and DQF-STMAS49,50 (17O) experiments. For more detailed experimental parameters see figure captions and Section S1 in the ESI.†Computation
Periodic planewave DFT calculations were carried out using the CASTEP code (version 16.12).51–53 Calculations were performed using the PBE exchange correlation functional54 and the semi-empirical dispersion correction scheme of Tkatchenko and Scheffler.55 Core-valence interactions were described by ultrasoft pseudopotentials, accounting for scalar relativistic effects using ZORA.56 Planewave energy cutoffs of 45 or 60 Ry were used for geometry optimisation, and 60 Ry for all NMR calculations, with the first Brillouin zone sampled using a Monkhorst–Pack grid57 with a reciprocal space grid spacing of 0.04 2π Å−1 in all cases. Initial structural models by generated by introducing vacancies into an undistorted (Al3O4) spinel structure and modified as described below. In the geometry optimization all atomic coordinates and unit cell parameters were allowed to vary for bulk models, while unit cell parameters were constrained for the surface models. Further models of bulk and surface structures were generated as described in the text and Sections S3–S6 of the ESI.†NMR parameters were calculated using the gauge-including projector augmented wave (GIPAW51) approach to reconstruct the all-electron wavefunction in the presence of a magnetic field. Calculations provide the absolute shielding tensor (σ), J coupling tensor (J) or electric field gradient tensor (V). Diagonalization provides their respective principal components, where σ11 ≤ σ22 ≤ σ33, J11 ≤ J22 ≤ J33 and |Vxx| ≤ |Vyy| ≤ |Vzz|. The isotropic shielding is given by σiso = (1/3)Tr(σ) and the predicted isotropic chemical shift by δiso = σref – σiso. Reference shieldings (σref) were determined by comparing experimental shift and calculated shielding for α-Al2O3 (27Al and 17O) and TMS (1H).42,58 The quadrupolar coupling constant, CQ = eQVZZ/h was obtained using nuclear quadrupole moments of 146.6 and −25.58 mb, for 27Al and 17O, respectively.59 From the quadrupolar magnitude, CQ, and asymmetry, ηQ = (VXX – VYY)/VZZ, the quadrupolar product PQ = CQ (1 + ηQ2/3)1/2 can be determined. The isotropic J coupling is given by (1/3)Tr(J).
Density matrix simulations were performed using SIMPSON.60 Details on the spin systems considered and the parameters used are provided in Section S10 of the ESI.†
Results and discussion
17O NMR spectroscopy: bulk structure of Al2O3
Fig. 1 shows structural models for γ-Al2O3 based on a cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m unit cell containing an fcc arrangement of O2− anions (on the 32e Wykoff positions), indicating both the spinel (8a and 16d) and non-spinel (48f and 16c) cation positions.13 In a spinel structure without cation vacancies, every O2− anion is tetrahedrally coordinated and surrounded by one AlIV and three AlVI cations (as shown in Fig. 1c). To aid the interpretation of local structure in the following discussion, this O environment will be denoted as OIV(6664). To maintain charge balance in the spinel-based model of Al2O3, 8/3 Al3+ vacancies are needed for every 32 O2− anions. Introducing an AlVI vacancy generates six three-coordinate O species (denoted OIII(664)), while an AlIV vacancy generates four OIII(666) sites. For non-spinel structures, there are 11 48f and 16c cations around each O2− potentially giving rise to many different O local environments, as seen in Fig. 1c.
m unit cell containing an fcc arrangement of O2− anions (on the 32e Wykoff positions), indicating both the spinel (8a and 16d) and non-spinel (48f and 16c) cation positions.13 In a spinel structure without cation vacancies, every O2− anion is tetrahedrally coordinated and surrounded by one AlIV and three AlVI cations (as shown in Fig. 1c). To aid the interpretation of local structure in the following discussion, this O environment will be denoted as OIV(6664). To maintain charge balance in the spinel-based model of Al2O3, 8/3 Al3+ vacancies are needed for every 32 O2− anions. Introducing an AlVI vacancy generates six three-coordinate O species (denoted OIII(664)), while an AlIV vacancy generates four OIII(666) sites. For non-spinel structures, there are 11 48f and 16c cations around each O2− potentially giving rise to many different O local environments, as seen in Fig. 1c.
Fig. 2a shows 17O MAS NMR spectra of γ-Al217O3 (500 °C), enriched for 48 h and then left open to the atmosphere, at three different B0 field strengths. The increase in field strength (and the consequent reduction in the second-order quadrupolar broadening) increases the spectral resolution and enables approximately four oxygen signals to be distinguished. The two sharper resonances at higher shift have been suggested to correspond to non-protonated oxygen species, while the two broader signals at lower shift have been previously assigned to protonated oxygens (i.e., adsorbed water and hydroxyl groups).42–45 The signals from the non-protonated oxygens do not become significantly sharper at higher field, suggesting that the major contribution to the line broadening is a distribution of chemical shifts as a result of small variations in the local environments, rather than from a significant second-order quadrupolar interaction. Early 17O NMR of γ-Al2O3 by Walter and Oldfield42 assigned the non-protonated 17O signal at higher shift (∼70 ppm at 20.0 T) to OIV and that at ∼55 ppm (at 20.0 T) to OIII. Although this assignment is generally accepted in the literature,43–46 it will be shown later that this is likely to be an oversimplification. However, the “OIV”![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) “OIII” ratio (1
“OIII” ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) obtained from 17O spectra in early work was later used46 to conclude that the cation vacancies in a spinel structure must be located exclusively at the octahedral positions (if they were at the tetrahedral sites, a 2
1) obtained from 17O spectra in early work was later used46 to conclude that the cation vacancies in a spinel structure must be located exclusively at the octahedral positions (if they were at the tetrahedral sites, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio would be obtained). This ratio was obtained by fitting the spectrum using Gaussian lineshapes, i.e., without considering the characteristic “tails” to low shift usually seen for quadrupolar nuclei in disordered environments,39 and neglecting any effects of differential relaxation rates or differing nutation rates (i.e., spectra were not acquired using a short flip angle pulse). Although the spectra in Fig. 2a in this work were acquired using sufficiently long recycle intervals (see Section S1 of the ESI†), it should be noted that the material was isotopically enriched by post-synthetic exchange with 17O2 (g), leading to possible non-quantitative incorporation of 17O. Although we would expect any differences to be insignificant, particularly when higher temperatures are used, any detailed structural conclusions in this work should not be based on spectral signal intensities alone but from the range and combination of experimental and computational evidence provided.
1 ratio would be obtained). This ratio was obtained by fitting the spectrum using Gaussian lineshapes, i.e., without considering the characteristic “tails” to low shift usually seen for quadrupolar nuclei in disordered environments,39 and neglecting any effects of differential relaxation rates or differing nutation rates (i.e., spectra were not acquired using a short flip angle pulse). Although the spectra in Fig. 2a in this work were acquired using sufficiently long recycle intervals (see Section S1 of the ESI†), it should be noted that the material was isotopically enriched by post-synthetic exchange with 17O2 (g), leading to possible non-quantitative incorporation of 17O. Although we would expect any differences to be insignificant, particularly when higher temperatures are used, any detailed structural conclusions in this work should not be based on spectral signal intensities alone but from the range and combination of experimental and computational evidence provided.
