Open Access Article
Open Access ArticleA review of designable deep eutectic solvents for green fabrication of advanced functional materials
Zheng
Wang
a,
Xinhui
Zhao
 b,
Yu
Chen
a,
Cong
Wei
b,
Yu
Chen
a,
Cong
Wei
 *a and
Jingyun
Jiang
*a and
Jingyun
Jiang
 *a
*a
aSchool of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450052, P. R. China. E-mail: weicong@zzu.edu.cn; jiangjingyun@zzu.edu.cn
bJiangsu Key Laboratory of Function Control Technology for Advanced Materials, School of Environmental and Chemical Engineering, Jiangsu Ocean University, Lianyungang 222005, P. R. China
First published on 20th December 2024
Abstract
Deep Eutectic Solvents (DESs) have become emerging green solvents within sustainable development and environmental protection. Characterized by their low toxicity, cost-effectiveness, environmental sustainability, and versatility, DESs are increasingly utilized across diverse sectors. Notably, in materials synthesis, these solvents offer the advantages of biodegradability and recyclability, bypassing high-temperature and high-pressure synthesis conditions, thus reducing environmental hazards and energy consumption while enhancing material performance. Consequently, adopting DESs as reactive or nonreactive media in nanomaterial synthesis has attracted significant attention. However, there are still knowledge gaps addressing the roles of DESs in developing and functionalizing advanced materials. This review regards these gaps by elucidating the unique chemical, thermal, and electrochemical properties of DESs. It then explores their recent applications in nanomaterial fabrication and discusses how DESs regulate material synthesis using three typical strategies, including chemical, thermal, and electrochemical processes. Additionally, it outlines the potential, key challenges, and limitations of using DESs in materials science. By providing a comprehensive analysis, this review aims to deepen understanding of DESs, broaden their use, and enhance their integration into materials synthesis practices.
Sustainability spotlightWith the rapid progress in green chemistry and clean energy, there is an increasing demand for scalable and sustainable methods to produce advanced energy materials. Deep eutectic solvents (DESs) are considered promising due to their low toxicity, affordability, environmental benefits, and versatility. However, while much research has explored their use in the synthesis and application of functional materials, the complex interactions between DESs and material structures remain insufficiently understood, limiting their effectiveness in guiding targeted material design. This demand for green fabrication aligns with the United Nations Sustainable Development Goals (SDGs), particularly Goal 7: Affordable and Clean Energy, and Goal 12: Responsible Consumption and Production. This review aims to advance scientific understanding, promote green energy innovations, support environmental stewardship, and inform policy development. |
1 Introduction
The choice of solvent is a crucial topic in material synthesis, as the properties of the solvent can significantly impact the feasibility of synthesis and the final material's properties. Among the approximately 600 existing volatile organic compounds widely used in research and industry, most are toxic and pose significant risks to both human health and the environment.1,2 Over the past two decades, ionic liquids (ILs) have emerged as clean, efficient, and environmentally friendly alternatives to volatile organic solvents in various fields.3 However, the limitations of ILs, such as toxicity, high cost, complex synthesis and purification processes, and non-renewability, are significant.4 Therefore, pursuing safe, stable, efficient, and sustainable solvents has become critical in fundamental research and practical applications.Deep Eutectic Solvents (DESs) share several solvent properties with ILs, including low vapor pressure, high thermal stability, non-flammability, and ease of recovery. Unlike ILs, which are entirely ionic, DESs are primarily composed of molecular components, often in dominant proportions.5 Due to their lower toxicity, reduced cost, simplicity in preparation, and broader solute applicability, DESs are increasingly regarded as environmentally favorable alternatives to ILs used in various applications.6,7 The concept of DES was introduced in 2003 by Abbott et al. to address low melting points in mixtures. The 1ChCl–2urea DES, exemplifies this, maintaining a liquid state at room temperature with a melting point of only 12 °C, significantly lower than those of its separate components.8 Typically, DES comprises combinations of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), forming complex supramolecular structures through extensive hydrogen bonding, which significantly lowers the melting point by altering the free energy of the solid phase.9 DESs' deviations from ideality are a result of their intrinsically cooperative intermolecular interactions, making them distinctly different from conventional ideal liquid mixtures. These features contribute to their unique properties, enabling their application in diverse fields such as catalysis, material synthesis, and electrochemistry. Abbott et al. classified the reported DESs into four types based on their components: type I (quaternary ammonium salts with metal chlorides), type II (quaternary ammonium salts with metal chloride hydrates), type III (quaternary ammonium salts with HBDs), and type IV (metal chloride hydrates with HBDs).10 Moreover, non-ionic systems like thymol–lidocaine, which also remain liquid at room temperature, demonstrate a negative deviation from ideality across a broad composition range, leading to their consideration as type V DESs.11 Despite these classifications, the categorization of DESs remains imperfect, with some systems involving Brønsted–Lowry acids and bases eluding conventional classification, highlighting the need for further refinement.12
Due to the diversity of DESs, they present significant advantages as reaction solvents in material synthesis. Firstly, they exhibit high solubility for diverse organic and inorganic precursors, facilitating uniform dispersion due to their polarity, hydrophilicity, and adaptability to various molecular sizes.13 Secondly, the tunability of DESs allows precise nanoscale adjustments of composition, molar ratios, and water content, enabling control over the size and shape of metal nanoparticles and, consequently, the properties of the synthesized materials.14 Thirdly, DESs fulfill multifunctional roles in synthesis, functioning as templates, directing agents, redox agents, stabilizers, or pH regulators, thereby preventing the need for additional reactants.15 Fourthly, their low surface tension diminishes nucleation barriers, facilitating the formation of smaller particles.16 However, the correlation between the properties of DESs and structures of target advanced materials is still vague, falling short of regulating the design paths towards high-performance materials. Therefore, it is necessary to advance the understanding of the chemical/thermal/electrochemical properties of DESs and depict their mechanism in material synthesis.
This review highlights recent progress in using DESs for materials synthesis, effectively linking their unique properties to the functionalization of advanced materials (Fig. 1). Initially, it discusses how the thermal properties of DESs control the formation of advanced materials through solvothermal, annealing, and microthermal techniques. Subsequently, the review addresses chemical strategies to synthesize advanced materials and illustrates how the chemical properties of DESs influence the structures of these materials. It also details the electrochemical properties of DESs and their mechanisms in the electrodeposition of functional materials. Utilizing the intrinsic properties of DESs, this review methodically examines their role in tailoring the functional properties of materials across various synthesis processes. As a comprehensive resource, this document supports green chemistry proponents and professionals in developing environmentally friendly and effective synthesis methods. Moreover, it delves into the versatile roles of DESs in materials science, evaluating their impact on material properties and highlighting the continual need for innovation.
2 Heat treatment
Heat treatment is a widely employed process in nanomaterials engineering that adjusts the crystal structure, grain size, and physical properties of materials through specific heating temperatures and cooling durations. Additionally, the composition and molar ratios of DESs are crucial in determining their thermal properties, significantly influencing the target materials' phase, morphology, and structure. The techniques utilized for heat treatment include solvothermal, annealing, and microwave synthesis, each distinguished by its unique experimental procedures.2.1 Solvothermal synthesis
Solvothermal synthesis involves mixing reactants with DES, heating in autoclaves, cooling, solidifying, and washing away non-reactive DESs to obtain nanomaterials. This process typically requires no additional solvents or catalysts. The strong hydrogen bonding in DESs increases precursor solubility, making them suitable for various nanostructures. Due to their unique microstructure, DESs often act as templates and structure-directing agents. The reported materials include inorganic/organic frameworks, carbon materials, non-carbon powder materials, and self-supporting electrodes.Beyond carbon dots, more extensive carbon materials are synthesized through a direct solvothermal process using DES. Lai et al. prepared solvothermal carbon (STC) using ChCl–citric acid (CA) DES and cotton stalk. Compared to hydrothermal carbon produced in water, the STCs exhibited higher carbonization and more significant amounts of carboxyl and phenolic groups.25 The ChCl–CA system can also be applied to obtain CoAl2O4/CNF, which exhibited enhanced electrochemical performance as a battery-type electrode. The charge storage capacity of CoAl2O4/CNFs is 2.2 times higher than that of CoAl2O4.26 N-Doped carbon nanotubes (N-CNTs), which were synthesized using an acidified CNTs-ChCl–urea system. These N-CNTs effectively support the uniform dispersion of Pt nanoparticles. Theoretical calculations indicate that the d-band center of Pt in Pt/N-CNTs is near its Fermi energy level, exhibiting superior stability during the methanol oxidation reaction (MOR).27 PtSnx/CNTs were synthesized using acetamide–[BMim]Br DES, where the Pt3Sn intermetallic compound modulates Pt's geometric and electronic structures, enhancing hydrogen molecule activation.28
Thiourea (TU) is widely used as a sulfur source to stabilize the M–S bonds during the fabrication of metal sulfides. TU-based DES is often utilized as an ideal medium for synthesizing these materials. For instance, Liu et al. synthesized a highly effective and bifunctional photocatalyst NiS2/CdS nanospheres in the 2PEG 200–1TU DES (Fig. 2A). In this process, TU decomposes at elevated temperatures, reacting with Cd2+ and Ni2+ to form metal sulfides.32 A similar situation was observed in Fe-doped NiS2 systems, where varying the Fe to Ni ratio in PEG–TU affected the related electrocatalytic oxygen evolution reaction (OER) performance.33 Other S-involved DESs, including ChCl–2thioacetamide, ChCl–2TU, and metal salt–TU, have also been employed to synthesize metal sulfides. For example, Zhang et al. utilized the 1ChCl–2thioacetamide DES to synthesize a range of metal sulfide nanocrystals, such as Sb2S3, Bi2S3, PbS, CuS, Ag2S, ZnS, and CdS. This DES functions as a polar capping agent, stabilizing nanoparticles through electrostatic interactions, which prevents further growth or aggregation and lowers nucleation barriers, thereby aiding in forming smaller particles. Additionally, varying the reaction temperature and the molar ratio of ChCl to thioacetamide can modify the size and morphology of the crystals.34 A similar temperature regulation was observed in the solvothermal synthesis of Cu3BiS3 (CBS) with 1D, 2D, and 1D–2D morphologies in the ChCl–TU DES at temperatures of 150, 180, and 250 °C, respectively (Fig. 2B). The ChCl–TU DES effectively combines the reactants through a restructuring effect induced by coordinative bonding and hydrogen bonding, resulting in different Cu3BiS3 morphologies.35
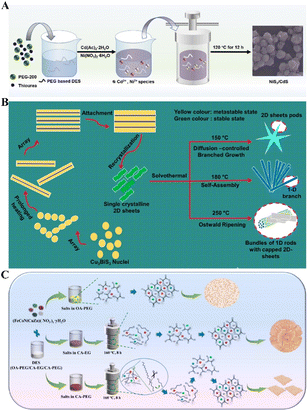 | ||
| Fig. 2 Fabrication of non-carbon powder materials: (A) schematic illustration of NiS2/CdS. Reprinted with permission from copyright© Wiley library, 2020.32. (B) Schematic illustration for plausible growth mechanism of CBS-1, CBS-2, and CBS-3. Reprinted with permission from copyright©American Chemical Society, 2021.35. (C) Schematic illustration of the synthetic process of (FeCoNiCuZn)(C2O4)·2H2O. Reprinted with permission from copyright© Elsevier Ltd, 2022.37 | ||
Some metal hydroxides and high-entropy oxalates can also be synthesized in DESs via solvothermal synthesis. Mo2C, Ni(NO3)2·6H2O, Fe(NO3)3·6H2O, and NH4F were dissolved in 1ChCl–2urea to produce a novel NiFe-layered double hydroxide (LDH)/MoC nanocomposite through the solvothermal process. The multiple nanosheets' interconnected structure provides a smooth surface and a large specific surface area.36 Fe(NO3)3·9H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Cu(NO3)2·3H2O, and Zn(NO3)2·4H2O were incorporated into PEG–CA and EG–CA DESs, leading to the formation of a single-phase multi-metal oxalate (FeCoNiCuZn)(C2O4)·2H2O (Fig. 2C). The DES's composition and structure critically influence the nucleation and growth of multi-metal oxalate salts. Notably, PEG supports the formation of 2D structures, whereas the lower molecular weight EG promotes the formation of 3D nanoparticles.37
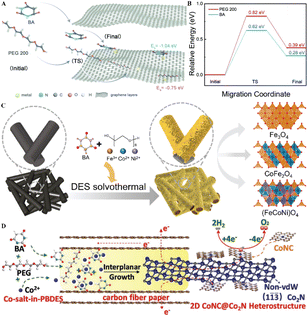 | ||
| Fig. 3 Fabrication of self-supporting electrodes. (A) Schematic illustrating the migration path of BA and PEG 200 entering the graphitic layers and the corresponding binding energy. (B) Migration energy diagram of BA and PEG 200 entering the graphitic layers. Reprinted with permission from copyright© Royal Society of Chemistry, 2021.39. (C) Schematic illustration of the universal preparation of 2D spinel oxides. Reprinted with permission from copyright© Elsevier Ltd, 2022.41. (D) Schematic illustration of the fabrication process of 2D CoNC@Co2N heterostructures for water splitting. Reprinted with permission from copyright© Wiley-VCH GmbH, 2020.43 | ||
Low-crystalline carbon cloth (CC) collectors were also applied in the DESs to fabricate self-supporting electrodes. For example, NiCl2·6H2O–CoCl2·6H2O–TU DES were introduced into an autoclave containing CC substrates to fabricate CoNi2S4/CC electrodes. Due to the DES's intense adhesion and liquid characteristics, CoNi2S4 nanosheets were uniformly deposited onto the CC substrate. The coupling of the two components enhanced electron transfer efficiency, preventing catalyst aggregation and corrosion during catalysis.44 Metal foams, including Ni and Fe foam, are also ideal substrates to further react with DES to obtain self-supporting electrodes. Bifunctional Ni–Fe–P catalysts in situ grown on FF (Ni–Fe–P/FF) electrode were developed in the 1ChCl–2EG containing NiCl2·6H2O, NaH2PO2, and FeCl3·6H2O systems. DES's unique solvent environment and the regulatory influence of Fe promote the formation of composition-controlled Ni–Fe–P.45
This chapter examines synthesizing diverse materials with substantial application potential using the solvothermal method in DESs. The materials produced encompass various frameworks, carbon-based substances, non-carbon powders (including metal oxides, sulfides, hydroxides, and oxalates), and self-supporting electrodes utilized across energy storage, catalysis, adsorption separation, sensing, and other fields. The solvothermal method with DESs allows for controlled morphology and structure, ensures high purity and excellent crystalline quality, and supports environmental sustainability.