 | ||
| Fig. 2 17O NMR of γ-Al2O3 enriched by post-synthetic exchange with 17O2 (g). (a) MAS NMR spectra of γ-Al217O3(500 °C), acquired at the magnetic fields shown. (b) 17O (20.0 T, 30 kHz) DQF-STMAS49 NMR spectrum of γ-Al217O3(950 °C). For detailed experimental parameters see Section S1 of the ESI.† | ||
Fig. 2b shows the 17O STMAS spectrum of γ-Al2O3(950 °C), a sample enriched by heating in a 70% 17O2 atmosphere at 800 °C for 6 h and then increasing the temperature to 950 °C before heating for further 14 h (Fig. S2.1 in the ESI† shows a direct comparison of the 17O MAS NMR spectra of γ-Al2O3 samples enriched at the two temperatures). The higher temperature leads to more efficient and more uniform enrichment (and therefore higher sensitivity) but results in small impurities of δ-Al2O3 and θ-Al2O3,9,10 as discussed in more detail in Section S2 of the ESI.† The spectrum shows two signals from the non-protonated O species – one centred at δ1 of ∼35 ppm and a second centred at higher δ1 (∼37. 5 ppm). Note that the signals from the protonated O species are not seen in the STMAS spectrum owing to very rapid relaxation (vide infra). To explore the likelihood of Al3+ cations occupying spinel or non-spinel environments, DFT calculations were used to predict 17O NMR parameters for two non-spinel structural models proposed by Paglia (using 3 × 1 × 1 supercells of the cubic and tetragonal unit cells).61 Schematics of the structural models used are shown in Section S3 of the ESI.† As shown in Fig. 3a, the oxygen sites present can be divided into two types based on their first coordination sphere: “spinel” sites (i.e., local environments that would occur in structures where all cations are restricted to spinel positions) and “non-spinel” sites (i.e., local environments that can occur when this restriction is not in place). Fig. 3b overlays the predicted positions (δ1, δ2) of the centre-of-gravity of the signal from each O species on the experimental 17O STMAS spectrum of γ-Al217O3(950 °C) from Fig. 2b. The vast majority of the signals predicted for “non-spinel” species fall outside of the regions where signal is observed experimentally, while the majority of the signals predicted for the “spinel” species are in much better agreement with the experiment, indicating that γ-Al217O3(950 °C) is unlikely to contain a high fraction of cations in non-spinel positions. It can also be seen that the signals from OIII(644), shown in bright yellow (and also present in the low level δ-Al2O3 and θ-Al2O3 impurities9,10), overlay well with the shoulder seen on the STMAS lineshape, suggesting that this part of the signal may have a contribution from these impurities, as discussed in the ESI.† A second non-spinel model that is widely used for computational studies (as it contains only 14 atoms in the primitive unit cell) is that of Digne et al.,62 which has three types of O environment (OIV(6664), OIV(6666), and OIII(664), shown in Fig. 3 in red, orange and cyan, respectively).
 | ||
| Fig. 3 (a) Plots showing the overlay of (δ1, δ2) centre-of-gravity shifts predicted (using DFT) for different O environments in 3 × 1 × 1 supercells of cubic and tetragonal non-spinel structural models of γ-Al2O3 from Paglia,61 with the 17O (20.0 T, 30 kHz) DQF-STMAS spectrum of γ-Al217O3(950 °C) from Fig. 2b. The colours denote the local oxygen environments present (shown in (b)), which have been separated into “spinel” and “non-spinel”, as defined in the text. See the ESI† for the full structural models. | ||
Given the poor agreement with 17O NMR experiments for O species that can only occur in non-spinel models of γ-Al2O3, only spinel models have been considered from this point forwards. Most previous computational studies on spinel-based models of γ-Al2O3 have used the smallest unit cell that contains an integer number of vacancies (i.e., a 3 × 1 × 1 supercell of the 14 atom primitive cell with two cation vacancies).11,14–20 These suggested that lower energy structures were obtained when the cation vacancies were on the AlVI sites and had the greatest separation between them. Here, we have used a larger model (a 3 × 1 × 1 supercell of the cubic spinel cell, with 160 atoms and eight cation vacancies). See the ESI† for more detail. Assuming the two requirements suggested above are retained, this model (Model SP1 in the ESI†) contains two types of O species; OIV(6664) and OIII(664). As shown in Fig. 4a, the DFT-predicted position of the 17O STMAS signals do not agree well with the experimental data. Although the points corresponding to OIV (red) match reasonably well with the ridge centred at lower δ1, the OIII (cyan) points match poorly with the second ridge of signal (appearing at much higher (δ1, δ2) than seen in experiment), suggesting that at least one of the assumptions stated above is incorrect. If AlVI vacancies are retained, but the requirement for maximum separation between them is not strictly enforced (see Model SP2 in the ESI†), the OIII signals predicted by DFT (see Fig. 4b) cover a much wider spectral range and overlap with some of the signal seen experimentally. The OIII(664) sites with lower δiso values correspond to O species which are next to one AlVI vacancy but are also close to a second. For O sites with δ2 between 44 and 58 ppm, (corresponding to δiso between 50-64 ppm) the next nearest vacancy is ∼3.4 Å away, while for those with δ2 > 62 ppm (corresponding to δiso > 68 ppm), this distance is ∼4.3 Å. This suggests that γ-Al217O3(950 °C) exhibits preferential clustering of the cation vacancies, contrary to the conclusions of previous computational studies.11,14–20 However, the O sites present in the models used for the DFT calculations have slightly larger PQ values than those seen experimentally (and are more similar to the OIII(664) sites in δ-Al2O3 and θ-Al2O3).
 | ||
| Fig. 4 Plots showing the overlay of (δ1, δ2) centre-of-gravity shifts predicted (using DFT) for different O environments in 3 × 1 × 1 supercells of cubic spinel structural models of γ-Al2O3, with the 17O (20.0 T, 30 kHz) DQF-STMAS spectrum of γ-Al217O3(950 °C) from Fig. 2b. In (a), vacancies appear only on AlVI sites with maximum separation between them (see Model SP1 in the ESI†), while in (b), the distance between the vacancies is allowed to vary (see Model SP2 in the ESI†). | ||
If the requirement for only AlVI vacancies in the spinel model is also relaxed, and AlIV vacancies are introduced, different O environments are then observed, as shown in Fig. 5 (see Models SP3-5 in the ESI†). Fig. 5a–c show the DFT-predicted position of the 17O STMAS signals for the O species in three such models. The signals seen for a new O species, OIII(666), shown in green, are very similar to the red points corresponding to OIV(6664), and in the 17O MAS spectrum would primarily contribute to the O signal at the highest δ (previously assumed by Walter and Oldfield42 to result only from OIV). It is also clear that OIII(664) signals fall into two distinct groups; those shown in cyan are very similar to those seen previously in Fig. 4 for structures where only AlVI vacancies are present, and those shown in magenta, which have a smaller PQ value (∼3 MHz compared to ∼4 MHz) and are much closer to the values seen experimentally, matching well with the second ridge centred at higher δ1. The difference between these two chemically similar species can be seen in Fig. 5, which shows that the second type of OIII(664) (magenta) is next to an AlVI vacancy, and the next nearest AlIV along the (110) planes is also missing. However, for the OIII(664) (cyan) this AlIV is present. The OIII(664) magenta sites match much better with experimental measurements than the OIII(664) cyan sites, suggesting that it is likely that alternating AlVI and AlIV vacancies are present in γ-Al217O3(950 °C), and that there are roughly equal numbers of these.
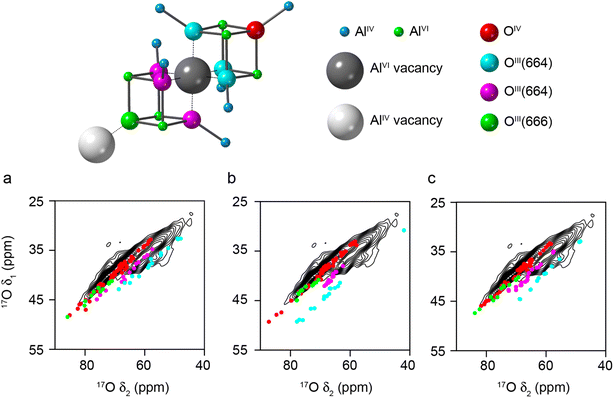 | ||
| Fig. 5 Plots showing the overlay of (δ1, δ2) centre-of-gravity shifts predicted (using DFT) for different O environments in three different structural models (Models SP3-5, for (a), (b) and (c), respectively) generated from 3 × 1 × 1 supercells of cubic spinel structural models of γ-Al2O3, with the 17O (20.0 T, 30 kHz) DQF-STMAS spectrum of γ-Al217O3(950 °C) from Fig. 2b. In each structure, vacancies are present on AlVI and AlIV sites with maximum separation between them (see Fig. S3.4 of the ESI†). The key above shows the atom types and vacancies present. | ||
Structural models with alternating AlVI and AlIV vacancies can be generated (see Models SP6-8 in the ESI†). However, by definition, these must contain a line of AlIV–AlVI–AlIV vacancies (see Fig. 5), although the next AlVI vacancy can then be in the direction of any of the three neighbouring O atoms. In a real crystal this would generate complex vacancy “trajectories” and lead to many different O species in the resulting disordered solid. However, when limited to a 160 atom supercell this results in only a small number of possibilities and ordered structural models, leading to a relatively small number of distinct O species, which do not represent well the range that would appear in reality. The DFT-predicted 17O STMAS signals for these more ordered models are shown in Fig. S3.5 of the ESI.† The DFT calculations reproduce the experimental PQ values well, but the δiso values show a poorer match to experiment for the OIII(664) sites shown in magenta in Fig. 5, owing to the limited cell size and the ordered structures that result. In these models the OIII(664) species are either very close to, or very far from, a third Al vacancy (giving rise to very low or very high δiso values, respectively). In a real material, a range of distances would be expected owing to the more disordered longer-range structure, leading to intermediate δiso values in better agreement with experiment; however, this would require much more computationally costly calculations to explore in detail.
Although it is well known that the surface of γ-Al2O3 is hydroxylated and hygroscopic, some studies have also suggested the presence of H in the bulk structure (from the boehmite precursor most commonly used in synthesis).63,64 The only NMR spectroscopic study that supports this proposition was carried out a number of years ago using continuous wave (CW) NMR of static samples, and concluded that 37% of all protons are located in the bulk γ-Al2O3.65 The more recent work of Paglia66 did not report any evidence for significant levels of bulk H, a conclusion which is supported further by the fact that γ-Al2O3 can also be produced from non-hydrogenous precursors.67 To investigate the possibility of significant levels of 1H in the bulk structure of γ-Al2O3, DFT calculations were carried out for four further structural models (Models SPH1-4), generated as described in Section S4 of the ESI† by replacing one Al3+ cation with three H+. The DFT-predicted positions of the 17O STMAS signals are shown in Fig. S4.1 of the ESI.† The substitutions generate a new type of O species that acts as a hydrogen bond acceptor (H–OIII(664)), which have similar NMR parameters to their non-hydrogen-bonded counterparts. No experimental 17O STMAS signals are seen for the protonated surface O species, owing to the rapid relaxation that results from dynamics between aluminols (i.e., Al–OH species) and exchange with water (see later). However, such dynamics are likely to be much less pronounced for H in the bulk, and the corresponding protonated O species may be expected to appear in the 17O STMAS spectrum. Fig. S4.1† shows no evidence for these signals (also predicted to be at much lower δ) experimentally. There is also little evidence for the presence of bulk H in the experimental 1H DEPTH MAS NMR spectrum of dehydrated γ-Al217O3(550 °C) shown in Fig. S4.2 of the ESI,† with no significant signal seen at the DFT-predicted δiso values. In general, therefore, there is no evidence that γ-Al2O3 contains a significant amount of H in the bulk structure.
Although it is generally accepted that the O2− anions form an fcc array, the presence of stacking faults has been reported68 and the presence of oxygen vacancies also proposed.69,70 To investigate the latter, two further models of γ-Al2O3 were considered, with the removal of 3 O2− and 2 Al3+ cations from Model SP6. In Model SPV1 (see Section S5 of the ESI†) the O vacancies are more remote in the structure, whereas in Model SPV2 an “Al2O3 unit” was removed with all of the vacancies clustered. In both cases, many new types of oxygen environments are created that exhibit NMR parameters in poor agreement with the signals in 17O STMAS spectra (see Fig. S5.1 in the ESI†). This suggests that if such vacancies are present in bulk γ-Al2O3, they most likely occur only at very low levels.
The results discussed so far present an overall picture of bulk γ-Al2O3 as having a fcc array of oxide ions with Al3+ occupying only spinel sites, with clusters and/or chains of Al vacancies on both AlIV and AlVI sites. The bulk appears to be anhydrous and there is little evidence of any significant disruption to the fcc array of O atoms (either stacking faults or clustered vacancies). While it is clear that OIV and OIII species are present, our computational work suggests that these signals may overlap more than had been previously suggested (and the assignments used in previous work may be a simplification).
17O NMR spectroscopy: surface structure of Al2O3
Although there has been considerable debate over the structure of bulk γ-Al2O3, an understanding of the surface structure is more relevant from a catalysis perspective, and has been the focus of much previous computational work.5,15,19,21,62,64 The three surface planes of γ-Al2O3, (100), (110) and (111) as indexed in the cubic spinel unit cell,5 are shown in Section S6 of the ESI,† where the surface arrangement of the O2− anions and Al3+ cations are also highlighted. Unlike the (100) and (100) planes, normal to the (111) planes there are alternating layers of O2− and Al3+ ions. Considering cation vacancies leads to a significantly larger number of inequivalent cleavages that could be performed. Structural models of “γ-Al2O3 slabs” for DFT calculations were generated from the bulk models of γ-Al2O3 by cleaving along the three surface planes as described in the ESI,† giving rise to 36 structural models (22 fully and 14 partially hydroxylated structures, each with two surfaces).Fig. 6a overlays the 17O DFT-calculated δ2 centre-of-gravity shifts (i.e., including both δiso and any second-order quadrupolar shift) on the experimental 17O MAS NMR spectrum of γ-Al217O3(500 °C). This is shown separately for oxygens in the surface layer, the sub surface layer and five layers from the surface. See Fig. S6.3 in the ESI† for the corresponding plots for O in layers three and four from the surface, and for plots of the (δ1, δ2) centre-of-gravity shifts for the non-protonated O in each of the five layers shown overlaid on the 17O STMAS spectrum of γ-Al217O3(950 °C). For O species that are four and five oxide layers from the surface (not including any water layer), the NMR parameters are very similar to those calculated for O in the bulk models of γ-Al2O3 discussed above, although the breaking of symmetry by the surface cleavage gives rise to a larger distribution of NMR parameters. The predicted shifts agree well with those seen experimentally. However, three layers from the surface (see Fig. S6.3 in the ESI†) a much greater number of different types of non-protonated O species are seen (owing to the different coordination numbers of the neighbouring Al cations, many of which are now reduced by the cleavage). Three layers from the surface there is also an increasing number of O with lower δiso (and δ2) shifts, providing insight into the observation above that the most downfield resonance in the 17O MAS NMR spectrum increases in intensity when higher enrichment temperatures are used, enabling diffusion of 17O deeper into the material. In the sub surface layer, there is a large variety of protonated and non-protonated O environments present, which span the experimentally observed shift range. However, there are several points that fall outside of this range, notably for OIV(6554) and OIII(655). These environments primarily occur in partially hydroxylated structures and so are not generally likely to be observed experimentally, where surfaces are fully hydroxylated and hydrated. Fig. 6b shows the overlay of the DFT-predicted (δ1, δ2) centre-of-gravity shifts for non-protonated O environments in the sub surface layers of the slab models of γ-Al2O3, with the 17O (20.0 T, 30 kHz) DQF-STMAS spectrum of γ-Al217O3(950 °C) from Fig. 2b. This shows relatively few points outside of the region where signals are observed experimentally, an observation which is perhaps illustrated more clearly in Fig. 6c, where Lorentzian lineshapes are applied to each of the points in Fig. 6b and summed. This result should not be interpreted as an indication that γ-Al217O3(950 °C) is enriched in 17O only as far as the sub surface layer but suggests that the variations in local geometry that arise from the presence of surfaces cannot be modelled well in the bulk models of γ-Al2O3 used above.