2.2 Annealing treatment
Annealing is a heat treatment process that occurs at higher temperatures than solvothermal. During annealing, components in DESs can decompose, producing bubbles that create pores and increase the surface area of the resulting materials. Additionally, DESs can serve as reactants, providing C or N/O/B/S/P sources for fabricating advanced carbon materials. The proportions of components in DESs and the calcination temperature significantly impact the structure and properties of the resulting materials.Firstly, biomass materials can be directly carbonized in DESs or pre-treated with DESs before carbonization. For example, Zou et al. dissolved bagasse in a DES containing ZnCl2, urea, and varying ratios of KCl to synthesize nitrogen-rich porous carbon (NDPC) through one-step calcination (Fig. 4A). The DES served as a nitrogen source and activator, promoting the formation of the porous structure. Increasing the amount of KCl in the DES led to higher nitrogen doping content, specific surface area, and total pore volume.46 Coconut shell can be dissolved in the FeCl3·6H2O–urea–NH4HB4O7·3H2O DES and then calcined to obtain N/B co-doped porous carbon (NBPCs). The DES acts as an activating agent and a source of N/B, further decomposing to produce NH3, H2O, and B2O3 at high temperatures, which leads to numerous pores on the surface of the carbon material.47
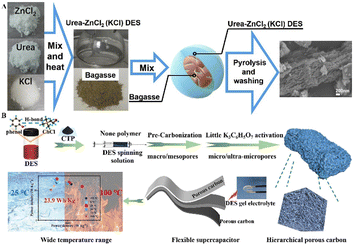 | ||
| Fig. 4 Fabrication of carbon materials through the DES annealing process. (A) Schematic illustration of the procedures of the synthesis of DES and NDPC. Reprinted with permission from copyright© Elsevier Ltd, 2020.46. (B) The schematic drawing for the preparation and application of CPCs. Reprinted with permission from copyright© Elsevier B.V, 2023.51 | ||
A two-step annealing treatment can enhance the structural properties of carbon materials. Pre-treating biomass in DESs initiates pre-carbonization through solvothermal or microwave processes, which is advantageous over direct calcination. Furthermore, a broader range of biomass types can be employed. For example, Huang et al. employed a 2ChCl–formic acid DES to solvothermal dry microalgal powder's pre-carbonization. Following this, KOH activation produced N, O dual-doped ultra-microporous carbon. This process leveraged the DES to enhance the Maillard reaction, promoting the incorporation of nitrogen and oxygen into the carbon structure while minimizing their loss during chemical activation.48 Amorphous activated carbon (AC) was obtained by calcinating the extracted lignin from the 1ChCl–9LA DES, resulting in large surface areas with micropores and mesopores.49 Furthermore, Xu et al. utilized a 1ChCl–10LA DES to extract lignin from wheat straw. Extending the duration of processing increased lignin extraction at lower temperatures, whereas elevating the DES-to-biomass mass ratio enhanced the extraction efficiency at higher temperatures. The extracted lignin underwent activation via calcination with KOH, resulting in a hierarchical porous structure comprising both micropores and mesopores and achieved a significant specific surface area of 3577.3 m2 g−1.50
Secondly, coal serves as a precursor for O/N-doped hierarchical porous carbon (HPC) via a process that combines electrospinning and calcination. Initially, coal tar pitch mixed with a ChCl–phenol DES forms a fishnet-like structure through electrospinning, where the high viscosity of the DES enhances pitch spinnability while also supplying nitrogen. Subsequent calcination with K3C6H5O7 activates the carbon structure, creating a range of pore sizes, including significant micro- and ultramicropores (Fig. 4B).51 In a separate process, Naomaohu coal undergoes microwave-assisted swelling with FeCl3–levulinic acid DES, which disrupts chemical bonds and expands aromatic interlayer spacing. This swelling is followed by high-temperature pyrolysis, where Fe and Fe2O3 composites facilitate bond cleavage and enhance redox reactions, converting solid products into liquid and gaseous forms.52
Thirdly, DESs demonstrate significant solubility for biomass, coal, and polymers. ACs were synthesized from polyethylene terephthalate (PET) using 1ChCl–2urea via a two-step pyrolysis process (ITP2). This method involves pre-carbonizing PET at low temperatures within the DES, followed by high-temperature annealing. This approach minimizes PET decomposition, allowing the 2D crystal structure to develop progressively along the a and c axes. The resulting PET-CU-A-ITP2 samples exhibited a uniform elemental distribution, high graphitization, and substantial nitrogen doping.53
Fourthly, DESs can directly serve as a carbon source for synthesizing carbon materials. The composition, comprising HBAs, HBDs, and their respective molar ratios, significantly shapes the morphology and structure of the resultant materials. For instance, Zhou et al. utilized a calcination method on glucose–urea DESs at different molar ratios (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3
5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) to produce nitrogen-doped carbon nanosheets. In this synthesis, glucose polymerizes to form a three-dimensional carbon framework. At the same time, urea, acting as a nitrogen source and blowing agent, decomposes to release ammonia, enhancing pore formation and nitrogen incorporation. Adjusting the glucose-to-urea ratio allows for control over the pore structure and nitrogen content, and substituting urea with thiourea facilitates simultaneous doping of sulfur.54
7) to produce nitrogen-doped carbon nanosheets. In this synthesis, glucose polymerizes to form a three-dimensional carbon framework. At the same time, urea, acting as a nitrogen source and blowing agent, decomposes to release ammonia, enhancing pore formation and nitrogen incorporation. Adjusting the glucose-to-urea ratio allows for control over the pore structure and nitrogen content, and substituting urea with thiourea facilitates simultaneous doping of sulfur.54
The versatility of DESs facilitates the efficient synthesis of heteroatom-doped nanocomposites. These carbon materials, enhanced with heteroatoms, serve as ideal substrates for anchoring metal-based catalysts and promote the in situ formation of composites containing metals, metal nitrides, metal (hydro)oxides, and metal sulfides. For example, a Co(NO3)2·6H2O–ChCl–urea–glucose acid DES was employed to synthesize Co@N-doped porous carbon (Co@NPC), yielding a material with a hierarchical porous structure and an expanded surface area. Nitrogen doping varies during the synthesis, improving the interaction with transition metals and ensuring a uniform distribution of metal particles.55 Another example involves the synthesis of ultrafine Co4N nanodots anchored onto an N-doped carbon framework (Co4N@NC) through calcination of a CoCl2–malonic acid–urea DES. Here, malonic acid and urea decompose to form the framework, with Co4N nanodots anchoring via hydrogen bonding, effectively preventing aggregation.56 Mou et al. developed a g-C3N4/Fe2O3 nanocomposite by calcining a ternary urea–melamine–FeCl3 DES. Adjusting DES ratios allows for controlled Fe2O3 loading, while the decomposition of organic components increases surface area and active site availability.57 Similarly, MoN synthesized from a 1ChCl–2urea containing MoO3, mixed with graphene aerogels (GA), forms a MoN@GA heterojunction that facilitates reduction and nitridation.58 In a related study, Yang et al. used 1ChCl–2urea to disperse H2PtCl6·6H2O, Ce(NO3)3, and acid-treated MWCNTs evenly, producing Pt2CeO2/CNT heterojunction nanoclusters through calcination that enhanced the Pt(0) component, revealing more active sites at the Pt and CeO2 interface.59
Metal sulfides@C composites can be synthesized via one-step or two-step annealing processes using DESs. Sulfur powder and thiourea are utilized as sulfide sources. For example, a mixture of sulfur powder and PEG–SnCl2·2H2O DES calcined produces sandwich-structured SnS@graphene composites, with SnS particles embedded between graphene layers. In this synthesis, DESs act as carbon sources and non-ionic surfactants, enhancing conductivity, reducing particle sizes, and preventing particle aggregation.60 In addition, Co9S8@graphene (Co9S8@G) nanocomposites were created by thermal sulfidation of a CoCl2·6H2O–PEG DES with sulfur powder at various temperatures, affecting the graphitization and morphology of Co9S8@G.61 Furthermore, TU, another common sulfur source, was used in the synthesis of an In2S3/CdS/N-rGO hybrid photocatalyst through single-step calcination of a CdCl2·2.5H2O–InCl3–EG DES. This process ensures optimal integration of CdS/In2S3 with N-rGO, forming a cohesive interface and heterostructures with tremella-like morphology.62 In another instance, 3D Co9S8@NSG (N/S-co-doped graphene) composites were produced through a one-step annealing of CoCl2·6H2O–urea–thiourea–PEG DES. The heterojunction interfaces within these materials are finely adjustable by modifying the DES composition.63 Zhang et al. synthesized Co9S8–Ni3S2@N, S, O-tri-doped carbon (NSOC) through one-step pyrolysis and sulfuration of a NiCl2·6H2O/CoCl2·6H2O–PEG 200–TU DES, with an increased proportion of Co altering the composite's phase behavior, diminishing the tubular Ni3S2 phase and enhancing the sheet-like Co9S8 structure.64
Firstly, a single metal can be dissolved in DESs to prepare the related functional metal oxides. Iwanow et al. prepared CuO by employing different DES compositions (glucose–urea and galactose–urea), using various copper precursors (Cu and CuO). The choice of DES influenced the grain size of Cu, particularly in the z-direction. Compact CuO nanoparticles around 100 nm were produced using Cu–DES systems, while copper oxide–DES systems yielded nanoparticles under 50 nm with larger pores and less ordered structures.65 In another synthesis, mixing Sn and Se in 1ChCl–2urea formed the precursor [(CH3)3N–(CH2)2OH]2[Sn3Se7]·H2O, which was calcined to produce mesoporous SnO2 (P-SnO2). The DES facilitated hydrogen bonding, aiding material dissolution and promoting chemical reactions. Extending the calcination can increase the size and decrease the BET surface area of P-SnO2.66
Secondly, DESs are particularly effective in dissolving metal oxides, thereby enabling their transformation into porous materials. For example, commercial Fe3O4 was dissolved in a ChCl–OA DES and exposed to microwave irradiation to produce Fe3O4 nanosheet precursors. These were subsequently calcined to generate porous Fe3O4 nanosheets (Fig. 5A).67 Moreover, bimetallic oxides such as MFe2O4 (where M = Mg, Co, Ni, Zn) have been synthesized by dissolving Fe2O3 and another metal oxide in a ChCl–maleic acid DES, followed by a two-step calcination process (Fig. 5B). The exceptional solubility of metal oxides in DESs stems from the complex formation with these oxides rather than the disruption of metal-oxide bonds, allowing dissolution at significantly lower temperatures than traditional solid-phase reactions, thus reducing the energy required for synthesis.68 A related study calcined a binary metal oxide-dissolved 1ChCl–2urea system to produce M2V2O7−δ (M = Zn and Cu). During pyrolysis, ammonia released from urea decomposition helped stabilize oxygen vacancies and maintain reduced oxidation states within the metal oxide matrix.69 Lastly, calcination of V2O5–VOHPO4·0.5H2O dissolved in a ChCl–glucose DES and a benzyl alcohol–isobutanol solution produced a vanadium phosphorus oxide (VPO) catalyst. In this process, the ChCl–glucose DES served as a structure-directing agent, facilitating the formation of active planes and resulting in highly crystalline precursors while concurrently reducing the acidity during calcination.70