 | ||
| Fig. 6 (a) Plots showing the overlay of the DFT-predicted NMR shifts (δ2) for all O in the slab models of γ-Al2O3, separated by the number of layers from the surface, with the 17O (20.0 T, 20 kHz) MAS NMR spectrum of γ-Al217O3(500 °C) from Fig. 2a. The red line results from co-adding Lorentzian lineshapes simulated for each O species, centred at the δ2 centre-of-gravity with a FWHH proportional to CQ. (b and c) Plots showing the overlay of (δ1, δ2) centre-of-gravity shifts predicted (using DFT) for non-protonated O environments in sub surface layer of the slab models of fully hydroxylated γ-Al2O3, with the 17O (20.0 T, 30 kHz) DQF-STMAS spectrum of γ-Al217O3(950 °C) from Fig. 2b. In (c), the red contours represent the spectrum simulated from the sum of Lorentzians for each of the points in (b), with a FWHH of 1.5 ppm in δ1 and CQ/MHz ppm in δ2 the ESI.† | ||
The 17O MAS NMR spectrum of γ-Al217O3(500 °C) acquired at 20.0 T, shown in Fig. 7a, contains two signals from primarily protonated O species; a broad resonance at ∼20 ppm and a sharper signal at −2 ppm (see Fig. S7.1 in the ESI† for results at 14.1 T). These signals have very short relaxation times (T1 of ∼1.6 and ∼0.4 ms, respectively, as measured by saturation recovery experiments, and T2′ of ∼0.6 and ∼0.1 ms, measured using rotor-synchronised single spin-echo experiments). This contrasts with the relaxation seen for the signals at higher shifts from non-protonated O (T1 of ∼1–1.5 s and T2′ of ∼10 ms), reflecting the involvement of the protonated O species in dynamic surface processes and their interactions with water. From Fig. 6a, the surface layers of the γ-Al2O3 models contain only protonated O, with the majority of calculated δ2 values lower than the signals seen experimentally. However, the δ2 values for the protonated O in the sub surface layer match very well with the experimentally observed broader resonance, suggesting this results from less accessible O sites. The sharper resonance then likely results from averaging of the more accessible surface sites (which is also reflected in the faster T1 and T2 relaxation times for this signal). This is also supported by the observation that only the broader signal is seen in 1H–17O cross polarisation (CP) spectra, as shown in Fig. 7a. Further evidence to support the assignment of the sharp signal at −2 ppm to O in the surface layers is given in Fig. S7.2 in the ESI,† where it can be seen to vary in intensity with time and storage conditions. The 17O MAS NMR spectra of γ-Al217O3(500 °C) acquired at different temperatures, and shown in Fig. 7b, reveal a significant change in linewidth of the sharp signal at lowest δ2, with broader lines seen at lower temperatures (suggesting dynamics are in the fast-intermediate regime). At −60 °C, the sharp resonance becomes overlapped with the broader signal at higher δ2. Very little change is seen in the corresponding CP MAS NMR spectra, which still contain only the broader signal.
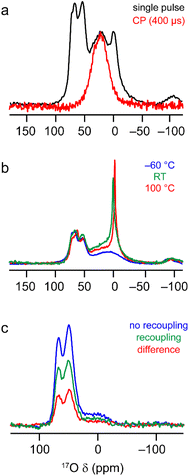 | ||
| Fig. 7 17O MAS NMR of γ-Al217O3(500 °C). (a) (20.0 T, 20 kHz) MAS and CP MAS NMR spectra acquired with τCP = 400 μs. (b) (20.0 T, 20 kHz) MAS NMR spectra acquired at varying temperatures. (c) (14. 1 T, 14 kHz) 17O–27Al TRAPDOR spectra acquired with τrec = 16 τR. See Section S7 of the ESI† for more detailed experimental parameters and information on normalisation and scaling. | ||
The computational results suggest that the broad signal for protonated O (at ∼20 ppm at 20.0 T) in the 17O MAS NMR spectrum arises primarily from aluminols (Al–OH species). However, there is an argument that this signal could, alternatively, be assigned to less mobile or strongly hydrogen bonded H217O molecules. These species are likely to be much further from Al3+ cations and so the two types of signal could potentially be distinguished using 17O-27Al dipolar dephasing measurements. Fig. 7c shows 17O-27Al TRAPDOR71 spectra of γ-Al217O3(500 °C), which demonstrate the presence of the broad signal from protonated O in the dephasing difference spectrum and confirm its assignment to aluminols. See the ESI† for a more detailed discussion of the recoupling experiments.
27Al and 1H NMR spectroscopy of Al2O3
As discussed briefly above, many of the previous spectroscopic studies of γ-Al2O3 have focussed on 27Al NMR, which is sensitive to the Al coordination number (with AlIV, AlV and AlVI species found at δ = 80 to 50 ppm, 40 to 20 ppm and 20 to −20 ppm, respectively, although exact shifts for a material are field dependent as I = 5/2 for 27Al). The 27Al MAS NMR spectrum (acquired with a short flip angle) of γ-Al2O3 in Fig. 8a shows two broad signals, corresponding to AlIV and AlVI, in a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, with both exhibiting the tails to low frequency characteristic of disordered materials.39 The 27Al MQMAS spectrum in Fig. 8c confirms distributions of both δiso and PQ are present (with average values as given in Section S8 of the ESI†). 27Al–17O HMQC72 experiments were performed both without (i.e., J-HMQC) and with (D-HMQC) dipolar recoupling (using SR421
2 ratio, with both exhibiting the tails to low frequency characteristic of disordered materials.39 The 27Al MQMAS spectrum in Fig. 8c confirms distributions of both δiso and PQ are present (with average values as given in Section S8 of the ESI†). 27Al–17O HMQC72 experiments were performed both without (i.e., J-HMQC) and with (D-HMQC) dipolar recoupling (using SR421![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73). For D-HMQC (as shown in Fig. 8d), all O signals correlate with AlIV and AlVI species as expected. However, for J-HMQC experiments the correlation is much stronger with AlIV than with AlVI. This somewhat surprising result may be understood by considering the values of the 27Al–17O J couplings for these two environments. These were calculated for Model SP6 of bulk γ-Al2O3, showing that AlVI species have much smaller J couplings (e.g., ∼3–10 Hz) than AlIV (e.g., ∼7–20 Hz), reflecting the higher ionicity (and consequently longer Al–O bonds) for AlVI. See Section S9 of the ESI† for more detail. This would require longer HMQC evolution periods for efficient magnetisation transfer for AlVI, although accessible values are ultimately limited by T2 (3–5 ms).
73). For D-HMQC (as shown in Fig. 8d), all O signals correlate with AlIV and AlVI species as expected. However, for J-HMQC experiments the correlation is much stronger with AlIV than with AlVI. This somewhat surprising result may be understood by considering the values of the 27Al–17O J couplings for these two environments. These were calculated for Model SP6 of bulk γ-Al2O3, showing that AlVI species have much smaller J couplings (e.g., ∼3–10 Hz) than AlIV (e.g., ∼7–20 Hz), reflecting the higher ionicity (and consequently longer Al–O bonds) for AlVI. See Section S9 of the ESI† for more detail. This would require longer HMQC evolution periods for efficient magnetisation transfer for AlVI, although accessible values are ultimately limited by T2 (3–5 ms).
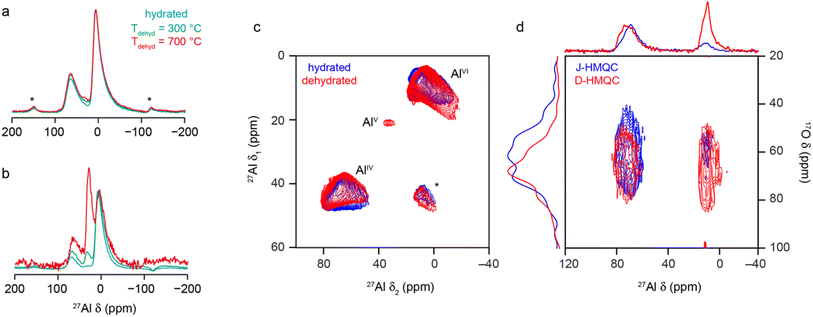 | ||
| Fig. 8 27Al (9.4 T, 14 kHz) (a) MAS and (b) CP MAS NMR spectra of γ-Al2O3, hydrated (cyan) and dehydrated at 300 °C (green) and 700 °C (red). (c) 27Al (14.1 T, 14 kHz) MQ MAS spectrum of γ-Al2O3 hydrated (blue) and dehydrated at 300 °C (red). (d) 27Al–17O (20.0 T, 20 kHz) J-HMQC (blue) and D-HMQC (red) spectra of γ-Al217O3(950 °C). For D-HMQC, SR421 recoupling was applied for 0.9 ms. For detailed experimental parameters see Section S1 of the ESI.† | ||
AlV species are not easily observed in the MAS or MQMAS spectra of hydrated bulk γ-Al2O3, although their low-level presence has been demonstrated previously.32,74–77 Early studies32 showed the concentration of AlV depends on the exact synthetic route and conditions used, and particularly on any grinding of the precursor prior to calcination. Most studies indicate AlV sites are preferentially, if not exclusively, located at the surface. As discussed above, there is little evidence in the materials studied here for H in the bulk, and so 1H–27Al CP experiments can be used to selectively probe the alumina surface. For the hydrated γ-Al2O3, a low intensity AlV signal can be seen between 20 and 40 ppm in the CP MAS spectrum in Fig. 8b. Dehydration will also affect the surface structure, increasing the relative intensity of AlIV and AlV species in the MAS and CP MAS NMR spectra (as shown in Fig. 8) with increasing dehydration temperature. Although not shown, the MAS spectrum does not change for samples dehydrated at 500 and 700 °C, suggesting the lower temperature is sufficient to remove the adsorbed water molecules. Dehydration yields a higher concentration of AlV and their observation (albeit with very low intensity, at δ1 = 20 ppm) in the 27Al MQMAS spectrum (as shown in Fig. 8c), with average NMR parameters as shown in Table S8.1 the ESI.†
Early work using IR spectroscopy78 suggested the presence of at least 5 different types of OH groups at the surface of γ-Al2O3. However, the 1H MAS NMR spectrum of hydrated γ-Al2O3 is dominated by a broad resonance from water (∼4.5 ppm) that obscures signals from surface aluminol groups, as shown in Fig. 9a. Upon dehydration, more complex spectral lineshapes are revealed. After dehydration at 300 °C, the lineshape is still relatively broad, but two distinct components (at ∼3.7 and 0.7 ppm) are resolved, with a shoulder at ∼5.1 ppm. When dehydration is carried out at a higher temperature (550 °C) a larger number of sharper signals are observed (with most between 0 and 4 ppm), although these are still significantly overlapped. The slow rehydration of the surface inside the NMR rotor (leading to increasing intensity at higher shifts) is shown in Fig. S10.1 in the ESI.† It should be noted that no significant decrease in linewidth was observed with increasing MAS rate or with the application of homonuclear decoupling, suggesting that the linewidth results primarily from a distribution of chemical shifts.
 | ||
| Fig. 9 (a) 1H (9.4 T, 14 kHz) DEPTH MAS NMR spectra of γ-Al2O3 hydrated (black) and dehydrated at 300 °C (blue) and 550 °C (red). (b and c) Plots showing the overlay of the DFT-predicted NMR shifts (δiso) for all H in the (b) fully and (c) partially hydroxylated slab models of γ-Al2O3, overlayed with the 1H (14.1 T, 20 kHz) DEPTH MAS NMR spectrum of γ-Al2O3 dehydrated at 550 °C. The red line represents the spectrum simulated from the sum of Lorentzians (FWHH of 150 Hz) added for each of the points shown. For detailed experimental parameters see the ESI.† | ||
The ranges of 1H δiso predicted using DFT for the 22 fully and 14 partially hydroxylated models of γ-Al2O3 described above are shown in Fig. 9b and c, respectively. The 1H environments are grouped according to the denticity of the hydroxyl groups (with μ1 being non bridging or terminal, μ2 bridging and μ3 a triply-bridged OH group), the coordination number of the Al3+ co-ordinated to the O, and whether the H is a hydrogen bond donor (HD) or the O a hydrogen bond acceptor (HA). The OH distance was considered a covalent bond when <1.2 Å. The spectra obtained from summing Lorentzians (FWHH of 150 Hz) of unit intensity applied to each data point do not match well with the experimental spectra in either case, but the range of shifts is reproduced well, and the spectra help in understanding the changes to the 1H MAS NMR spectrum seen with hydration. For a group of μn1H, those H that act as hydrogen bond donors have higher shifts in each case. It is also noticeable that signals between 0 and 1 ppm result primarily from μ1 and μ2 hydrogen bond acceptor aluminols), in good agreement with the previous work of Deng and co-workers.43 Similar plots considering predicted shifts for μ1, μ2 and μ3 separately are given Fig. S10.2 in the ESI† (where the coordination numbers of the coordinated Al3+ are also denoted in each case). Comparison of these plots confirms that the average 1H shift increases with denticity, negative shifts are only observed for non-bridging aluminols and that there is little dependence of the 1H shift on the coordination number of the Al3+ around O.
The denticity of the aluminol groups that produce different signals in the 1H MAS NMR spectrum was investigated experimentally using RESPDOR79 to measure 1H–27Al dipolar couplings (see Section S1 of the ESI† for the experimental details). The 1H MAS NMR spectrum was decomposed into 10 separate Voigt components, nine of which were then used for analysis (see Section S10 of the ESI†), as shown in Fig. 10a. Fig. 10b shows the nine RESPDOR fractions ((S0 – S)/S0) as a function of τrec. Three groups of signals can be clearly distinguished, corresponding to components 1–2, 3–7 and 8–9. In Fig. 10c, the RESPDOR curves for components 2 and 4 are compared with numerical simulations for a 1H(27Al)2 spin system, with distances d1 and d2 determining the two different heteronuclear dipolar couplings. For all simulations, the 27Al CQ = 5 MHz and the angle between the two internuclear vectors was fixed at 60°. Variation in these parameters has only a small effect on the RESPDOR fraction calculated (as shown in the ESI†). For component 2, a good match is obtained when d1 < d2 (i.e., a μ1 aluminol), which agrees with the DFT-predicted shift of these species. For component 4, simulation shows the best match is for d1 = d2 ≈ 2.4 Å, suggesting this group of signals result from μ2 aluminols, which make up the most abundant H environments on the γ-Al2O3 surface. Finally, as shown in Fig. 10d RESPDOR curves were simulated for the two most common types of μ3 aluminols (spin system geometries were directly extracted from the structural models described above, with d1, d2 and d3 set to 2.4 Å). The simulation is in good agreement with the experimental result, confirming that components 8–9 (δiso = 4–5 ppm) can be attributed primarily to triply-bridging OH groups. These results agree with those of Taoufik et al.,77 who were able to identify μ1 and μ2 aluminols, but also provides the assignment of signals from μ3 aluminols, which have previously only been tentatively assigned based on their predicted higher shifts.