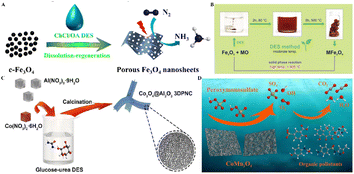 | ||
| Fig. 5 Fabrication of metal oxides through the DES annealing process. (A) Schematic illustration for the synthesis process of porous Fe3O4-300 nanosheets. Reprinted with permission from copyright© Elsevier Inc, 2021.67. (B) Schematic illustration for the synthesis process of MFe2O4. Reprinted with permission from copyright© WILEY-VCH Verlag GmbH, 2016.68. (C) Schematic representation of the synthesis of Co3O4@Al2O3 3D PNC. Reprinted with permission from copyright© Tsinghua University Press, 2023.74. (D) Schematic representation of HPNSs processing peroxydisulfate and organic pollutants. Reprinted with permission from copyright© Tsinghua University Press, 2023.75 | ||
Thirdly, inorganic and organic salts can be integrated into DESs to create metal oxides. For example, titanium butoxide (TBT), an organic salt, is soluble in ChCl–phenol DES and facilitates the synthesis of TiO2 nanoparticles. As the calcination temperature rises, the nanoparticles' surface area and pore volume decrease due to pore collapse and disordered nanoparticle aggregation. Adjusting the ratio of TBT to DES allows for the control of TiO2's exposed facets.71 TiO2 nanoparticles can be synthesized by combining tetra butyl titanate and tetrabutylammonium bromide–glycerol DES. The hydrogen bond network within the DES influences TiO2's nucleation and growth by preferentially adsorbing on the nanoparticle surfaces.72
Inorganic salts are frequently employed to synthesize mono- or multi-metal oxides and their composites. Jaihindh et al. used varying molar ratios of 1ChCl–2urea and Cu(NO3)3·3H2O aqueous solution to produce Cl-doped CuO. Acting as both a green solvent and a Cl-source, the DES facilitated the synthesis of CuO catalysts.73 The Co3O4@Al2O3 3D mesoporous nanocomposite was synthesized by calcining a glucose–urea DES containing Al(NO3)3·9H20 and Co(NO3)2·6H2O (Fig. 5C). This unique composite, featuring a hierarchical structure with open 3D helical microstructures and well-distributed Co3O4 particles, enhances active site accessibility and reduces mass transport distances.74 Furthermore, CoMn2O4 hierarchical porous nanosheets (HPNSs) were synthesized through a microwave-assisted process involving preheating and calcination of Co(NO3)2·6H2O and Mn(NO3)2·4H2O dissolved in a glucose–urea DES (Fig. 5D). These nanosheets are characterized by their hierarchical porous structure.75 Separately, Luke et al. synthesized honeycomb-layered Na3Ca2BiO6 and orthorhombic Na3Ni2BiO6 using a betaine–glucose DES, where DES decomposition at high temperatures facilitated the formation of porous structures.76 Notably, LaCl3 NiCl2, LaCl3, and CoCl2 can dissolve in ChCl–malonic acid DES, in which a series of La-containing perovskite LaTO3 (T = Mn, Mn/Ni, or Co) was achieved by a two-step calcining process. The DES enables the uniform mixing of metal precursors and facilitates the formation of the active intermediate LaOCl. The intermediate further reacts with binary metal oxides (Mn3O4, NiO, or Co3O4) to form their respective perovskites.77
Fourthly, direct calcinating metal salts-based DESs can also yield metal oxides, and adding surfactants during the calcination process can improve the materials' specific surface area and pore size distribution. For example, CeOx nanoparticles were synthesized by calcining a 1Ce(NO3)3·6H2O–3.5urea DES with various surfactants. Cationic surfactants, particularly those with longer chain lengths, increased porosity, and surface area more effectively than non-ionic types.78 Additionally, 1SnCl2–2urea DES can mixed with tetraethyl orthosilicate to form a gel, which is then calcined to obtain SnO2/SiO2. Increasing the DES content in the sol–gel process reduces surface tension, shortens intermolecular colloidal interactions, and promotes the formation of smaller SiO2 particles with greater surface area.79
High-entropy oxides (HEOs), composed of five or more metallic elements, have attracted considerable attention for their unique structures and properties, distinguishing them from conventional oxides. These HEOs can be synthesized using DESs through a two-stage process. For example, Guan et al. utilized DESs of PEG–TU, EG–CA, and EG–urea with NiCl2·6H2O, MnCl2·4H2O, CoCl2·6H2O, FeCl3, and AlCl3, to synthesize diverse FeCuNiMnAl HEMs. By adjusting the composition and ratios of DESs, they achieved control over the morphology, influencing the metals' aggregation and structural form.80 Additionally, a microwave pretreatment followed by calcination of La(NO3)3·6H2O, Co(NO3)2·6H2O, Cr(NO3)3·9H2O, Fe(NO3)3·9H2O, Mn(NO3)2·4H2O, and Ni(NO3)2·6H2O in a glucose–urea DES facilitated the synthesis of La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3, alongside lithium- and sodium-doped variants. Including Li+ and Na+ improved surface oxygen vacancies and induced lattice distortions, resulting in finer grain sizes.81 This glucose–urea DES also enabled the microwave-assisted synthesis of various 1D and 2D HEOs such as rock-salt (CoCuMgNiZn)O, spinel (CoCrFeMnNi)3O4, and perovskite La(CoCrFeMeMiNi)O3. These HEOs exhibited uniform metal dispersion, substantial surface areas, and consistent single-phase structures enhanced by configurational entropy, making them suitable as negative electrode materials for LIBs.82
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, favoring the formation of stable Fe–F bonds. Additionally, the selective presence of Fe3+ and Co2+ in the DES prevents the creation of cobalt fluoride compounds, transforming HTB-type FeF3 into a 3D porous brick structure of interconnected nanoparticles.84
O, favoring the formation of stable Fe–F bonds. Additionally, the selective presence of Fe3+ and Co2+ in the DES prevents the creation of cobalt fluoride compounds, transforming HTB-type FeF3 into a 3D porous brick structure of interconnected nanoparticles.84
With the development of DES, more and more novel DESs were fabricated and utilized to prepare functional materials. Amino acid-based DESs demonstrate significant potential. Jiang et al. used a FeCl3·6H2O–CoCl2·6H2O–NiCl2·6H2O–L-cysteine DES to synthesize hierarchical FeCoNi-based nitro-sulfide (FeCoNi-NS), which uniformly incorporates N and S heteroatoms into Fe, Co, and N. This hierarchical porous structure enhances catalytic surface area and transport capability, which increases and diversifies active sites.85 In another study, Wei et al. explored the effects of amino acid structure on metal reduction by synthesizing Ni/Ni(OH)2 and metallic Ni from NiCl2·6H2O–serine NiCl2·6H2O–threonine DES, respectively. The presence of a methyl group in serine enhances its reduction capability, leading to complete reduction to metallic Ni, whereas threonine yields a unique ultrathin heterostructure of Ni (0) embedded in Ni(OH)2 nanosheets.86
The calcination of DESs effectively promotes the formation of stable, porous structures, thereby increasing the specific surface area and enhancing performance in catalysis, adsorption, and other applications. Key aspects include: (i) DES' strong eutectic properties enable effective integration with reactants, producing uniformly distributed mixtures; (ii) DES decomposition facilitates pore creation and influences the structural transition from crystalline to amorphous forms; (iii) the multi-component nature of DESs supports the multifunctionality of the resulting materials; (iv) the inherently low-toxic and environmentally friendly nature of DES components favors large-scale applications.
2.3 Microwave synthesis
Microwave synthesis, another one-pot process, involves directly loading all reagents into the microwave reactor. Unlike solvothermal and annealing, this method efficiently transmits energy via radiation interactions, significantly shortening reaction times. In this technique, DES precursors are uniformly and rapidly heated within microwave fields, capitalizing on conduction, polarization effects, and molecular interactions.DESs can dissolve organic compounds and biomass, facilitating microwave-assisted synthesis of carbon materials. For instance, glucose dissolved in various ChCl-based DESs produces CDs, with the DESs acting as passivating agents, dopants, and solvents. Modifying DES components allows for the tailored adjustment of heteroatom doping levels. Shorter carbon chains in the DES and higher concentrations of functional groups (–NH2, –OH) improve quantum yields.87 Kaur et al. processed powdered wheat straw with LA–ZnCl2 DES under microwave irradiation, converting it into oxidized graphitic material. This method includes using water to regenerate graphene, promoting DES recycling, and enhancing process sustainability.88 Xie's microwave-assisted pretreatment of bamboo holocellulose in a ChCl–zinc acetate (ZAC) DES yielded fermentable sugars and nanocellulose, preserving equipment and improving sustainability by altering the crystalline and microstructure of cellulose.89 Additionally, Adeyemi et al. synthesized AgInS2 and other compounds, adjusting concentrations to control crystallinity and particle sizes, using a two-step heating profile for thorough dissolution and size control.90,91 Therefore, microwave techniques in DES systems shorten reaction times, enhance yields, and allow precise control of material properties, though research in this area remains limited. Future studies should explore the potential of DESs and microwave synthesis for creating functional materials, potentially revolutionizing material synthesis and applications.
Solvothermal synthesis, microwave synthesis, and calcination are pivotal thermal treatment methods for synthesizing materials using DESs. These techniques allow meticulous control over reaction conditions—temperature, duration, and DES composition—tailoring various functional materials' microstructure and macroscopic properties. Solvothermal synthesis involves heating precursors with DESs in a sealed vessel, typically at lower temperatures than calcination, ensuring superior control over reaction conditions and product purity. This method is ideal for temperature-sensitive materials such as MOFs, COFs, metal sulfides, and phosphides. Conversely, calcination subjects materials to high temperatures to promote transformation and crystallization, which is crucial for developing specific crystal structures in porous carbon materials and metal oxides. Microwave synthesis leverages microwave radiation to expedite reactions, offering rapid processing at lower temperatures, thus enhancing safety and reducing energy consumption. It ensures even heating, which may result in more uniformly structured materials. While all three methods are effective for material synthesis, their application varies depending on the specific needs and properties of the target products.