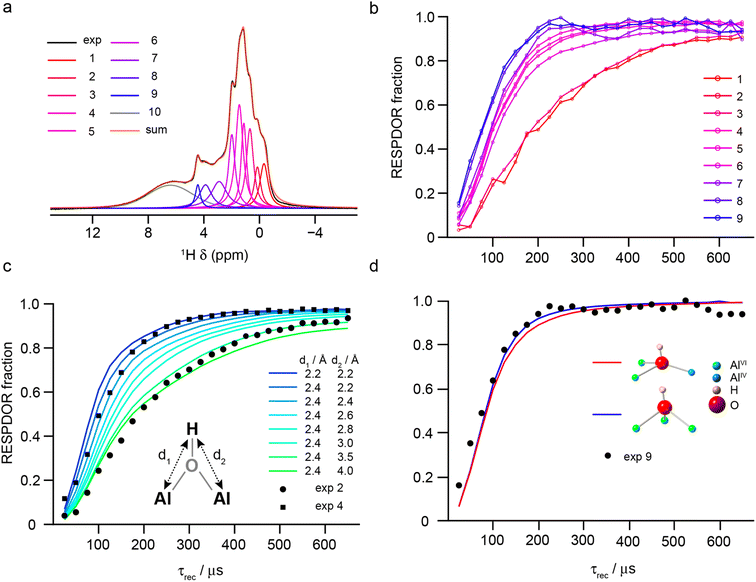 | ||
| Fig. 10 (a) Decomposition of the 1H (14.1 T, 40 kHz) DEPTH MAS NMR spectrum of γ-Al2O3 dehydrated at 550 °C (blue) into the 10 components used in fits of the RESPDOR spectra. (b) Experimental RESPDOR curves (acquired using R1235 recoupling) plotted as a function of τrec. (c) Overlay of experimental RESPDOR curves for components 2 and 4 with corresponding plots from numerical simulations of a three-spin (H2Al) system with varying H–Al distances, as shown. (d) Overlay of the experimental RESPDOR curve for component 9 with corresponding plots from numerical simulations of two four-spin (H3Al) systems for μ3 aluminols extracted from the structural models above, as shown. In (c) and (d), the 27Al CQ was 5 MHz and in (c) the angle between the internuclear vectors was also fixed at 60°. For detailed experimental parameters see Section S1 of the ESI.† | ||
The coordination numbers of the Al cations attached to the aluminols were determined using 1H–27Al D-HMQC experiments (with TRAPDOR recoupling), as shown in Fig. 11a. Sum projections for shift regions corresponding to AlIV, AlV and AlVI show that μ1 aluminols exhibit a weaker correlation with AlV/VI than with AlIV (a result consistent with previous work by Taoufik et al.77 and Szeto et al.80). This reflects not only the higher abundance of AlIV on partially dehydroxylated surfaces, but the increased difficulty of removing OH groups from these species (as opposed to AlV and AlVI) species, as this would generate metastable AlIII environments. The existence of AlIII sites on alumina surfaces has been hypothesised in a range of previous work considering the reaction of N2, H2, CH4, pyridine, CO and CO2 with alumina, and studied using computational approaches, NMR spectroscopy and IR spectroscopy.81–85 The AlIII species are thought to result from high-temperature pre-treatment of the surface (typically at ∼700 °C) and to be largely responsible for the adsorption of N2 or pyridine and the splitting of CH4 and H2.82–85 However, it has also been shown that the level of water present plays an important role both in the changing the reactivity seen and in varying the coordination number of the Al species at the surface.81–83 Direct detection of AlIII species (if present) using NMR spectroscopy would be extremely challenging, owing the large quadrupolar couplings expected. In the current work, for μ2 aluminols, coordination to all three types of Al is observed, but at higher δH the correlation to AlV and AlVI is weaker, suggesting high levels of μ2-OH (Al-44), while the reverse is true at lower δH, suggesting these 1H signals result from species such as μ2-OH (Al-56) and μ2-OH (Al-66). For 1H signals corresponding to μ3 aluminols, correlations to AlIV are weaker, suggesting the most abundant species are μ3-OH (Al-666), μ3-OH (Al-665), etc. The spatial distribution of aluminols can be investigated using 1H DQ MAS spectroscopy,86 as in previous work.77Fig. 11b shows that γ-Al2O3 dehydrated at 550 °C correlations between most types of aluminol 1H are observed, although with differing intensities. This suggests no sites are exclusively isolated, but that the closest proximities are between bridging aluminols coordinated to AlIV and/or AlV species (traces C and D). This is perhaps not surprising given these are the most abundant species present on the surface, and these species also show correlations with all other types of 1H (traces A, B, E and F). This observation can also be understood by considering the topology of the (100) and (110) surfaces (as shown in Fig. S6.1†). The (100) surface contains chains of AlV species which can, upon hydroxylation, accommodate neighbouring μ2-OH (Al-66) environments that do not share Al3+ cations. At the (110) surface, chains of AlIV sites (generated from AlVI) are present, which can give rise to continuous chains of μ2-OH (Al-66) sharing AlVI cations, upon hydroxylation. This can also give rise to μ2-OH (Al-56) and μ2-OH (Al-55) at the ends of, or at breaks in, the chains. These topologies also explain the lower abundances of μ1-OH (Al-6) and μ1-OH (Al-5) relative to μ1-OH (Al-4). Upon hydroxylation of one such AlV or AlIV environment, μ1-OH (Al-6) or μ1-OH (Al-5) are formed, respectively, with two neighbouring coordinatively unsaturated aluminium sites. A small displacement of the oxygens of these hydroxyls generates a μ2 aluminol, which is energetically more favourable. Chains of μ1-OH therefore give rise to corresponding chains of μ1-OH (Al-6) or μ1-OH (Al-5), which also do not correspond to the 1H signals at lower δ. Trace A shows autocorrelations between μ1-OH, while trace G demonstrates correlations between H that are hydrogen-bond donors and μ1-OH. The intensity of these cross peaks decreases significantly with recoupling time (see Fig. S10.4 of the ESI†), suggesting close proximity of the hydrogen-bond donors H and μ1-OH on the surface. Trace H confirms the autocorrelation of two hydrogen-bond donors (a signal which also decreases with recoupling time), suggesting this most likely arises from water. The only autocorrelation that is not observed is for μ3-OH, most likely because of their low overall concentration.
 | ||
| Fig. 11 (a) 1H–27Al (14.1 T, 40 kHz) D-HMQC spectrum (with TRAPDOR recoupling) of γ-Al2O3 dehydrated at 550 °C, with sum projections (extracted parallel to δ2) over the regions corresponding to AlIV, AlV and AlVI (as shown), overlaid with the 1H DEPTH (14.1 T, 40 kHz) DEPTH MAS NMR spectrum of γ-Al2O3 dehydrated at 550 °C. (b) 1H (14.1 T, 40 kHz) DQ MAS spectrum (acquired using BABA recoupling) of γ-Al2O3 dehydrated at 550 °C, with cross sections (extracted parallel to δ2 and vertically expanded as shown) extracted at the δ1 positions indicated. For detailed experimental parameters see Section S1 of the ESI.† | ||
Conclusions
In this work we have used a multinuclear NMR crystallographic approach, combining periodic planewave DFT calculations with experimental NMR spectroscopy of isotopically enriched materials, to probe the detailed atomic-level structure of γ-Al2O3. Although each of these approaches has been used in previous work, the combination of all three in a single study here has demonstrated the complexity of the bulk material and range of surface species that may be present, and the challenge of unambiguously assigning (and quantifying) the experimental signals observed (an approach on which some previous literature conclusions have depended). This complexity has potentially limited prior analysis, and the insight obtained in this work provides new opportunities to interrogate this important system and related materials using NMR crystallography.Comparison of experiment and computation suggested that the bulk structure of γ-Al2O3 contained Al cations primarily in “spinel-like” sites, with roughly equal numbers of alternating AlVI and AlIV vacancies in disordered “chains” throughout. This contrasts with some previous work where Al vacancies were thought to occur exclusively on AlVI sites, with a maximum separation between them. The use of a larger unit cell for the calculations enabled a wide range of local environments to be generated, and the inclusion of AlIV vacancies produces many new types of 17O signals, and notably OIII sites with very similar parameters to OIV signals. This highlights potential problems with previous work, where many conclusions relied on an unambiguous assignment (and quantification) of OIII and OIV signals and correlations of these with other species to determine local structure. There was no evidence for the presence of H in the bulk structure of γ-Al2O3, or for any significant levels of O vacancies.
While catalysis is often a surface phenomenon, the surface structure is inherently related to the position, type and ordering of Al vacancies found in the bulk. Applying an NMR crystallographic approach to three separate surfaces made from a set of our structural models with different cation vacancies and different levels of hydration, enabled the prediction of 17O NMR spectra for surface, sub surface and bulk species. This showed that even at the third oxide layer from the surface many different types of O species are formed owing to the different coordination numbers of the neighbouring Al cations, many of which are now reduced by the cleavage, providing additional support for the assignment and interpretation of the complex resonances in the 17O NMR spectra.
Relaxation measurements highlighted the involvement of protonated O species in dynamic surface processes and their interactions with water, with the rapid relaxation seen accounting for the absence of these signals in STMAS (and MQMAS) spectra. Through VT, CP and TRAPDOR experiments, and by comparison to DFT calculations, it was possible to assign the two signals seen, with the sharper and broader resonances attributed to more accessible surface sites that often interact strongly with water and less accessible aluminols, respectively. 17O-27Al D-HMQC spectra showed that all O signals correlate to AlIV and AlVI species but, somewhat surprisingly, J-HMQC experiments suggested that the correlations are much stronger with AlIV than with AlVI. However, this is likely to result from the different J couplings, with O–AlVI couplings much smaller (3–10 Hz) than O–AlIV couplings, reflecting the different bond lengths, rather than a difference in the presence of these atom pairs.
DFT-predicted 1H NMR parameters confirmed the 1H shift generally increases with denticity (in agreement with previous work), and that, although a wide range of shifts are seen depending on the coordinated atoms, there is little direct dependence of the 1H shift on the Al coordination number. Spectral assignments were also supported by 1H–27Al RESPDOR experiments, which identified spectral components resulting from μ1, μ2 and μ3 aluminols. Combining these with 1H–27Al D-HMQC experiments showed that (i) μ1 aluminols are more likely to be bound to AlIV, (ii) μ2 aluminols are coordinated to all three types of Al, but with a higher proportion bound to similar types of Al, e.g., μ2-OH (Al-44), μ2-OH (Al-56) and μ2-OH (Al-66) and (iii) μ3 aluminols are most likely bound to higher coordinated Al species, e.g., μ3-OH (Al-666) and μ3-OH (Al-665). DQ MAS spectroscopy confirmed no types of aluminols exist exclusively in isolation but showed that the closest proximities are between bridging aluminols coordinated to AlIV and/or AlV species, reflecting the likely topology of the surfaces.
The combination of multinuclear NMR spectroscopy with both isotopic enrichment and DFT calculations has provided more detailed insight into both the bulk and surface structure of γ-Al2O3. The complexity of both bulk and surface structures that this reveals highlights the challenges faced by any study, and the need to combine approaches to obtain a much fuller picture. The different results observed in this work from some previous studies may reflect the natural advances in both computation and experiment over the years but are also likely to result from the varied nature of aluminas depending on the route by which they are synthesised and how they have been treated or stored. While this adds to the challenge of control for catalytic applications, it also offers an exceptional opportunity to fine tune the properties of the surfaces and their modification if the detailed atomic-level structure of these materials can be understood. The insight achieved here will provide a basis for future work both on the reactivity of aluminas themselves and on their modified surfaces.
Data availability
Detailed experimental parameters, structural models used for DFT calculations, further 17O, 27Al and 1H NMR spectra and calculated 27Al–17O J couplings are given in the ESI.† The research data (and/or materials) supporting this publication can be accessed at https://doi.org/10.17630/5020123c-d803-4bfd-8228-3e2507f077e4.87 In order to meet institutional and research funder open access requirements, any accepted manuscript arising shall be open access under a Creative Commons Attribution (CC BY) reuse licence with zero embargo.Author contributions
SEA and PBW co-conceived and funded the project and had oversight and supervision of the work. MLB carried out the experimental and computational work. DMD supported the acquisition, analysis and curation of the NMR data and contributed to the preparation of the figures. SEA wrote the initial manuscript and all authors contributed to the final draft.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Sasol for partial funding of a studentship for MBL, and EPSRC for support through the Collaborative Computational Project on NMR Crystallography (CCP-NC), via EP/M022501/1 and EP/T026642/1. PBW also thanks the Royal Society for the award of a Royal Society Industry Fellowship. The authors would like to thank Dr Andrew Rankin for help with preliminary experiments. The UK High-Field Solid-State NMR Facility used in this research was funded by EPSRC and BBSRC (EP/T015063/1) as well as the University of Warwick including via part funding through Birmingham Science City Advanced Materials Projects 1 and 2 supported by Advantage West Midlands (AWM) and the European Regional Development Fund (ERDF). Collaborative assistance from the Facility Manager Team (Dinu Iuga, University of Warwick) is acknowledged.References
- M. Trueba and S. P. Trasatti, Eur. J. Inorg. Chem., 2005, 3393–3403 CAS.
- G. Busca, Chem. Rev., 2007, 107, 5366–5410 CrossRef CAS.
- Fischer-Tropsch Technology, ed. A. P. Steynberg and M. E. Dry, Elsevier, 1st edn, 2004 Search PubMed.
- W. H. Shearon and W. R. Fullem, Ind. Eng. Chem., 1959, 51, 720–726 Search PubMed.
- G. Busca, Catal. Today, 2014, 226, 2–13 CrossRef CAS.
- G. Busca, Catal. Today, 2020, 357, 621–629 CrossRef CAS.
- K. Wefers and C. Misra, Alcoa Tech. Pap., 1987, 19, 1–100 Search PubMed.
- I. Levin and K. C. Brandon, J. Am. Ceram. Soc., 2005, 81, 1995–2012 Search PubMed.
- L. Kovarik, M. Bowden, A. Genc, J. Szanyi, C. H. F. Peden and J. H. Kwak, J. Phys. Chem. C, 2014, 118, 18051–18058 CrossRef CAS.
- L. Kovarik, M. Bowden, D. Shi, N. M. Washton, A. Andersen, J. Z. Hu, J. Lee, J. Szanyi, J. H. Kwak and C. H. F. Peden, Chem. Mater., 2015, 27, 7042–7049 CrossRef CAS.
- C. Wolverton and K. C. Hass, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 63, 024102 CrossRef.
- E. J. W. Verwey, Z. Kristallogr. Cryst. Mater., 1935, 91, 65–69 CrossRef CAS.
- B. C. Lippens and J. H. de Boer, Acta Cryst., 1964, 17, 1312–1321 CrossRef CAS.
- S.-D. Mo, Y.-N. Xu and W.-Y. Ching, J. Am. Ceram. Soc., 2005, 80, 1193–1197 CrossRef.
- C. Y. Ouyang, Ž. Šljivančanin and A. Baldereschi, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 235410 CrossRef.
- F. H. Streitz and J. W. Mintmire, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 773–777 Search PubMed.
- G. Gutiérrez, A. Taga and B. Johansson, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 65, 012101 CrossRef.
- A. Vijay, G. Mills and H. Metiu, J. Chem. Phys., 2002, 117, 4509–4516 CAS.
- H. P. Pinto, R. M. Nieminen and S. D. Elliott, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 125402 Search PubMed.
- E. Menéndez-Proupin and G. Gutiérrez, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 035116 Search PubMed.
- S. Blonski and S. H. Garofalini, Surf. Sci., 1993, 295, 263–274 CAS.
- M.-H. Lee, C.-F. Cheng, V. Heine and J. Klinowski, Chem. Phys. Lett., 1997, 265, 673–676 CAS.
- K. P. Sinha and A. P. B. Sinha, J. Phys. Chem., 1957, 61, 758–761 CAS.
- J. A. Wang, X. Bokhimi, A. Morales, O. Novaro, T. López and R. Gómez, J. Phys. Chem. B, 1999, 103, 299–303 CAS.
- Y. G. Wang, P. M. Bronsveld, J. T. M. DeHosson, B. Djuričić, D. McGarry and S. Pickering, J. Am. Ceram. Soc., 2005, 81, 1655–1660 Search PubMed.
- G. Kryukova, D. Klenov, A. Ivanova and S. Tsybulya, J. Eur. Ceram. Soc., 2000, 20, 1187–1189 CAS.
- B. Ealet, M. H. Elyakhloufi, E. Gillet and M. Ricci, Thin Solid Films, 1994, 250, 92–100 CAS.
- R. Dupree, M. H. Lewis and M. E. Smith, Philos. Mag. A, 1986, 53, L17–L20 CAS.
- H. Saalfeld, Clay Miner., 1958, 3, 249–257 CAS.
- V. Jayaram and C. G. Levi, Acta Metall., 1989, 37, 569–578 CrossRef CAS.
- C. Pecharromán, I. Sobrados, J. E. Iglesias, T. González-Carreño and J. Sanz, J. Phys. Chem. B, 1999, 103, 6160–6170 CrossRef.
- F. Chen, J. G. Davis and J. J. Fripiat, J. Catal., 1992, 133, 263–278 CrossRef CAS.
- D. Iuga, J. Magn. Reson., 2011, 208, 225–234 CAS.
- G. Paglia, C. E. Buckley, A. L. Rohl, R. D. Hart, K. Winter, A. J. Studer, B. A. Hunter and J. V. Hanna, Chem. Mater., 2004, 16, 220–236 Search PubMed.
- G. Paglia, C. E. Buckley, A. L. Rohl, B. A. Hunter, R. D. Hart, J. V. Hanna and L. T. Byrne, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 144110 Search PubMed.
- V. A. Ushakov and E. M. Moroz, React. Kinet. Catal. Lett., 1984, 24, 113–118 Search PubMed.
- R.-S. Zhou and R. L. Snyder, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 617–630 CrossRef.