3 Precipitation synthesis
Precipitation synthesis is a technique for preparing nanomaterials under ambient pressure and temperature, significantly reducing energy requirements. In this method, DESs act predominantly as inert solvent media, simplifying their recovery and reuse. Furthermore, DESs can engage with various reactants, such as acids, bases, oxidizers, and reducers, to facilitate coprecipitation. This approach also enables modifying materials within DESs to improve their existing properties or impart new functionalities. DESs serve three primary functions in precipitation synthesis: reaction media, coprecipitation facilitators, and surface-modifying agents.3.1 Reaction media
DESs, as green and economical reaction media, provide distinct advantages in precipitation synthesis. They facilitate reactions at lower temperatures than conventional methods, enabling efficient synthesis of desired products. DESs exhibit high solubility and diffusivity, ensuring uniform solute distribution and enhancing reaction efficiency and product purity. Additionally, their stability and recyclability contribute to minimal waste, aligning with the principles of green chemistry.Combining covalent organic frameworks (COFs) using DESs significantly reduces energy consumption, shortens reaction times, and eliminates the need for hazardous solvents. The composition of DESs and the controlled reaction parameters, such as time and temperature, are crucial in determining the structural characteristics of COFs. Deng et al. demonstrated the high yield and crystallinity of imine-linked COFs synthesized using a ChCl–hexafluoroisopropanol (HFsIP) DES, which also acted as a catalyst (Fig. 6A). They observed that a DES ratio of 1ChCl–4HFIP produced the highest crystallinity in TPB-TPDD-based COFs. Variations in solubility and acidity among different DES compositions affect these outcomes, with reaction conditions further refining crystallinity.92 Qiu et al. synthesized various 2D (keto-enamine, azine, and hydrazone-linked) and 3D imine-linked COFs using 1ChCl–2Gly, 1ChCl–2urea, 1ChCl–2EG DESs, achieving superior crystallinity and surface areas within 2 h at room temperature (Fig. 6B). Additionally, these DESs could be recycled up to three times with minimal loss in efficacy.93 Ce–Fe Prussian blue analogs (Ce–Fe PBAs) were synthesized in the ChCl–CA DES containing Ce(NO3)3·6H2O, K3[Fe(CN)6], water, and ethanol. The competitive complex of Ce3+–citrate ions and Ce3+–ferricyanide ions moderated the nucleation rate, yielding uniformly structured Ce–Fe PBAs. The crystallization of PBAs, influenced by the ethanol-to-water volume ratio and the molar ratio of DES to metal ions, dictated the morphology, dimensions, and chemical composition of the resultant Ce–Fe oxides following calcination.94
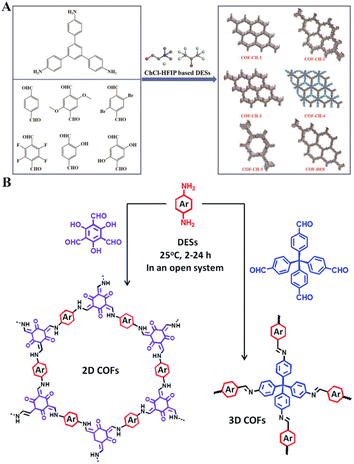 | ||
| Fig. 6 Framework materials fabrication via precipitation, utilizing DESs as the reaction medium. (A) Preparation processes of the selected imine-linked COFs. Reprinted with permission from copyright© American Chemical Society, 2023.92. (B) Schematic illustration of the syntheses of 2D and 3D COFs. Reprinted with permission from copyright© Royal Society of Chemistry, 2020.93 | ||
Chloride ions in DES significantly affect the reduction potential of noble metals, thereby enhancing their reduction rates. This makes ChCl-based DESs containing halide ions particularly effective for synthesizing noble metal nanomaterials. Kim et al. utilized 1ChCl–2acrylamide DES to produce a series of nanoparticles, including Au, Ag, Pd, Pd@Ag, and Au@Pd. In this process, acrylamide undergoes radical polymerization upon heating, transitioning from liquid to solid phase. This change facilitates the stabilization of high concentrations of Au NPs and ensures their uniform dispersion.95 Additionally, intermetallic PtPd nanocrystals were obtained in 1ChCl–2EG DES, in which the DES served dual roles as a reducing agent and shape-directing agent. This approach efficiently reduced Pt4+/Pd2 to PtPd nanocrystals within six minutes, producing octahedral nanoparticles.96 Moreover, DESs can act purely as reaction media without participating directly in the reactions. For example, when added to a DES solution containing zinc complexes, EG induces desolvation, promoting the aggregation and growth of ZnO crystals along the c-axis. In cases where ZnO is dissolved in a 1ChCl–2urea DES, the addition of EG results in the precipitation of uniquely shaped double-cone ZnO mesocrystals.97
External agents such as reducing agent hydrazine (N2H4·H2O), oxidizing agent (Na2S2O3·5H2O), and alkali (KOH) can be combined with metal salts in DESs to synthesize metal oxides or sulfides. For instance, adding N2H4·H2O to a ChCl–D-lactic acid DES containing ZnCl2 leads to the formation of rod-like and spherical ZnO nanoparticles.98 Furthermore, introducing N2H4·H2O into 1ChCl–2urea or 1ChCl–2EG DES results in the synthesis of HgS, ZrO2, MnO2, and CuO nanoparticles, which range in size from 50 to 150 nm after a specified reaction time.99 Na2S2O3·5H2O serves as both an oxidant and a sulfur source, facilitating the oxidation of NiCl2·6H2O into Ni3S4/NiS2 composites within 1ChCl–2EG DES. The DES provides structural stability and functions as a solvent and a template during synthesis. Adjusting the concentrations of NiCl2·6H2O and Na2S2O3·5H2O yields various structural forms of Ni3S4/NiS2, including wrinkled, lamellar, and layer-rolled morphologies.100
In polymerization, DESs often negate the need for additional initiators, simplifying the reaction setup, reducing costs, and enhancing controllability and repeatability. For instance, poly(furfuryl alcohol) (PFA) was polymerized from FA in ChCl–2ZnCl2 DES, eliminating the need for external solvents or initiators (Fig. 7A). The polymerization efficiency of FA increased with higher reaction temperatures and DES/FA weight ratios. Moreover, the DESs proved recyclable, demonstrating their potential for sustainable and large-scale PFA production.101 In another example, zwitterionic eutectogels were produced from acrylamide in various betaine (Bet)-based DESs, such as Bet–Gly, Bet–EG, and Bet–urea, facilitated by noncovalent interactions (Fig. 7B). The eutectogel from Bet–Gly DES exhibited superior properties, including an ion conductivity of 0.23 mS cm−1, an elongation capability of 1400%, and a self-healing property of 82.01%. These features made the eutectogel ideal for wearable self-adhesive strain sensors, capable of adhering to skin and sensitively monitoring body movements over a wide temperature range.102
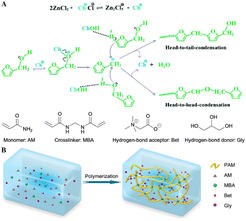 | ||
| Fig. 7 Polymer fabrication via precipitation, utilizing DESs as the reaction medium. (A) Proposed mechanism for the polymerization of FA catalyzed by DES. Reprinted with permission from copyright© Royal Society of Chemistry, 2022.101. (B) Schematic illustration of the polymerization of the PAM/Bet–Gly eutectogel. Reprinted with permission from copyright© American Chemical Society, 2023.102 | ||
DESs offer numerous advantages as reaction media: they facilitate synthesis under mild conditions, increase solubility and stability, enable recyclability, and simplify the system. These characteristics make DESs highly promising for diverse applications, including synthesizing COFs, noble metal nanoparticles, metal oxides and sulfides, and polymers. Ongoing research is anticipated to highlight their importance as green solvents further.
3.2 Coprecipitate agents
DESs facilitate coprecipitation with reactants, allowing for controlled nanoparticle synthesis by adding acids or bases. ChCl–EG, ChCl–glucose, and ChCl–EG–glucose DESs serve as reducing agents to convert KMnO4 into nanostructured manganese oxides.103 Amorphous MnO cathodes, synthesized using a betaine–urea DES, display short-range atomic order and numerous defects, with the betaine-to-urea ratio critically affecting the MnO2 structure (Fig. 8A). This optimization leads to cathodes with extensive surface areas and a mix of meso- and micropores, improving ion/electron transfer rates and overall performance.104 Additionally, the introduction of alkali into metal salt-based DESs triggers the formation of metal oxides/hydroxides, with the morphologies influenced by the decomposition temperatures of the metal salts. Diverse morphologies like rod-bundle FeO(OH), plate-like Co(OH)2, rod-like ZnO, and wedge-like nanoplate CuO were synthesized using ChCl–MClx (M = Fe, Zn, Co, Cu) DESs combined with KOH (Fig. 8B). The high viscosity of DESs slows ion diffusion, ensuring dense surface coverage and selectively inhibiting growth along specific axes to favor low-dimensional structures.105 Layered hydroxy salt Zn5(OH)8Cl2(H2O), a mixture of ZnO and Zn5(OH)8(CH3COO)2·2H2O, and flower-like ZnO were obtained from ZnCl2–3.54urea, Zn(NO3)2–3.54urea, and Zn(CH3COO)2–3.54urea DESs by adding NaOH at temperatures below 85 °C, respectively.106 The introduction of KMnO4 into metal-based DESs is another method for synthesizing metal oxides. CoMnO was produced using a CoCl2·6H2O–ChCl–glycerol DES, where the DES acted as a reductant, solvent, template, and cobalt source (Fig. 8C). Adding water modulated these interactions and controlled the reaction kinetics, transitioning from nanosheets to nanoparticles, leading to aggregation into larger clusters.107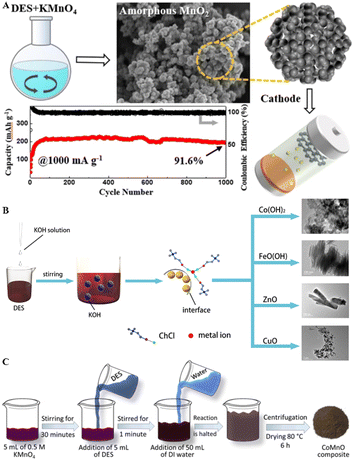 | ||
| Fig. 8 Metal oxide fabrication via precipitation, utilizing DESs as the coprecipitate agents. (A) Schematic illustration for the preparation of MnO2. Reprinted with permission from copyright© Springer Nature, 2024.104. (B) Nanomaterials formed by metal-based DESs: Mechanism of formation. Reprinted with permission from copyright© Royal Society of Chemistry, 2023.105. (C) Synthesis of CoMnO Nanostructures. Reprinted with permission from copyright© American Chemical Society, 2023.107 | ||
3.3 Surface modifying agents
DESs serve as a versatile platform for enhancing material performance through surface modification. For example, Liu et al. employed a 2ChCl–1DL-alanine–35water DES to modify magnetic COFs (MCOFs), significantly changing their surface properties (Fig. 9A). Despite a reduction in specific surface area and an increase in average pore size, the DES introduced numerous amino groups to the adsorbent's surface. This modification significantly enhanced the adsorption sites and improved the efficiency of Cu2+ adsorption.108 Similarly, Noorani et al. increased CO2/N2 separation efficiency in NH2-MIL-53(Al) MOFs by treating them with amine-functionalized DESs, such as ChCl–ethanolamine (DES1), ChCl–ethanolamine–diethanolamine (DES2), and ChCl–ethanolamine–methyl diethanolamine (DES3). The DES1-treated NH2-MIL-53(Al) doubled the CO2 adsorption capacity compared to its untreated counterpart.109 In another instance, Wang used ChCl–1,2-butanediol (BD) DES at varying ratios to treat copper tetrakis(4-carboxyphenyl) porphyrin (CuTCPP) membranes, archiving nanoconfined DES electrolytes (Fig. 9B).110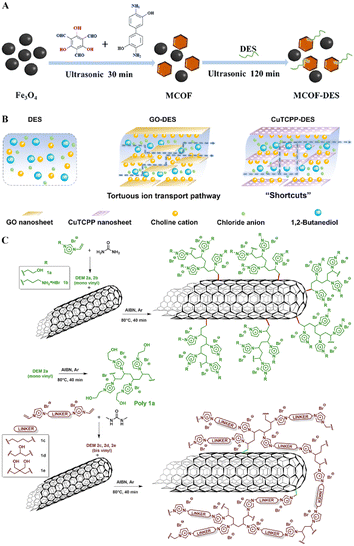 | ||
| Fig. 9 Materials fabrication via precipitation, utilizing DESs as the surface modifying agents. (A) Synthesis process of MCOF-DES for the extraction of Cu2+. Adapted with permission from copyright© Elsevier B.V., 2024.108. (B) Schematic illustration of DES, GO-DES, and CuTCPP-DES. Reprinted with permission from copyright© Royal Society of Chemistry, 2023.110. (C) Synthetic procedure for the preparation of DES-modified MWCNTs. Reprinted with permission from copyright© The Elsevier B.V., 2023.113 | ||
Moreover, typical carbon materials such as cellulose nanofibrils, graphite felt, and CNTs can be modified with DESs to improve their properties. Ye et al. used a phytic acid–sulfamic acid–urea DES for the pretreatment and modification of cellulose nanofibrils. This treatment generated phytates and sulfate-containing holocellulose nanofibrils (PHCNFs) that incorporated multiple P/N/S groups into the cellulose structure, significantly enhancing fibrillation efficiency and improving the fire resistance, mechanical strength, and optical properties of the PHCNFs.111 Similarly, graphite felt surfaces were modified using NH4Cl–FeCl3 or 1ChCl–2urea DES, enhancing the surface area by 46% and increasing disorder and defects within the graphite structure.112 Valentino et al. introduced a novel subclass of polymerizable deep eutectic monomers (DEMs) consisting of mono- and bis-vinyl imidazolium salts equipped with various functional groups (–OH, –NH2, –NH3+Br−). These DEMs served as both a dispersing medium and a structure-directing agent, facilitating free radical-induced polymerization directly on the MWCNTs' surfaces, which also acted as templates to guide the orderly polymer network formation (Fig. 9C).113
In addition, various DES-modified metal compounds with abundant functional groups have been developed. Ahmad et al. used tetra butyl phosphonium bromide and various carboxylic acids (hexanoic, pentanoic, butyric, propanoic, acetic, and formic acid) in a phosphoryl DES to enhance the surface of cerium oxide nanoparticles (CeNPs), achieving high CO2 adsorption at low pressure.114 Lauric acid-based DESs, combining lauric acid with capric acid, octoic acid, nonanoic acid, and thymol, were employed to modify Cs3Cu2I5, creating sensitive NH3 fluorescence sensors. These sensors effectively adsorb and react with NH3 on their surface, drastically reducing fluorescence intensity due to interactions with the –COOH group in DES.115 Furthermore, Fe(CO)5 was processed in a TBAC–decanoic DES to produce TBAC acid DES@Fe, subsequently enhanced with N, S-doped amorphous carbon to create N, S-doped amorphous carbon/TBAC acid DES@Fe. This design inhibits particle aggregation and leverages π–π interactions for adsorption.116
This chapter delves into the multifaceted roles of DESs in synthesis, underscoring their effectiveness as reaction solvents, coprecipitation agents, and surface modifiers, all under environmentally benign conditions that adhere to the principles of green chemistry. Initially, we discuss the role of DESs as reaction media. Their distinctive properties enable the dissolution of a broad spectrum of chemical entities, thereby boosting reaction yields and facilitating the recovery of solvents post-reaction, which minimizes environmental footprint. Furthermore, DESs actively engage in the synthesis process as coprecipitation agents, forming materials such as ZIFs, metal oxides, and sulfides. Their capacity as reducing agents demonstrates remarkable flexibility, broadening their application in creating innovative materials. In their capacity as surface modifiers, DESs bolster the performance of MOFs and carbon materials by enhancing stability, conductivity, and adsorption capabilities. This versatile functionality highlights the pivotal role of DESs in pushing the boundaries of material science within eco-friendly frameworks, offering groundbreaking solutions for sustainable advancement.