- X. Krokidis, P. Raybaud, A.-E. Gobichon, B. Rebours, P. Euzen and H. Toulhoat, J. Phys. Chem. B, 2001, 105, 5121–5130 CAS.
- R. F. Moran, D. M. Dawson and S. E. Ashbrook, Int. Rev. Phys. Chem., 2017, 36, 39–115 Search PubMed.
- P. Euzen, P. Raybaud, X. Krokidis, H. Toulhoat, J.-L. Le Loarer, J.-P. Jolivet and C. Froidefond, in Handbook of Porous Materials, ed. F. Schüth, K. Sing and J. Weitkamp, Wiley-VCH, Weinheim, Germany, 2002, ch. 4.7.2, pp 1591–1677 Search PubMed.
- S. E. Ashbrook and M. E. Smith, Chem. Soc. Rev., 2006, 35, 718–735 CAS.
- T. H. Walter and E. Oldfield, J. Phys. Chem., 1989, 93, 6744–6751 CAS.
- Q. Wang, W. Li, I. Hung, F. Mentink-Vigier, X. Wang, G. Qi, X. Wang, Z. Gan, J. Xu and F. Deng, Nat. Commun., 2020, 11, 3620 CAS.
- W. Li, Q. Wang, J. Xu, F. Aussenac, G. Qi, X. Zhao, P. Gao, C. Wang and F. Deng, Phys. Chem. Chem. Phys., 2018, 20, 17218–17225 CAS.
- L. Shen, Y. Wang, J. Du, K. Chen, Z. Lin, Y. Wen, I. Hung, Z. Gan and L. Peng, ChemCatChem, 2020, 12, 1569–1574 CrossRef CAS.
- R. Prins, Angew. Chem., Int. Ed., 2019, 58, 15548–15552 CrossRef CAS PubMed.
- H. Nagashima, J. Trebosc, O. Lafon and J.-P. Amoureux, J. Magn. Reson. Open, 2024, 21, 100177 CrossRef.
- L. Frydman and J. S. Harwood, J. Am. Chem. Soc., 1995, 117, 5367–5368 CrossRef CAS.
- H. T. Kwak and Z. Gan, J. Magn. Reson., 2003, 164, 369–372 CrossRef CAS PubMed.
- S. E. Ashbrook and S. Wimperis, Prog. Nucl. Magn. Reson. Spectrosc., 2004, 45, 53–108 CrossRef CAS.
- C. J. Pickard and F. Mauri, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245101 CrossRef.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr. Cryst. Mater., 2005, 220, 567–570 CrossRef CAS.
- S. A. Joyce, J. R. Yates, C. J. Pickard and F. Mauri, J. Chem. Phys., 2007, 127, 204107 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 1865–1868 CrossRef.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef.
- J. R. Yates, C. J. Pickard, M. C. Payne and F. Mauri, J. Chem. Phys., 2003, 118, 5746–5743 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- H. J. Jakobsen, J. Skibsted, H. Blidsoe and N. C. Nielsen, J. Magn. Reson., 1989, 85, 173–180 CAS.
- P. Pyykkö, Mol. Phys., 2018, 116, 1328–1338 Search PubMed.
- M. Bak, J. T. Rasmussen and N. C. Nielsen, J. Magn. Reson., 2000, 147, 296–330 Search PubMed.
- G. Paglia, Determination of the Structure of γ-Alumina using Empirical and First-Principles Calculations Combined with Supporting Experiments, PhD thesis, Curtin University of Technology, 2004.
- M. Digne, P. Sautet, P. Raybaud, P. Euzen and H. Toulhoat, J. Catal., 2004, 226, 54–68 CrossRef CAS.
- D. A. Dowden, J. Chem. Soc., 1950, 242–265 RSC.
- K. Sohlberg, S. J. Pennycook and S. T. Pantelides, Chem. Eng. Commun., 2000, 181, 107–135 CrossRef CAS.
- R. Pearson, J. Catal., 1971, 23, 388–394 CrossRef CAS.
- G. Paglia, C. E. Buckley, T. J. Udovic, A. L. Rohl, F. Jones, C. F. Maitland and J. Connolly, Chem. Mater., 2004, 16, 1914–1923 CrossRef CAS.
- L. Smrcok, V. Langer and J. Krestan, Acta Crystallogr. Sect. C Cryst. Struct. Commun., 2006, 62, 83–84 CrossRef.
- G. Paglia, E. S. Božin and S. J. L. Billinge, Chem. Mater., 2006, 18, 3242–3248 CrossRef CAS.
- S. K. Sharma, P. Das and B. K. Sanfui, Mol. Syst. Des. Eng., 2022, 7, 67–91 RSC.
- M. E. Miller and S. T. Misture, J. Phys. Chem. C, 2010, 114, 13039–13046 CrossRef CAS.
- C. P. Grey and A. J. Vega, J. Am. Chem. Soc., 1995, 117, 8232–8242 CrossRef CAS.
- A. Lesage, D. Sakellariou, S. Steuernagel and L. Emsley, J. Am. Chem. Soc., 1998, 120, 13194 CrossRef CAS.
- A. Brinkmann and A. P. M. Kentgens, J. Am. Chem. Soc., 2006, 128, 14758–14759 CrossRef CAS PubMed.
- R. Wischert, P. Florian, C. Copéret, D. Massiot and P. Sautet, J. Phys. Chem. C, 2014, 118, 15292–15299 CrossRef CAS.
- J. H. Kwak, J. Hu, A. Lukaski, D. H. Kim, J. Szanyi and C. H. F. Peden, J. Phys. Chem. C, 2008, 112, 9486–9492 CrossRef CAS.
- J. H. Kwak, J. Hu, D. Mei, C. -W. Yi, D. H. Kim, C. H. F. Peden, L. F. Allard and J. Szanyi, Science, 2009, 325, 1670–1673 CrossRef CAS PubMed.
- M. Taoufik, K. C. Szeto, N. Merle, I. Del Rosal, L. Maron, J. Trébosc, G. Tricot, R. M. Gauvin and L. H. Delevoye, Chem.–Eur. J., 2014, 20, 4038–4046 CrossRef CAS.
- H. Knözinger and P. Ratnasamy, Catal. Rev., 1978, 17, 31–70 CrossRef.
- L. Chen, Q. Wang, B. Hu, O. Lafon, J. Trébosc, F. Deng and J. P. Amoureux, Phys. Chem. Chem. Phys., 2010, 12, 9395–9405 RSC.
- K. C. Szeto, N. Merle, J. Trébosc, M. Taoufik, R. M. Gauvin, F. Pourpoint and L. Delevoye, J. Phys. Chem. C, 2019, 123, 12919–12927 CAS.
- V. Sanchez Escribano, G. Garbarino, E. Finocchio and G. Busca, Top. Catal., 2017, 60, 1554–1564 CAS.
- R. Wishert, P. Laurent, C. Coperet, F. Delbecq and P. Sautet, J. Am. Chem. Soc., 2012, 134, 14430–14449 Search PubMed.
- R. Wishert, C. Coperet, F. Delbecq and P. Sautet, Angew. Chem., 2011, 50, 3202–3205 Search PubMed.
- J. Joubert, A. Salameh, V. Krakoviack, F. Delbecq, P. Sautet, C. Coperet and J.-M. Basset, J. Phys. Chem. B, 2006, 110, 23944–23950 CAS.
- I. B. Moroz, K. Larmier, W.-C. Liao and C. Coperet, J. Phys. Chem. C, 2018, 122, 10871–10882 CAS.
- H. Geen, J. J. Titman, J. Gottwald and H. W. Spiess, Chem. Phys. Lett., 1994, 227, 79–86 CAS.
- M. B. Legrady, D. M. Dawson, P. B. Webb and S. E. Ashbrook, Insight into the Atomic-Level Structure of γ-Alumina Using a Multinuclear NMR Crystallographic Approach (dataset), University of St Andrews Research Portal, 2025, DOI:10.17630/5020123c-d803-4bfd-8228-3e2507f077e4.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc01198a |
| ‡ Currently at: School of Chemistry, University of Southampton, University Road, Southampton, SO17 1BJ, UK. |
| This journal is © The Royal Society of Chemistry 2025 |