4 Electrodeposition
DESs have gained prominence in electrodeposition due to their exceptional physicochemical properties. These solvents are characterized by low volatility, high solubility, and a wide operational temperature range, enabling efficient dissolution of metal precursors and providing excellent conductivity and a stable electrochemical window. In electrodeposition, DESs enable precise deposition of nanomaterials, including alloys and carbon-based materials. Strategic inclusion of additives during the process tailors material morphology and incorporates beneficial impurities to enhance the quality of the deposits. Additionally, the environmental sustainability and tunability of DESs support the green chemistry objectives of contemporary industries.4.1 Precise composition control of alloys
ChCl-based DESs are extensively utilized in electrodeposition due to their superior ion conductivity, low viscosity, and excellent electrochemical stability. The composition of HBDs like urea, EG, and Gly significantly impacts the formation of metal complexes, influencing the structure of resultant metal alloys during electrolysis. Sales et al. demonstrated this by synthesizing Fe–Mn alloys using DESs composed of 1ChCl–2EG, ChCl–Gly, or 1ChCl–2urea DES. Variations in coordination complexation between Mn2+ and the HBDs led to significant differences in manganese content and morphology of the alloys.117 1D tellurium (Te) rods, preferentially growing along the [001] axis from the (100) planes, were electrodeposited onto a gold surface using 1ChCl–2urea and 1ChCl–2EG DESs. In these systems, chloride ions and EG or urea coordinated with Te4+ to form complexes facilitating one-dimensional growth under sufficiently negative potentials. The viscosity of the DES impacted the diffusion coefficients of these complexes, thereby influencing the microtopography and diameter of the Te rods.118Pd, Rh, and PdRh NPs were electrodeposited from 1ChCl–2urea DES containing respective metal chlorides, showcasing the adaptability of DESs in generating advanced materials with tailored properties.119 Notably, a concave triple-octahedral Au–Pd alloy was deposited using 1ChCl–2urea DES, exemplifying the potential of DESs in fine-tuning material properties for specific applications.120 Concave nanocubes of Pt–RE alloys (RE = La, Y, Sc) featuring high-index facets were also synthesized in DES, enhancing nitrogen reduction activity by strengthening the bond with reaction intermediates.121 Additionally, porous Ni and Fe NPs were electrolytically deposited onto stainless steel mesh from a 1ChCl–2urea containing NiCl2 and FeCl3. The resultant NiFe@SS electrode demonstrated a significantly larger ECSA than Ni@SS alone, delivering enhanced OER performance.122 Ni(OH)2/Ni/carbon felt composite electrodes were also produced via single-step electrodeposition in a water-involved 1ChCl–2urea system. Water content influenced the crystal structures and morphologies: with 10% water, nanosheets were evenly distributed, whereas at 20% water, they began to clump into irregular clusters.123
1ChCl–2EG DES is also frequently utilized for the electrochemical deposition of alloys. The composition of these alloys is determined by the concentration of metal precursors in the DES. For instance, Pd and Sn were electrodeposited onto SS using 1ChCl–2EG DES containing PdCl2 and SnCl2, forming Pd–Sn/SS electrodes. These electrodes consistently achieved nitrate conversion rates exceeding 90%, demonstrating that the preparation method in the DES has a significant impact on nitrate reduction.124 Deomar et al. used 1ChCl–2EG DES to dissolve Zn and Co salts for the electrodeposition of Zn, Co, and Zn–Co coatings. These DES-based electrolytes demonstrated high conductivity, abundant metal ions, resistance to high temperatures, and biodegradability. The atomic ratio of Zn to Co in the deposits accurately reflected their molar ratios in the electrolyte, highlighting DES's capability to dictate the composition of Zn–Co coatings precisely.125 Further research in the 1ChCl–2EG DES led to the electrodeposition of ternary Zn–Sn–In coatings on 1020 carbon steel substrates, enhancing corrosion resistance. Increasing the concentration of In3+ refined the grain size and improved deposition efficiency, affecting current density and charge time.126 Additionally, a Ni–Co–Zn alloy was pulse-electrodeposited from 1ChCl–2EG DES containing ZnCl2, CoCl2, and NiCl2. The resulting alloy coating was uniformly dense with spherical particles.127
Ternary ChCl–EG–Gly DES containing AgNO3 was applied to electrodeposit Ag and subjected to a galvanic replacement reaction with PdCl2 and HAuCl4 to fabricate a hollow dendritic Au/Pd/Ag electrode. The viscosity of the DES influences the transport rate of Pd2+ ions, affecting the speed of substitution reactions and the formation of the hollow structure. The duration of this substitution is critical in determining the electrode's morphology and composition.128 RuRhPdPtAu NPs can be fabricated through electrodeposition from 1ChCl–2urea DES containing RuCl3, RhCl3, PdCl2, K2PtCl6, and HAuCl3 (Fig. 10A). These NPs form spherical aggregates characterized by extensive defects, providing high entropy and numerous active sites essential for their functionality.129 Furthermore, FeCoNiCrMo is processed through electrochemical dealloying in a ChCl–TU DES, altering their surface and internal structures (Fig. 10B). This technique yields a 3D porous structure with particle diameters of approximately 100 nm, which optimizes the electronic configuration crucial for OER activity.130
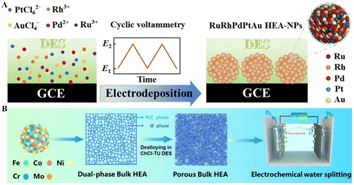 | ||
| Fig. 10 Fabrication of HEOs through the DES electrodeposition process. (A) Illustration of the programmed electrodeposition method for the preparation of RuRhPdPtAu HEA-NPs. Reprinted with permission from copyright© Royal Society of Chemistry, 2023.129. (B) Process outline for electrochemical dealloying in ChCl–TU DES of FeCoNiCrMo HEA. Reprinted with permission from copyright© American Chemical Society, 2023.130 | ||
4.2 Carbon-metal composites
Electrodeposition in DES electrolytes is an effective method for preparing and modifying carbon-metal materials. Liu et al. utilized a mixture of 1ChCl–2EG DES and ammonium chloride for pulse electrodeposition, producing amorphous carbon coatings (ACC) on 316L stainless steel. This technique facilitated strong carbon–iron bonding, significantly improving the substrate's corrosion resistance.131 Similarly, a film of tin-reduced graphene oxide (Sn-rGO) was electrodeposited onto copper plates using 1ChCl–2EG DES solution containing tin(II) chloride and graphene oxide, ensuring even dispersion of graphene oxide and seamless integration into the tin matrix. The presence of rGO influenced the crystal growth direction of Sn, suppressing growth along the (101), (211), (301), and (112) planes and promoting it on the (200), (220), (400), (321), (420), and (501) planes, thereby enhancing the composite's corrosion resistance.132 Furthermore, CNT has been modified effectively through electrodeposition. Employing a pulse reverse current electrochemical method in a ChCl–Gly DES containing CNTs enabled the deposition of Ag NPs, which significantly improved specific capacitance and capacitance retention.133 CNTs were templates for fabricating Ni/Sb co-doped TiO2 electrodes via electrolysis in a 1ChCl–2urea DES composed of NiCl2·6H2O, SbCl3, SnCl2·2H2O, and CNTs. This electrode exhibited a rough surface, high coating hardness, and adhesion strength, a uniform coating structure, abundant adsorbed hydroxyl groups, enhanced active oxygen species presence, and improved charge transfer rates.1344.3 Electropolymerized polymers
Specific polymer monomers are capable of undergoing electropolymerization in DESs. The polymers produced range from single-component polymers to composites, synthesized using binary DESs with or without additional water. Additionally, introducing acids into these DESs promotes polymerization.1ChCl–2urea DES, for example, was used to electropolymerize poly(1,5-diaminonaphthalene) (PDAN) onto CC electrodes, yielding PDAN/CC composites. As electropolymerization progressed, PDAN's morphology evolved from granular to compact, enhancing its electrochemical charge storage capabilities due to its amine/imino groups' redox activities and electrolyte anions' (de)intercalation.135 Pyrrole (Py) was electropolymerized in a ChCl–propionic acid DES with Fe3O4 NPs to produce a PPy/Fe3O4 nanocomposite, where the acid facilitated redox processes within the PPy membranes.136 Pyrrole was also polymerized in a ChCl–LA DES, incorporating graphene sheets into the polymer matrix on a TiZr substrate, creating TiZr–PPy–GS. This composite, serving as an effective drug reservoir, showed enhanced hydrophilicity and improved antibacterial properties.137 Melamine was electropolymerized onto MWCNT-modified GCE in 1ChCl–2EG DES, forming a PME/MWCNTs/GCE composite.138 Additionally, polymethylene blue (PMB) and Au NPs were sequentially electropolymerized and deposited onto screen-printed carbon electrodes (SPCE) with zinc oxide nanorods in 1ChCl–2EG DES, enhancing the electrode's functionality.139
Acid doping in DES has been shown to boost polymer growth more effectively than undoped systems. For instance, phenazine was electropolymerized in acid-doped 1ChCl–2EG DES and deposited on a Fe2O3 NP-modified GCE, resulting in a nanostructured poly(phenazine)/Fe2O3 film compelling for hydrogen peroxide biosensing.140 Furthermore, poly(cresyl violet) was electropolymerized on a CNT-modified GCE in 1ChCl–2EG DES mixture with acetic acid, where water enhanced the polymer's redox activity.141 Another complex, poly(thionine-methylene blue) (PTH-MB), was formed in a ChCl–malic acid–water system on electrochemically reduced graphene oxide-modified GCE (ERG/GCE), displaying notable electrocatalytic activity towards nicotinamide adenine dinucleotide.142 Similarly, polyneutral red (PNR) was developed in an acid-doped ChCl–ethylene glycol–fructose DES on a CNT-modified GCE, showing improved electrochemical performance and selectivity for acetaminophen detection.143 Hydrochloric acid (HCl) was incorporated to modulate the viscosity and improve the ionic conductivity of the ChCl–OA–EG DES, facilitating the formation of a robust polymethylene green (PMG) polymer.144
4.4 Non-carbon powder materials
Metal oxides, hydroxides, and phosphates can be electrodeposited from DES. For instance, γ-Fe2O3 is synthesized through two distinct methods: one involves electrolyzing iron anodes in 1ChCl–2urea either with145 or without146 phosphate. In the first method, iron atoms on the anode are oxidized to Fe3+, while in the second method, Fe2+ in the electrolyte is electrochemically converted to Fe3+. The resulting Fe3+ reacts with active oxygen produced during DES decomposition, forming iron oxide nanocrystals. Concurrently, amino group radicals generated from urea decomposition are grafted onto these particles, improving their dispersibility. Ni–Co–P films electrodeposited onto CC substrates from a methyl triphenylphosphonium bromide–EG DES display a surface rich in both amorphous and crystalline structures.147 Similarly, Ni–Mo–P coatings are obtained by electrolyzing a complex system containing NaH2PO2, citric acid, ammonium molybdate, NiCl2, and 1ChCl–2EG DES. The ionic nature of the DES promotes the formation of a rough Ni–Mo–P surface, providing abundant active sites for HER.148 Additionally, a nanoporous Fe-doped Ni(OH)2 catalyst with adjustable oxygen vacancies is synthesized through electrodeposition from an 1ChCl–2EG DES solution containing Ni(NO3)2 and NaH2PO2. Introducing water into the DES adjusts the hydrogen bonding between ChCl and EG, thus enhancing electrical conductivity and control over oxygen vacancies.1494.5 Strong coatings with organic and inorganic additives
In electrodeposition, incorporating organic compounds and inorganic salts is essential for improving the mechanical properties of metal coatings. These additives enhance smoothness and hardness and affect the growth and heteroatom doping within the coatings.Hasan et al. used 1ChCl–2EG DES to dissolve ZnCl2 with additives such as nicotinic acid (NA), boric acid, and benzoquinone for zinc electrodeposition. These additives, serving as brighteners, refined the smoothness and fineness of the zinc deposits. However, boric acid and benzoquinone adsorb on the cathodic electrode, inhibiting zinc nuclei growth and reducing film thickness. Typically, zinc grows along the (002), (101), and (102) planes, but NA and boric acid redirect their growth to the (100), (101), and (110) planes.150 Using a method involving supercritical carbon dioxide (SC-CO2)-assisted electrodeposition from 1ChCl–2urea DES with ZnCl2 and additives like threonine (Thr) or a NA/ascorbic acid (AA) mixture, the hardness of Zn coatings improved by 30% compared to conventional methods (Fig. 11A). This approach also altered the crystal structure, eliminating the hexagonal closest-packed zinc (100) diffraction peak and reducing grain size and hydrogen bubble formation, thus smoothing the cathode surface.151 Additionally, introducing 0.06 M NA during ZnO2 electrodeposition transformed the zinc morphology, significantly enhancing surface density and smoothness.152
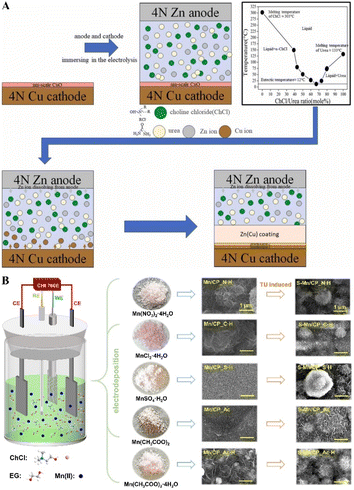 | ||
| Fig. 11 Fabrication of coatings through the DES electrodeposition process with additives. (A) Process outline for using SC–CO2-assisted electrodeposition to synthesize Zn coatings. Reprinted with permission from copyright© Elsevier Ltd, 2023.151. (B) Schematic diagram of Mn/CP and S-Mn/CP electrodes electrodeposited from 1ChCl–2EG DES containing different anions. Reprinted with permission from copyright© Elsevier B. V., 2023.155 | ||
Inorganic salts also significantly enhance the performance of electrodeposited coatings. For example, Ag is electrodeposited from 1ChCl–2EG DES electrolyte containing AgCl and KBr, which negatively shifts the reduction peak potential of Ag+, resulting in denser coatings and a preferred orientation on the Ag (111) crystal plane.153 Similarly, a mixture of boric acid and CuSO4·5H2O in 1ChCl–2EG DES produces a mirror-like nickel surface with improved quality and reduced roughness. The presence of Cu2+ in the DES enhances nickel electrodeposition uniformity.154 During the electrodeposition of S-doped Mn on carbon paper (S-Mn/CP) in 1ChCl–2EG DES containing various manganese salts and thiourea, nitrate anions and crystal water facilitate the formation of manganese oxide/hydroxide (Fig. 11B). In contrast, sulfate anions remain stable and do not participate in the reaction. The introduction of crystal water and thiourea partially replace chlorine in manganese complexes, forming [Mn(Cl)6−x(H2O)x]x−4 and [Mn(Cl)6−x−y(TU)y(H2O)x]x+y−4 complexes, which accelerate the electrodeposition process.155
This chapter delves into the electrodeposition of alloys, metal oxides, phosphides, hydroxides, carbon materials, and polymers using DESs. These solvents serve as a versatile medium for tailoring the properties of materials. The process exploits the unique electrochemical properties of DESs to control the synthesis of materials with precision, optimizing the deposition dynamics. Researchers utilize DESs to enhance materials' structural and functional attributes, often surpassing traditional methods' capabilities. Additionally, incorporating organic compounds or inorganic salts into DESs modulates nucleation and growth, aiding in grain refinement, surface tension adjustment, doping, and inhibitory effects. Consequently, electrodeposition in DESs facilitates the meticulous design of materials with bespoke properties, fostering innovative techniques and furthering advancements in materials science.
5 Outlook and summary
Deep Eutectic Solvents (DESs) have emerged as a promising class of green solvents with significant potential for material synthesis applications. These solvents serve as media and actively participate in the formation and structural regulation of materials, introducing innovative dimensions to materials science research. DESs outperform their pure components in material fabrication by offering enhanced solubility, improved reaction control, and superior material properties, while also being more sustainable and cost-effective. These advantages make DESs a promising choice for the development of advanced materials across various applications. It is crucial for future research to explore the potential, challenges, and innovations of DESs from diverse perspectives.Firstly, DESs offer unique synthesis capabilities that provide extensive opportunities for developing new materials through solvothermal synthesis, annealing, microwave synthesis, precipitation, and electrodeposition. Although known for their multifunctionality and environmental benefits, further research is necessary to understand their adaptability and selectivity across various material systems.
Secondly, understanding the interaction between DESs and materials' structural and functional properties is essential. The influence of DESs' molecular structure and composition on material synthesis remains under-explored, particularly in complex multi-component systems. Future studies should aim to elucidate the microscopic reaction mechanisms of DESs and their impact on the resulting materials' crystal structure, morphology, and properties, enabling precise control over material synthesis and the development of high-performance materials.
Thirdly, the role of DESs in material functionalization is receiving increasing attention. As modifiers, DESs can influence the surface properties of materials, impacting their performance in catalysis and energy storage. Current research is often limited to specific instances, and systematic studies are needed to understand the surface modification effects of DESs across different material systems and their overall impact, which could lead to the development of novel functional materials and broaden the applications of DESs.
Fourthly, the advantages of DESs make them promising for green chemistry applications, but their potential limitations highlight the need for further research. Efforts to lower viscosity, such as the incorporation of co-solvents, may mitigate some drawbacks but require careful evaluation to avoid compromising their environmental benefits. Similarly, expanding our understanding of DES stability and environmental impact will be critical for their wider adoption. While DESs represent a significant step forward in sustainable solvent technology, their current drawbacks necessitate targeted innovations to optimize their performance and broaden their industrial applicability.
Finally, the potential of DESs in emerging fields such as energy storage, environmental remediation, and biomaterials is only beginning to be realized. Future research should focus on developing innovative functional materials based on DESs and evaluating their practical applications. As knowledge of DESs expands, more potential applications may emerge, advancing the field of materials science.
In conclusion, DESs are poised to influence the future of material synthesis significantly. Although substantial progress has been achieved, many challenges must be addressed. It is crucial to investigate the applications of DESs across various synthesis methods, elucidating their mechanisms of action and enhancing their industrial applications. These efforts will establish DESs as a critical tool in advancing materials science. Future research is anticipated to produce transformative outcomes, driving the development of DESs within materials science and engineering.
Data availability
The data that support the findings of this study are available in the request from the corresponding author (E-mail: jiangjingyun@zzu.edu.cn) upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21903070), the Advantageous Discipline Cultivation Joint Fund of Henan Province (No. 22610031), and the China Scholarship Council (202207045006).References
- N. Winterton, Clean Technol. Environ. Policy, 2021, 23, 2499–2522 CrossRef PubMed.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Brohl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- G. Kaur, H. Kumar and M. Singla, J. Mol. Liq., 2022, 351, 118556 CrossRef CAS.
- S. S. de Jesus and R. Maciel Filho, Renewable Sustainable Energy Rev., 2022, 157, 112039 CrossRef CAS.
- F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33 CrossRef CAS.
- S. P. Ijardar, V. Singh and R. L. Gardas, Molecules, 2022, 27, 1368 CrossRef CAS.
- S. Azmi, M. F. Koudahi and E. Frackowiak, Energy Environ. Sci., 2022, 15, 1156–1171 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 1, 70–71 RSC.
- C. Florindo, F. S. Oliveira, L. P. N. Rebelo, A. M. Fernandes and I. M. Marrucho, ACS Sustain. Chem. Eng., 2014, 2, 2416–2425 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- D. O. Abranches, M. A. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. Coutinho, Chem. Commun., 2019, 55, 10253–10256 RSC.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich and B. W. Doherty, Chem. Rev., 2020, 121, 1232–1285 CrossRef PubMed.
- Y. Ma, Y. Yang, T. Li, S. Hussain and M. Zhu, Green Chem., 2024, 26, 3627–3669 RSC.
- R. Deng, M. Gao, B. Zhang and Q. Zhang, Adv. Energy Mater., 2024, 14, 2303707 CrossRef CAS.
- D. Yu, Z. Xue and T. Mu, Cell Rep. Phys. Sci., 2022, 3, 100809 CrossRef CAS.
- N. N. Nam, H. D. K. Do, K. T. L. Trinh and N. Y. Lee, Nanomaterials, 2023, 13, 1164 CrossRef CAS.
- M. Teixeira, R. A. Maia, L. Karmazin, B. Louis and S. A. Baudron, CrystEngComm, 2022, 24, 601–608 RSC.
- R. A. Maia, B. Louis and S. A. Baudron, Dalton Trans., 2021, 50, 4145–4151 RSC.
- R. A. Maia, A. Fluck, C. Maxim, B. Louis and S. A. Baudron, Green Chem., 2023, 25, 9103–9108 RSC.
- X. Wang, L. Wang, K. K. Rani, X. Peng, Y. Ning, X. Liu, Y. Fan, D.-H. Chen and W. Chen, Inorg. Chem. Front., 2023, 11, 98–106 RSC.
- H. Ren, Y. Liu, R. Zhang, Y. Zheng, T. Zhao, J. Han, C. Chen and E. Duan, J. Environ. Chem. Eng., 2023, 11, 109988 CrossRef CAS.
- R. Zhang, Y. Zheng, Q. Zhang, Z. Wu, L. Wang, J. Zhang, H. Ren and E. Duan, J. Environ. Chem. Eng., 2024, 12, 112391 CrossRef CAS.
- M. Tian, D. Wang, Q. Liu, L. Wang, Y. Tao, J. Wang, Y. Zou, Y. Yang, Q. Zhou, L. Li, M. Wang, X. Li and D. Gao, J. Mol. Liq., 2024, 397, 112391 CrossRef.
- G. Fu, C. Gao, K. Quan, H. Li, H. Qiu and J. Chen, Anal. Bioanal. Chem., 2023, 415, 4255–4264 CrossRef CAS PubMed.
- P. Lai, H. Zhou, Z. Niu, L. Li, W. Zhu and L. Dai, Chem. Eng. J., 2023, 457, 141255 CrossRef CAS.
- S. Chinnapaiyan, H. T. Das, S.-M. Chen, M. Govindasamy, R. A. Alshgari, C.-H. Fan and C.-H. Huang, J. Alloys Compd., 2023, 931, 167553 CrossRef CAS.
- X. Liu, C. Huang, M. Waqas, L. Wang, D. Huang, Q. Huang, Z. Yang, Y. Fan, C. Hou and W. Chen, Mol. Catal., 2023, 551, 113612 CrossRef CAS.
- R. Wang, X. Ma, D. Ding, B. Huang, Z. Zhu, T. Su, W. Liao, H. Lue and K. Yang, Inorg. Chem. Front., 2023, 10, 5420–5429 RSC.
- S. K. Shahi, S. Sandhu, N. Kaur, J. S. Shahi, M. Kaur, V. Singh and V. Singh, New J. Chem., 2022, 46, 18865–18873 RSC.
- G. Yao, N. Zhang, Y. Zhang and T. Zhou, J. Alloys Compd., 2022, 892, 162205 CrossRef CAS.
- M. M. Stanley, V. A. Sherlin, S.-F. Wang, B. Sriram, J. N. Baby and M. George, J. Environ. Chem. Eng., 2023, 11, 110185 CrossRef CAS.
- S. Liu, B. Zhang, Z. Yang, Z. Xue and T. Mu, Green Chem., 2023, 25, 2620–2628 RSC.
- H. Zhao, W. Li and R. Wang, Mater. Adv., 2022, 3, 7125–7131 RSC.
- T. Zhang, T. Doert and M. Ruck, Z. Anorg. Allg. Chem., 2017, 643, 1913–1919 CrossRef CAS.
- B. Mahto, A. A. Khan, A. Barhoi and S. Hussain, ACS Appl. Nano Mater., 2023, 6, 6784–6797 CrossRef CAS.
- T. Kokulnathan, T.-J. Wang, F. Ahmed and S. Kumar, J. Mol. Liq., 2023, 369, 120785 CrossRef CAS.
- H. Yang, Z. Cheng, P. Wu, Y. Wei, J. Jiang and Q. Xu, Electrochim. Acta, 2022, 427, 140879 CrossRef CAS.
- W. Chen, X. Bai, Z. Xue, H. Mou, J. Chen, Z. Liu and T. Mu, New J. Chem., 2019, 43, 8804–8810 RSC.
- Z. Cheng, H. Yang, Y. Xu, J. Jiang and Q. Xu, Mater. Today Chem., 2022, 26, 101214 CrossRef CAS.
- H. Zhang, Y. Zhao, Z. Cheng, J. Jiang, J. Fu and Q. Xu, Small, 2024, 20(47), 2405225 CrossRef CAS.
- Y. Xu, Z. Cheng, J. Jiang, J. Du and Q. Xu, Chem. Commun., 2021, 57, 13170–13173 RSC.
- Y. Wei, Y. Xu, H. Zhang, J. Jiang and Q. Xu, New J. Chem., 2024, 48, 11206–11210 RSC.
- J. Jiang, P. Yan, Y. Zhou, Z. Cheng, X. Cui, Y. Ge and Q. Xu, Adv. Energy Mater., 2020, 10, 2002214 CrossRef CAS.
- X. Su, X. Shao, Y. Wang, W. Fan, C. Song and D. Wang, ACS Appl. Nano Mater., 2023, 6, 23029–23036 CrossRef CAS.
- R. Xue, R. Deng, Y. Li, M. Gao, J. Wang and Q. Zhang, Green Chemical Engineering, 2024, 6(1), 93–101 CrossRef.
- K. Zou, Z. Guan, Y. Deng and G. Chen, Carbon, 2020, 161, 25–35 CrossRef CAS.
- D. Zhang, X. Zhan, T. Zhou, J. Du, K. Zou and Y. Luo, J. Mater. Sci. Technol., 2024, 193, 22–28 CrossRef CAS.
- R. Huang, A. Zhong, K. Huang, Y. Yu, Y. Tang and P. Xia, J. Environ. Chem. Eng., 2023, 11, 111474 CrossRef CAS.
- D. Aydemir, M. E. Ergun, S. K. Gulsoy, Z. E. Ozan and G. Gunduz, Biofuels Bioproducts & Biorefining-Biofpr, 2024, 18, 251–264 Search PubMed.
- M. Xu, X. Zhu, Y. Lai, A. Xia, Y. Huang, X. Zhu and Q. Liao, Appl. Energy, 2024, 353, 122095 CrossRef CAS.
- Q. Liu, T. Wang, C. Wang and D. Wu, Chem. Eng. J., 2024, 481, 148292 CrossRef CAS.
- C. Wang, T. Wang, Q. Liu and D. Wu, Fuel, 2024, 362, 115965 Search PubMed.
- C. O. Ehi-Eromosele, C. N. Onwucha, S. O. Ajayi, G. Melinte, A.-L. Hansen, S. Indris and H. Ehrenberg, RSC Adv., 2022, 12, 34670–34684 RSC.
- R. Zhou, C. Xu, J. Yang, D. Guan and J. Cai, Chem. Lett., 2020, 49, 585–588 CrossRef CAS.
- D. Li, Y. Huang, Z. Li, L. Zhong, C. Liu and X. Peng, Chem. Eng. J., 2022, 430, 132783 CrossRef CAS.
- D. Zhang, C. Sun, D. Liu, C. Song and D. Wang, Sci. China Mater., 2023, 66, 1362–1372 CrossRef CAS.
- H. Mou, J. Wang, D. Zhang, D. Yu, W. Chen, D. Wang and T. Mu, J. Mater. Chem. A, 2019, 7, 5719–5725 RSC.
- M. M. Stanley, V. A. Sherlin, S.-F. Wang, J. N. Baby, B. Sriram and M. George, J. Mol. Liq., 2023, 375, 121308 CrossRef.
- P. Yang, X. Wei, L. Zhang, S. Dong, W. Cao, D. Ma and Y. Ouyang, Molecules, 2023, 28, 2995 CrossRef CAS PubMed.
- H. Li, S. Xu, H. Qi, X. Cao, D. Ju, C. Chen, Y. Chen, S. Li and L. Chang, Energy Technol., 2023, 11, 2201400 CrossRef CAS.
- S. Xu, X. Cao, F. Li, H. Li, H. Qi, J. Zhang, C. Chen and D. Ju, J. Alloys Compd., 2023, 936, 168080 CrossRef CAS.
- M. Zhang, X. Wu, X. Liu, H. Li, Y. Wang and D. Wang, Molecules, 2023, 28, 7878 CrossRef CAS.
- Y. Li, Y. Bao, B. Han, Y. Zhuang, H. Li, C. Chen and D. Ju, Flatchem, 2024, 45, 100635 CrossRef CAS.
- Y. Zhang, Z. Xue, X. Zhao, B. Zhang and T. Mu, Green Chem., 2022, 24, 1721–1731 RSC.
- M. Iwanow, J. Seidler, L. Vieira, M. Kaiser, D. Van Opdenbosch, C. Zollfrank, T. Gaertner, M. Richter, B. Koenig and V. Sieber, Catalysts, 2021, 11, 542 CrossRef CAS.
- S. Zhang, M. Sun, K.-Y. Wang, L. Cheng, S. Zhang and C. Wang, ACS Sustain. Chem. Eng., 2021, 9, 2358–2366 CrossRef CAS.
- H. Ying, T. Chen, C. Zhang, J. Bi, Z. Li and J. Hao, J. Colloid Interface Sci., 2021, 602, 64–72 CrossRef CAS.
- A. Soeldner, J. Zach, M. Iwanow, T. Gaertner, M. Schlosser, A. Pfitzner and B. Koenig, Chem.–Eur. J., 2016, 22, 13108–13113 CrossRef CAS PubMed.
- S. Hong, R. M. Doughty, F. E. Osterloh and J. V. Zaikina, J. Mater. Chem. A, 2019, 7, 12303–12316 RSC.
- B. He, Y. Li, T. Zhang, Y. Shi, K. Li, F. Dai, R. Zhang, R. Liu and S. Zhang, J. Phys. Chem. B, 2020, 124, 3743–3753 CrossRef CAS.
- S. Sandhu, M. Kaur, N. Sharma, N. Kaur and V. Singh, Catal. Sci. Technol., 2022, 12, 6717–6727 RSC.
- W. Song, C. Liu, J. Yan, L. Zhou and L. Zhang, J. Photochem. Photobiol., A, 2024, 451, 115482 CrossRef CAS.
- D. P. Jaihindh, P. Anand, R.-S. Chen, W.-Y. Yu, M.-S. Wong and Y.-P. Fu, J. Environ. Chem. Eng., 2023, 11, 109852 CrossRef CAS.
- Y. Wang, K. Rong, J. Wei, S. Chang, D. Yu, Y. Fang and S. Dong, Nano Res., 2023, 16, 11430–11443 CrossRef CAS.
- J. Wei, K. Rong, Y. Wang, L. Liu, Y. Fang and S. Dong, Nano Res., 2023, 16, 10381–10391 CrossRef CAS.
- E. J. Luke, J. Potticary, S. Friedemann and S. R. Hall, Dalton Trans., 2023, 52, 3188–3194 RSC.
- S. Hong, A. M. Diez, A. N. Adeyemi, J. P. S. Sousa, L. M. Salonen, O. I. Lebedev, Y. V. Kolen'ko and J. V. Zaikina, ACS Appl. Mater. Interfaces, 2022, 14, 23277–23284 CrossRef CAS PubMed.
- I. Manasi, M. R. Andalibi, R. Castaing, L. Torrente-Murciano and K. J. Edler, J. Mater. Chem. A, 2022, 10, 18422–18430 RSC.
- X.-y. Liu, X.-p. Li, R.-x. Zhao and H. Zhang, New J. Chem., 2021, 45, 15901–15911 RSC.
- S. Guan, B. Xu, J. Wu, J. Han, T. Guan, Y. Yang, K. Li and J. Wang, Fuel, 2024, 358, 130315 CrossRef CAS.
- M. Bao, S. Chen, X. Shao, H. Deng, A. Mao and J. Tan, Chin. J. Chem., 2024, 82, 303–313 CAS.
- J. Wei, K. Rong, X. Li, Y. Wang, Z.-A. Qiao, Y. Fang and S. Dong, Nano Res., 2022, 15, 2756–2763 CrossRef CAS.
- C. Lai, K. Chen, M. Lei, J. Hu, S. Chen and C. Li, Adv. Funct. Mater., 2024, 34, 2312415 CrossRef CAS.
- C. Lai, K. Chen, Y. Zheng, J. Meng, J. Hu and C. Li, J. Energy Chem., 2023, 78, 178–187 CrossRef CAS.
- J. Jiang, L. Chang, W. Zhao, Q. Tian and Q. Xu, Chem. Commun., 2019, 55, 10174–10177 RSC.
- Y. Wei, J. Jiang, J. Dong, Y. Xu, J. Fu and Q. Xu, Green Chem., 2022, 24, 8014–8020 RSC.
- R. Tabaraki and F. Nazari, J. Photochem. Photobiol., A, 2023, 444, 114891 CrossRef CAS.
- H. Kaur, M. Singh, K. Singh, A. Kumar and T. S. Kang, Green Chem., 2023, 25, 5172–5181 RSC.
- Y. Xie, J. Zhao, P. Wang, Z. Ji, Z. Ling and Q. Yong, Ind. Crops Prod., 2023, 191, 115965 CrossRef.
- A. N. Adeyemi, R. A. Earnest, T. Cox, O. I. Lebedev and J. V. Zaikina, Molecules, 2022, 27, 1815 CrossRef CAS.
- A. N. Adeyemi, M. Clemente, S. J. Lee, A. Mantravadi and J. V. Zaikina, ACS Appl. Energy Mater., 2022, 5, 14858–14868 CrossRef CAS.
- L. Deng, L. Wang, S. Liu, Q. Liu, J. Wang, Y. Tao, M. Tian, Y. Yang, Y. Zou, H. Niu, D. Wang and D. Gao, Macromolecules, 2023, 56, 7707–7720 CrossRef CAS.
- J. Qiu, P. Guan, Y. Zhao, Z. Li, H. Wang and J. Wang, Green Chem., 2020, 22, 7537–7542 RSC.
- S. Wei, H. Li, K. Li, R. Zhang, G. Wang and R. Liu, Ind. Eng. Chem. Res., 2022, 61, 17842–17853 CrossRef CAS.
- Y. H. Kim, J.-H. Oh and J.-S. Lee, J. Ind. Eng. Chem., 2022, 112, 182–192 CrossRef CAS.
- B. Li, H. Zhang, J. Kaelin, S. Gao, F. Xia, B. An, K. Lu and Y. Cheng, ACS Appl. Nano Mater., 2023, 6, 3184–3190 CrossRef CAS.
- H. Wagata, G. Harada, E. Nakashima, M. Asaga, T. Watanabe, Y. Tanaka, M. Tada and K. Yubuta, Crystengcomm, 2021, 23, 8367–8378 RSC.
- H. Jabeen, S. Shaukat and H. M. A. U. Rahman, J. Mol. Liq., 2024, 395, 123783 CrossRef CAS.
- R. Balaji and D. Ilangeswaran, Mater. Today: Proc., 2022, 56, 3366–3375 CAS.
- Y. Zhang, J. Ru, P. Huang, Y. Jiang and E. Wu, Adv. Powder Technol., 2023, 34, 104200 CrossRef CAS.
- C. Thanh Phuong, H. Chuc Nguyen, V.-Q. Hieu, D. M. Kabtamu, S. Kumar, N. Van Cuong and C. Xuan Thang, New J. Chem., 2022, 46, 3786–3793 RSC.
- Q. Lu, H. Li and Z. Tan, ACS Appl. Mater. Interfaces, 2023, 15, 34055–34063 CrossRef CAS.
- K. Aruchamy, N. Maalige, M. M. Halanur, A. Mahto, R. Nagaraj, D. Kalpana, D. Ghosh, D. Mondal and S. Kotrappanavar Nataraj, Chem. Eng. J., 2020, 379, 122327 CrossRef CAS.
- X. Peng, P. Liu, T. Zeng, H. Cui, M. Wang, J. Li, K. Liu, Q. Wang and Y. Liu, J. Sol-Gel Sci. Technol., 2024, 109, 695–706 CrossRef CAS.
- H. Chen, W. Jiang, N. Zhao, X. Zhang, X. Ma, H. Jia, Y. Zhuang and M. Guan, New J. Chem., 2023, 47, 7903–7909 RSC.
- L. Gontrani, D. T. Donia, E. M. Bauer, P. Tagliatesta and M. Carbone, Inorg. Chim. Acta, 2023, 545, 121268 CrossRef CAS.
- A. Samage, K. Pramoda, M. Halakarni and N. S. Kotrappanavar, ACS Appl. Energy Mater., 2023, 6, 2412–2422 CrossRef CAS.
- Y. Liu, X. Yang, J. Hu, N. Lu, D. He, H. Chi, Y. Liu, S. Yang and X. Wen, Anal. Chim. Acta, 2024, 1290, 342197 CrossRef CAS.
- N. Noorani and A. Mehrdad, Sci. Rep., 2023, 13, 13012 CrossRef CAS.
- X. Wang, Y. Wang, Y. Kang, B. Yao and X. Peng, Nanoscale, 2023, 15, 15626–15634 RSC.
- J. Ye, Y. Gao, Q. Xu, Z. Jin, G. Wu, S. Wang, Z. Cai, K. Yang, Q. Wu and Q. Li, Chem. Eng. J., 2023, 477, 147142 CrossRef CAS.
- L. M. Murillo-Herrera, E. S. Aguilar, M. W. Thielke and A. Jorge Sobrido, Chem.–Asian J., 2023, 18, e202201208 CrossRef CAS PubMed.
- L. Valentino, R. Di Forti, A. Morena, C. Aprile, M. Gruttadauria, F. Giacalone and V. Campisciano, Chem. Eng. J., 2024, 489, 151447 CrossRef CAS.
- T. Ahmad, J. Iqbal, M. A. Bustam, M. Babar, M. B. Tahir, M. Sagir, M. Irfan, H. M. A. Asghar, A. Hassan, A. Riaz, L. F. Chuah, A. Bokhari, M. Mubashir and P. L. Show, Environ. Res., 2023, 222, 115314 CrossRef CAS PubMed.
- Q. Zheng, Y. Zhou, J. Huang, H. Lu, S. Wu, J. Zhang, Y. Li, W. Hong, X. Tan, J. Lin, Q. Chen, F. Li, Z. Cai and M. Zhang, J. Alloys Compd., 2024, 986, 174155 CrossRef CAS.
- A. Bazzaz Dilmaghani, M. R. Afshar Mogaddam, F. Monajjemzadeh and M. A. Farajzadeh, Anal. Sci., 2022, 39, 169–178 CrossRef.
- V. Sales, C. Paternoster, D. Mantovani and G. Kolliopoulos, Journal of Ionic Liquids, 2024, 4, 100086 CrossRef.
- L. P. M. dos Santos, R. M. Freire, S. Michea, J. C. Denardin, D. B. Araujo, E. B. Barros, A. N. Correia and P. De Lima-Neto, J. Mol. Liq., 2019, 288, 111038 CrossRef CAS.
- V. A. Medina, M. G. M. de Oca, J. I. Aldana, M. A. Romero and M. E. Palomar, ECS Trans., 2023, 110, 103 CrossRef.
- F. Liu, C. Chen, X. Jiang, J.-Y. Guo, Y. Wei, J.-W. Li, T. Sheng, X. Zhao and L. Wei, ACS Sustain. Chem. Eng., 2023, 11, 1631–1637 CrossRef CAS.
- Y.-J. Mao, F. Liu, Y.-H. Chen, X. Jiang, X.-S. Zhao, T. Sheng, J.-Y. Ye, H.-G. Liao, L. Wei and S.-G. Sun, J. Mater. Chem. A, 2021, 9, 26277–26285 RSC.
- P. M. Viyanni, T. N. J. I. Edison and M. G. Sethuraman, Materials Today Sustainability, 2023, 24, 100565 CrossRef.
- Y.-H. Liu, H.-W. Guo and F.-Y. Zeng, J. Power Sources, 2023, 570, 233043 CrossRef CAS.
- W.-F. Kuan, C.-L. Chen, M. S. Ahmad, C.-H. Hsieh, H. M. Chen and J. F. Su, Adv. Sustainable Syst., 2024, 8, 2300425 CrossRef CAS.
- D. N. Rodrigues-Junior, N. G. Sousa, F. M. T. Luna, T. M. B. F. Oliveira, D. S. Abreu, W. Schwarzacher, P. de Lima-Neto and A. N. Correia, J. Electroanal. Chem., 2023, 947, 117785 CrossRef CAS.
- J. C. Pereira, L. P. M. dos Santos, A. A. C. Alcanfor, O. S. Campos, P. N. S. Casciano, A. N. Correia and P. de Lima-Neto, Electrochim. Acta, 2022, 407, 139647 CrossRef CAS.
- B. Li, C. Zheng, C. Pan, F. Pan, T. Lv, X. Wang, X. Ju, K. Gong, W. Zou and G. Yi, Mater. Chem. Phys., 2024, 317, 129071 CrossRef CAS.
- C.-W. Lin, Y.-H. Chen, P.-C. Chou and Y.-T. Hsieh, Microchem. J., 2024, 200, 110328 CrossRef CAS.
- C. Chen, J. Guo, J. Liu, W. Li, Y. Wei, H. Wang, X. Zhao and L. Wei, Chem. Commun., 2023, 59, 12863–12866 RSC.
- Y.-c. Xu, W.-j. Chen, J.-f. Zhou, C.-b. Hu, S.-w. He, H. Liu and Z.-s. Hua, Langmuir, 2024, 40, 14291–14302 CrossRef CAS PubMed.
- W. Liu, W. Li, W. Dong, L. Guo, Y. Zou, C. Yu, C. Sun, N. Huang and X. Sun, J. Power Sources, 2024, 606, 234518 CrossRef CAS.
- S. Costovici, A. Pantazi, D. Balan, A. Cojocaru, T. Visan, M. Enachescu and L. Anicai, Metals, 2023, 13, 203 CrossRef CAS.
- A. T. S. C. Brandão, S. Rosoiu, R. Costa, O. A. Lazar, A. F. Silva, L. Anicai, C. M. Pereira and M. Enachescu, J. Mater. Res. Technol., 2021, 15, 342–359 CrossRef.
- Z. Zhang, Z. Wang, Y. Sun, S. Jiang, L. Shi, Q. Bi and J. Xue, J. Electroanal. Chem., 2022, 911, 116225 CrossRef CAS.
- X. Lai, Z. Dang, L. Wang, P. Li, Y. Yang and C. Wang, J. Energy Storage, 2024, 91, 112032 CrossRef.
- I. B. Qader, H. K. Ismail, H. F. Alesary, J. H. Kareem, Y. T. Maaroof and S. Barton, J. Electroanal. Chem., 2023, 951, 117943 CrossRef CAS.
- M. E. Voicu, F. Golgovici, M. Prodana, D. Draganescu and I. Demetrescu, Materials, 2023, 16, 4387 CrossRef CAS.
- Y. H. Chang, P. M. Woi and Y. B. Alias, Electrocatalysis, 2021, 12, 238–250 CrossRef CAS.
- T. Atici, M. B. Kamac, M. Yilmaz and A. Y. Kabaca, Electrochim. Acta, 2023, 458, 142484 CrossRef CAS.
- W. da Silva, A. C. Queiroz and C. M. A. Brett, Electrochim. Acta, 2020, 347, 136284 CrossRef CAS.
- R. M. Buoro, J. M. S. Almeida and C. M. A. Brett, Electrochim. Acta, 2024, 490, 144305 CrossRef CAS.
- M. Ding, T. Hou, H. Niu, N. Zhang, P. Guan and X. Hu, J. Electroanal. Chem., 2022, 920, 116602 CrossRef CAS.
- X. Liang, Y. Zhou, J. M. S. Almeida and C. M. A. Brett, J. Electroanal. Chem., 2023, 936, 117366 CrossRef CAS.
- J. M. S. Almeida, Z. S. B. Pedro, R. M. Buoro and C. M. A. Brett, Chem.–Eur. J., 2024, 30(58), e202401752 CrossRef CAS.
- H. Jia, J. Sun, M. Dong, H. Dong, H. Zhang and X. Xie, Nanoscale, 2021, 13, 19004–19011 RSC.
- H. Jia, F. Zhang, J. Sun, S. Jiang, Q. Yin, Y. Zhang and X. Xie, Colloids Surf., A, 2023, 660, 130864 CrossRef CAS.
- Y. Wu, Z. Zhou, Q. Yao, J. Wang, Y. Tian, S. Liu and C. Wang, J. Alloys Compd., 2023, 942, 169070 CrossRef CAS.
- Y. Hu, W. Li, L. Li, J. Wu, S. Sheng and H. Wang, Appl. Catal., A, 2023, 662, 119267 CrossRef CAS.
- Q. Li, M. Gao, M. Cheng, H. Li, Y. Hua, Q. Zhang and J. Ru, Electrochim. Acta, 2024, 478, 143859 CrossRef CAS.
- H. F. Alesary, S. Cihangir, A. D. Ballantyne, R. C. Harris, D. P. Weston, A. P. Abbott and K. S. Ryder, Electrochim. Acta, 2019, 304, 118–130 CrossRef CAS.
- C.-Y. Lee, W.-Y. Chen, J.-K. Chang and H.-B. Lee, Mater. Today Commun., 2024, 38, 107941 CrossRef CAS.
- S. Xu, F. Li, C. Chen, N. Bai, D. Ju, J. Zhang and X. Li, J. Mol. Liq., 2024, 397, 124094 CrossRef CAS.
- C. Zhan, R. Zhang, X. Fu, H. Sun, X. Zhou, B. Wang, H. Li and J. Sun, Ionics, 2023, 29, 4325–4335 CrossRef CAS.
- G. Qadr, M. I. Awad, K. Haji, J. A. Jumaa and H. H. Abdallah, J. Mol. Liq., 2023, 378, 121584 CrossRef CAS.
- M. Guo, B. Zhang and Q. Zhang, Appl. Surf. Sci., 2024, 645, 158843 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |