Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advancing laccase-catalysed depolymerisation of lignocellulosic biomass with the help of ionic liquids or deep eutectic solvents
Yumiko Takagi
 a and
Toshiyuki Itoh
a and
Toshiyuki Itoh
 *b
*b
aDepartment of Science, Faculty of Education, Kagawa University, 1-1 Saiwai-Cho, Takamatsu, Kagawa 760-8522, Japan. E-mail: takagi.yumiko@kagawa-u.ac.jp
bBiomass Green Innovation Centre, Kanazawa University, Kakuma-cho, Kanazawa City, Ishikawa 920-1192, Japan. E-mail: toshiyuki1111@mac.com
First published on 4th April 2025
Abstract
Selective oxidative depolymerization of a lignocellulosic biomass is the first step in the valorisation process. Chemical oxidations generally require hazardous reagents and harsh reaction conditions; thus, the resulting produced compounds are low-value molecules or complicated mixtures. Laccases are copper ion-containing oxidases and catalyze the oxidation of polyphenol or amine derivatives using molecular oxygen; laccase-mediated reaction systems thus allow the depolymerization of lignocellulosic compounds and the decomposition of aromatic pollutants in wastewater. However, due to the short distance between the active site and the surface of the laccase, the reactivity of laccase is influenced by the reaction conditions, in particular, the solvent system. Ionic liquids (ILs) and deep eutectic solvents (DESs) have now been acknowledged as not only new reaction media but also as activating agents of biocatalysts. In order to improve the activity or increasing the tolerance of laccases against ILs or DESs, three methods have been developed: the first is the direct evolution of the enzyme that is a very powerful tool for tailoring the enzyme, the second is the design of supporting materials including ILs for the immobilization of a laccase, and the third is modification of the surface of a laccase protein by chemical methods or protein engineering. This review examines laccase-mediated reactions in ILs and DESs focusing on how laccase contributes to sustainable chemistry; using laccase-mediated reactions, the depolymerization of lignocellulosic compounds, phenolic compounds, and synthetic dyes has now been accomplished. Since the reactions were accomplished under hazardous chemical reagent-free conditions, it is expected that investigation in this field of laccase-mediated oxidation might become even more important in sustainable chemistry.
Sustainability spotlightLignocellulosic compounds are highly branched amorphous polymers composed of polyphenol derivatives and moieties connected to polyhydroxylated alkanes and sugars via C–O and C–C bonds with very complex networks. This makes it difficult to achieve their valorisation, though they are viewed as important bio-renewable sustainable resources of aromatic compounds. Laccases allow hazardous reagent-free highly selective oxidative chemical conversions of lignocellulosic compounds with the help of ionic liquids (ILs) or deep eutectic solvents (DESs). |
1 Introduction
Lignocellulosic biomass consists of three components,1–3 cellulose, hemicellulose, and lignin, as illustrated by the schematic model in Fig. 1.1–3 Although the lignocellulose industry has a long history, only cellulose has been used in our life in its pure form. On the other hand, the difficulty of separating each component limits utilization of lignin and hemicellulose and prevents their economically feasible conversion into value-added products.4–8 It is generally easy to depolymerize cellulose and hemicellulose into the corresponding monomers since they consist of glycosyl bonding once these moieties are isolated from lignocellulosic materials.1–3 Lignin is found in most terrestrial plants in the approximate range of 15 to 40% dry weight and provides structural integrity.1–3 This compound is a highly branched amorphous polymer composed of polyphenol derivatives. It is thus viewed as an important bio-renewable resource of aromatic compounds.1–3 However, lignin consists of phenyl propanoid units and moieties connected to polyhydroxylated alkanes via C–O and C–C bonds with very complex networks as illustrated in Fig. 1. This makes it difficult to achieve the selective depolymerization of lignin.7,8 For this reason, the major industrial use of lignin is as a low-grade fuel; more than 50 million tons of lignin is produced per year as a black syrup byproduct during the paper production process, and almost all is just burned as an inefficient fuel.4 However, it is obvious that lignin may become not a low-grade energy source but a high-value aromatic resource for the pharmaceutical and chemical industries, if selective depolymerization of lignin is achieved (Fig. 1).4–12 The key to the valorisation of lignin is how to achieve selective depolymerization, in particular, by means of an environment-friendly methodology.7,8 Therefore, converting lignin to valuable small molecules by environmentally friendly means is an important and very challenging issue from the standpoint of sustainable chemistry.4–26 As shown in Fig. 1, lignin mainly consists of three aromatic alcohols with C–O–C and C–C bondings, while cellulose and hemicellulose connect the mono sugars by glycosidic bonding or ester bonding. Depolymerization of the glycosidic bond and ester bond is easily accomplished by a hydrolysis reaction, while it is difficult to depolymerize C–O–C and C–C bonded products; it is essential to apply an oxidation process to disconnect these bonds present in lignin. However, chemical-depolymerization processes via oxidation processes of organic compounds require hazardous reagents and harsh reaction conditions. Enzymatic depolymerization of lignocellulosic biomass thus represents an emerging research frontier.7–11,16,18–26 The combination of laccase-catalysed oxidation with a chemical or enzymatic hydrolysis reaction seems to be an important method to achieve the depolymerization of lignocellulosic compounds.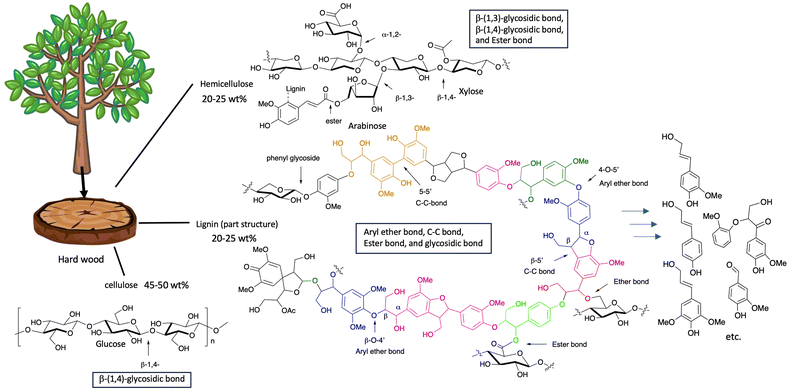 | ||
| Fig. 1 Schematic image of the “structure model of the core part of lignocellulose”.4–12 The tree image was obtained from the following site: https://tegakisozai.com/archives/29099 (accessed 17th February 2025). | ||
It is known that four types of lignin-degrading oxidases, i.e., laccases (EC 1.10.3.2),27–31 lignin peroxidases (EC 1.11.1.14),27 manganese peroxidases (EC 1.11.1.13),27,33 and aryl alcohol oxidases (EC 1.1.3.7),27,34 play a key role in the depolymerization of lignocellulosic materials in nature.32–45 Among lignin-degrading oxidases, we are fascinated by laccases, because these enzymes are expected to be the most promising tools for the oxidative depolymerization of polyphenol derivatives; in fact, a wide variety of applications have been reported for laccase-mediated reactions.28–45 However, there is a serious difficulty in achieving the selective depolymerization of lignocellulosic materials; these compounds show a poor solubility in water, while enzymatic reactions generally take place in an aqueous solution under optimal pH conditions. For biocatalytic reactions, the optimization of reaction media and supporting materials of the enzymes must be specifically performed in parallel with the effort to develop new enzymes.
Ionic liquids (ILs)46 possess very good properties as reaction media in chemical reactions; they are less-volatile and less-flammable and have a low toxicity and unique solubility for organic and inorganic materials.46–51 ILs are now being used as reaction media for biotransformations. Some hydrolases, in particular, lipases, can work even in a pure IL.51 A high number of applications have been reported for biocatalytic reactions in ILs, and the IL-mediated activation of enzymes was also reported.50,51 In fact, Nishihara et al. reported the amazing recyclable use of IL-supported lipase with more than 200 repeated reactions over two years in ILs.52
Deep eutectic solvents (DESs)53,54 are now widely acknowledged as a new class of ionic liquid (IL) analogues because they share many characteristics and properties with ILs.55 Due to their green properties, biological acceptance, and large variation, DESs have recently attracted strong interest as the reaction media of biocatalysis.55–58
Swatloski, Rogers and co-workers reported the first example of the dissolution of cellulose using an IL, 1-butyl-3-methylimidazolium chloride ([C4mim]Cl), and this opened the door to sustainable biomass engineering using ILs.59 Hinckley and co-workers reported the first example of a laccase-catalysed reaction in the presence of two types of ILs in 2002; the authors reported that laccase worked in the presence of a buffer aqueous solution containing a low concentration of 1-butyl-4-methylpyridinium tetrafluoroborate ([4-MBP][BF4]) and 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]), while no reaction took place in a pure IL.60 Turner and Rogers et al. produced an IL-regenerated cellulose film entrapping laccase and this immobilized enzyme exhibited a significant activity in an aqueous buffer solution.61 Barreca et al. demonstrated the oxidation of 4-methoxybenzyl alcohol as a lignin model compound using laccase in the presence of four types of mediator molecules in a mixed solvent of a buffer aqueous solution and organic solvent.62 Moniruzzaman and Ono attempted to isolate cellulose from wood biomass using ILs.63 Encouraged by these trials, an investigation started for both the chemical- and enzymatic-depolymerization of lignocellulosic materials in ILs or DESs. The laccase-catalysed reaction with the aid of ILs or DESs has recently become a significant topic in the field of biocatalysis.6,7,17,19,24–26,55,64–79
We previously published a review article regarding the laccase-catalysed reaction in ILs in 2021.68 Further interesting results have been accumulated after publication of our article, and a number of review papers about laccases in ILs and DESs have also been published in recent years,64–79 because the valorisation of lignocellulosic biomass is regarded as a particularly important topic in the field of sustainable chemistry. This article comprehensively reviews the field of the laccase-mediated depolymerization of lignocellulosic biomass and several persistent chemicals. ILs and DESs have now been acknowledged as the activating agents of biocatalysts. The aim of this review is to clarify the key points to improve laccase-mediated reactions with the help of ILs and DESs and provide an insight to readers on how to use laccase-mediated reactions for sustainable chemistry.
2 Structure of laccase and typical laccase-catalysed reactions
Laccase (EC 1.10.3.2) was first discovered 140 years ago by a Japanese chemist, Dr Hikorokuro Yoshida, as an enzyme which catalyses the coagulation of the sap of the Japanese lacquer tree Rhus vernicifera (Urushi).80 Laccases are glycoproteins with molecular weights of 50–130 kDa and belong to copper-containing oxidases that mediate the oxidative degradation or polymerization of the polyphenol formation in plants by inducing the radical polymerization of polyphenol derivatives.81,82,84–86 These enzymes truly play a key role in the sustainability of life on this planet. Therefore, laccases are widely found in plants, fungi, bacteria, and insects.80–82,84–86 More than 100 types of laccases have been isolated from nature.87Fig. 2 shows a visible representation of laccase from Trametes versicolor.44,81,82 This enzyme protein can be divided into three domains (D1, D2, and D3) and contains four copper ions, and these copper ions allow the oxidation of the substrate molecules. Four copper ions make a cluster in which three copper ions (T2, T3a, and T3b) and the T1 copper ion are separated, but the T1 copper ion connects with the T2, T3a, and T3b copper ion cluster through a His–Cys–His tripeptide moiety; the T1 copper was responsible for the blue color of this enzyme and the T1 and T2 coppers were paramagnetic and thus detectable by EPR spectroscopy. On the other hand, the T3a–T3b coppers were an antiferromagnetically coupled di-nuclear copper–copper pair and were thus EPR silent. The substrate is first bound to the T1 copper ion and then electron transfer occurred through cooperation of the T3a–T3b and T2 copper ions.81,82
 | ||
| Fig. 2 Ribbon diagram of laccase from Trametes versicolor (PDB ID: IGYC). The bottom part is a representation model of the Cu cluster in the active site. This picture was produced using data reported in ref.81 and 82. | ||
Laccase can thus convert substrates to corresponding radicals using one oxygen molecule as an oxidant through a single electron oxidation process and produce two water molecules. The T1 copper ion is embedded 6.5 Å from the surface of the enzyme;82 the distance between the entrance part and the active center is shorter than that in many enzymes such as an esterase. For instance, it was reported that the distance between the entryway from the active site of Candida antarctica lipase (triglycerol acylhydrolase) (EC 3.1.1.3) is estimated to be 8 Å.83 This indicates that laccase-catalysed reactions are more easily influenced by the solvent system compared to hydrolases, because the active site is located nearer to the surface of the enzyme. On the other hand, the short distance from the entrance part to the active center of laccase enables this enzyme to accept very large molecules, and this is the origin of the reason why laccases can accept such a large lignocellulosic molecule as substrates. Laccase mediated reactions are basically the single electron oxidation of phenols or amines using molecular oxygen.18,29,88
Laccases thus allow the four-proton reduction of oxygen to water and produce water as the only by-product without the use of hydrogen peroxide. During this pathway, laccase can oxidize various persistent phenolic compounds to produce radical intermediates as illustrated in Fig. 3 (eqn (1)). Furthermore, laccases allow the oxidation of aryl-ether compounds to afford the corresponding radical products (Fig. 3, eqn (2)) with the help of redox mediator molecules, i.e., 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1-hydroxybenzotriazole (HOBT), 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO), etc. (Fig. 3).17,29,76–78 Therefore, the laccase-catalysed reaction of lignocellulosic compounds affords at least six types of oxidation products (A to F in Fig. 4).68,75 Compounds A, B, and F should be produced via Cα–Cβ bond-cleavage and subsequent Cα oxidation as shown in eqn (1) and (2). Compound D should be produced by the aryl ether bond cleavage and subsequent Cα-oxidation via the process illustrated in eqn (2). Compounds C and E should be produced via Cα-oxidation in eqn (1) and (2) in Fig. 3. Of course, repolymerization of the radical products also takes place to produce complex polymers. The depolymerisation of lignocellulosic compounds might be accomplished through these oxidation pathways.
 | ||
| Fig. 3 Laccase-mediated oxidation.17,29,76–78 | ||
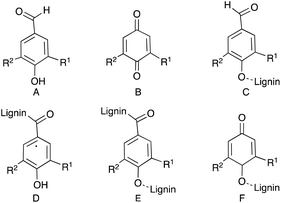 | ||
| Fig. 4 Products of laccase-mediated oxidation of lignocellulosic compounds.68,75 | ||
As already mentioned, the important characteristic of laccase is the short distance from the entrance part to the active center of laccase; this enables laccase to accept very large molecules such as lignocellulosic compounds. However, this indicates that the activity of a laccase is strongly influenced by the solvent system. In fact, Nowak et al. reported that laccase was inhibited by common polar organic solvents, i.e., dimethyl sulfoxide, methanol, acetonitrile, ethanol, and acetone, in the reaction of 4,4′-[(1E,2E)-1,2-hydrazindiylidendi(E)methylyliden]bis(2,6-dimethoxyphenol))(syringaldazine: SYR) and 2,6-dimethoxyphenol (2,6-DMOP); the inhibitory effect was higher for SYR than for 2,6-DMOP.89 The results suggest that the laccase activity is modified by both the substrate and solvent system, and the laccase-catalysed reaction of a large substrate is critically influenced by the solvent system.
Roohi and co-workers investigated the interaction of Trametes versicolor laccase (TvL) against three ILs, i.e., N-decyl-N,N,N-trimethylammonium bromide ([N1,1,1,10]Br), 1-decyl-3-methylimidazolium chloride ([C10mim]Cl), and cholinium decanoate ([Ch][Dec]), by a molecular dynamics (MD) simulation study.90 Both the cationic and anionic parts of the ILs affect the laccase structural dynamics. Compared to [N1,1,1,10]Br, [Ch][Dec] generated more hydrogen bonds with the laccase residues. Both the [N1,1,1,10] and [C10mim] cations bind to the enzymes more strongly than the [Ch] cation. However, the decanoate, [Dec] anion, modified the three-dimensional structure of this enzyme and destabilized it by interacting with the positively charged regions. The authors suggest that the long-chain alkyl groups of the ILs form a cluster near the T1 copper site and entrance part of the active site. Even at low [Ch][Dec] concentrations, the [Dec] anion group penetrates the enzyme around the copper T1 site, and a huge cluster of [Dec] anions occupies the active site entrance (Fig. 5). Therefore, [Ch][Dec] had a greater direct and indirect effect on the laccase enzyme as a solvent than the other two ILs and decreased the laccase activity.90 These results clearly indicated that the appropriate design of ILs or DESs might be the key to realize the desired laccase-mediated reaction.
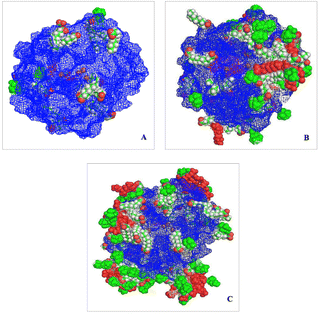 | ||
| Fig. 5 The ionic liquid interaction with the laccase protein and [Ch][Dec] at different concentrations. (A) 50 mM; (B) 100 mM; (C) 250 mM. Red spheres: [Ch] cation, white-green spheres: [Dec] anion, and orange spheres: Cu ions.90 Copyright Elsevier, 2024. | ||
3 How to improve the laccase activity
3.1 Reactivity of laccase-catalysed reaction in ILs
Prior to discussing laccase-mediated reactions, possible means to enhance the laccase activity using solvent engineering were initially examined. As already mentioned, the activity of laccases was generally strongly influenced by the reaction media.Hinckley et al. demonstrated the oxidation of veratryl alcohol using laccase C which was isolated from Trametes sp. in a mixed solvent of ILs with a citrate buffer (pH 5.6) solution; veratryl aldehyde was thus obtained in 86% yield when the reaction was carried out in the presence of a 25% (v/v) [4-MBP][BF4] buffer solution (pH 5.6) using N-hydroxyphtalimide (NHPI) as the mediator (Fig. 6).60 Anthraquinone was also attained in 15% yield by the laccase-catalysed oxidation of anthracene in a mixed solvent of a 25% (v/v) [4-MBP][BF4] buffer solution using 1-hydroxybenzotriazole as the mediator, while no product was obtained in the mixed solvent of 20% (v/v) tert-butanol in the buffer solution. It was further found that the enzyme showed no catalytic activity in the anhydrous ILs.60
 | ||
| Fig. 6 Laccase-catalysed oxidation of veratryl alcohol in a mixed solvent of [4-MBP][BF4] (25% v/v) and citrate buffer (pH 5.6) in the presence of NHPI as the mediator.60 | ||
Tavares and co-workers investigated the stability of laccase in a mixed solvent of a buffer with water soluble ILs and organic solvents, such as CH3CN or DMSO, under various pH conditions by the oxidation of ABTS.91 Since a clear and quick colour change from ABTS to ABTS++ was attained and the change was easily detected using a UV/visible detector at 420 nm, this reaction has been employed as an indicator of the activity of oxidases.92 Laccases generally worked well under weak acidic conditions. Tavares et al. tested the additive effect of ILs under different pH conditions and found that the enzyme exhibited an activity even at pH 9.0 in the presence of the IL, 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy)ethyl sulfate ([C2mim][C1(OC2)2OSO4]), and found that this IL effectively stabilized the enzyme (Fig. 7).91
 | ||
| Fig. 7 Additive effect of an IL ([C2mim][C1(OC2)2OSO3]) on the laccase-mediated oxidation of ABTS.91 | ||
Shipovskov et al. investigated laccase activity using the ABTS oxidation as a model reaction; the relative activity of Agaricus bisporus laccase (AbL)-catalysed oxidation of catechol increased ca. 1.9-fold when using a 15% (v/v) of [C4mim]Br solution of sodium phosphate-citrate buffer (pH 6.0).93 An approx. 1.5-fold enhanced activity was also obtained for the Trametes versicolor laccase (TvL)-catalysed reaction in a 20% (v/v) [C4mim]Br mixed solvent. However, the concentration of ILs over 60% (v/v) completely inhibited these enzymes. For these reactions, since the Km values were significantly increased by the addition of the ILs, it was assumed that the affinity of the enzyme against a substrate decreased in the IL mixed solvent system. A very unique additive effect was also obtained for 1-butyl-3-methylimidazolium dicyanamide ([C4mim][N(CN)2]); the relative activity of AbL decreased to half at 30% (v/v), but it increased thereafter and reached a maximum level of ca. 1.25-times higher at 50% (v/v) compared to the control reaction but the activity was completely lost at over a 70% (v/v) concentration.93
Domínguez et al. reported the additive effect of ILs on the TvL-catalysed oxidation of ABTS.94 The authors found that the addition of 1-ethyl-3-methylimidazolium ethylsulfate ([C2mim][EtSO4]) and [C4mim]Cl inhibited the laccase activity at a different rate, 10-fold more [C2mim][EtSO4] than [C4mim]Cl was required to cause the same degree of inhibition. However, [C2mim][EtSO4] appeared to have a stabilizing effect on the laccase at low concentrations.94 Rodriguez et al. further investigated the activity of the TvL-catalysed oxidation of ABTS in the presence of three imidazolium chloride ILs; 10% (v/v) of [C4mim]Cl effectivity stabilized TvL, while [C10mim]Cl inhibited the enzyme under the same conditions. The alkyl chain length of the imidazolium ring influenced the activity and inactivation of the laccase increased with the length of the alkyl chain in the ILs: [C10mim]Cl > [C8mim]Cl > [C4mim]Cl.95
Rehmann and co-workers conducted a very careful study of the additive effect of ILs against a laccase. They examined 63 types of water-miscible and water-immiscible ILs against TvL using the oxidation of ABTS as the model reaction.96 The formation of the ABTS radical cation was monitored at 420 nm every 25 s. The reaction was then allowed to reach completion, and subsequently, after 110 min, an additional aliquot of ABTS solution (5 μl) was added, to confirm that the reaction had previously stopped due to substrate depletion and to check for enzyme inactivation during the reaction (see, Fig. 8(a)). As shown in Fig. 8, laccase activity depended on the ILs. They evaluated the additive effect of ILs on the laccase-mediated ABTS oxidation and found that the addition of the ILs generally resulted in increased Km values that indicated reduced substrate selectivity, and an enhanced Vmax value means an increased reactivity. The laccase activity tended to be retained in water-immiscible ILs, such as the [AOT] and [NTf2] anions. Among their tested ILs, [P6,6,6,14][NTf2] was found to be the best IL that supports the laccase activity (Fig. 8).96 Three groups also reported that ILs contributed to an enhanced activity and improved thermal stability.97–99
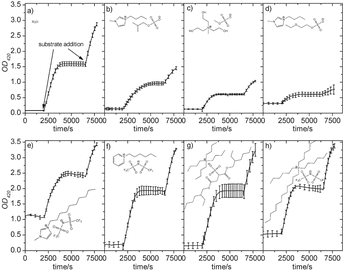 | ||
| Fig. 8 Time course of laccase-catalysed ABTS oxidation in the presence of ILs.96 (a) Control. (b) [C4mim][C3(2-C1)OSO3], (c) [N1,(2OH)3][C1OSO3], (d) [C4mim][C2OC2OSO3], (e) [C10mim][NTf2], (f) [C6py][NTf2], (g) [N4,4,4,4][AOT], and (h) [P6, 6,6,14][NTf2]). (b), (c), and (d) are aqueous buffer (pH 4.5) solutions of the water-miscible ILs (20% (v/v). (e), (f), and (g) are biphasic mixtures of buffer (pH 4.5) with water-immiscible ILs (20% (v/v)). Copyright RSC, 2012. | ||
As already mentioned, several types of mediator molecules have been employed for the laccase-catalysed reaction (see Fig. 3). However, mediator molecules generally deactivated the enzyme. Rehmann and co-workers solved this problem using a biphasic ionic liquid/water reaction system (Fig. 9).100 Three ionic liquids, i.e., [N1,8,8,8][AOT], [C6mim][AOT], and [N1,8,8,8][NTf2], dramatically improved the stability of the laccase in the presence of phenothiazine (Fig. 9b). Without the ionic liquids, the enzyme lost 93% of its activity after only 92 h, and there was no residual activity after 188 h. In the presence of [N1,8,8,8][AOT], [N1,8,8,8][NTf2], or [C6mim][AOT], the loss of activity was much slower, and 35, 31, or 35% of the initial activity was still retained after 188 h, respectively (Fig. 9b). On the other hand, for the reaction using TEMPO, only [N1,8,8,8][Sac] stabilized the enzyme (Fig. 9e). It was postulated that by choosing a suitable ionic liquid, the reactive mediator is preferentially partitioned into the ionic liquid phase away from the enzyme in the aqueous phase.100
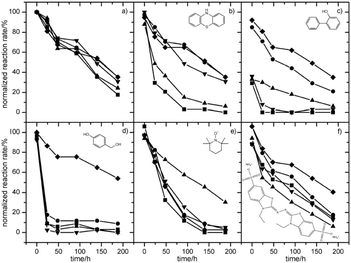 | ||
Fig. 9 Relative activity of laccase in the presence of mediators and ionic liquids. (a) no mediator, (b) phenothiazine, (c) 2-hydroxybiphenyl, (d) 4-hydroxybenzylalcohol, (e) TEMPO, and (f) ABTS in biphasic systems containing [N1,8,8,8][AOT] (●), [N1,8,8,8][Sac] (▲), [N1,8,8,8][NTf2] (▼), or [C6mim][AOT] (◆) and compared to controls without ionic liquids (■). The volume ratio of the ionic liquid–water was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The reaction rates are expressed as a percentage of the rate in a control sample taken immediately after mixing the laccase with a buffer solution (no mediator and no ionic liquid, time = 0, V0 = 0.113 μM s−1).100 Copyright RSC, 2014. 10. The reaction rates are expressed as a percentage of the rate in a control sample taken immediately after mixing the laccase with a buffer solution (no mediator and no ionic liquid, time = 0, V0 = 0.113 μM s−1).100 Copyright RSC, 2014. | ||
Harwardt et al. reported that the presence of 15% (v/v) [C2mim][EtSO4] helped stabilize the laccase activity using ABTS as a mediator.101 The authors postulated that the increased conductivity at low IL concentrations expectedly leads to an increasing reaction rate of the mediator with a substrate, whereas further increasing the IL concentrations leads to higher viscosities and, correspondingly, lower reaction rates. Munk et al. reported the influence of mediators on laccase catalysed radical formation.102 These results clearly indicate that use of ILs as an additive is effective in maintaining the enzyme activity during laccase-catalysed oxidation using mediator compounds.
Ferreira et al. demonstrated an interesting laccase-catalysed reaction using thermos-reversible aqueous biphasic systems composed of water-soluble ammonium-based zwitterions (ZI: N,N,N-tripentyl-3-sulfonyl-1-propaneammonium ([N5,5,5C3S]) and PEG400 in the presence of ABTS as a mediator (Fig. 10).103 This reaction system showed a homogeneous state at rt, while small changes in the temperature induced the formation of two phases and caused the complete separation of the enzyme from the products. The system also allowed the recovery and reuse of the enzyme, and five repetitions of the reaction were accomplished using their system. The oxidation reaction took place in a homogeneous medium, followed by the enzyme and product separation in liquid–liquid systems promoted by small changes in the temperature. It should be noted that this system avoids vigorous stirring to improve the mass transfer, as typically carried out in heterogeneous reactions.
 | ||
| Fig. 10 Oxidation of ABTS using laccase in a mixed solvent of PEG and IL, N5,5,5C3S. Selective separation from the reaction product and catalyst by changes in the temperature.103 Copyright RSC, 2018. | ||
It is known that a high concentration of imidazolium ILs inhibits the laccase. Due to the short distance from the entrance part and active site of the laccase, ILs can easily incorporate into the active site of the laccase. Sun and co-workers reported insight into the inhibition mechanisms of imidazolium ILs against Myceliophora thermophila laccase (MtL) by a combination of a kinetics analysis and molecular simulation.104 The docking test of MtL with ILs suggests that alkylimidazolium cations are competitively bound to the T1 Cu active pocket in the laccase through hydrophobic interactions (Fig. 11).104 Based on the results, the authors concluded that cations with shorter alkyl chains (C2–C6) entered the channel inside the pocket, exhibiting a high compatibility with laccase (competitive inhibition constant Kic = 3.36–3.83 mM). Under the same conditions, [C8mim]Cl (Kic = 2.15 mM) and [C10mim]Cl (Kic = 0.18 mM) with longer alkyl chains bound with Leu296 or Leu297 near the pocket edge and Leu429 around T1 Cu resulted in a stronger inhibition. The authors postulated that complexation with alkylimidazolium cations shifted the pH optima of laccase to the right by 0.5 units, which might lead to invalidation of the Hofmeister series of anions. The EtSO4 anion showed a higher biocompatibility than the acetate anion ([OAc]) or Cl ion, probably due to its binding near the T1 Cu and hindering the entry of alkylimidazolium cations.104
 | ||
| Fig. 11 Results of the docking test of ABTS (a) and five alkylimidazolium cations (b–f) bound to the TI Cu active pocket of Myceliophora thermophila laccase.104 MDPI, 2017, Open Access. | ||
Patel et al. investigated the inhibitory effect of ILs against Trametes versicolor laccase (TvL) using four types of imidazolium chloride ILs ([C2mim]Cl, [C4mim]Cl, [C6mim]Cl, and [C8mim]Cl) and four types of 1,1,3,3-tetramethylguanidinium amino acids [TMG][AA] ([TMS][Ser], [TMG][Thr], [TMG][Asp], and [TMG][Glu]) using the ABTS oxidation as a model reaction.105 The authors found that the inhibitory effect of the imidazolium ILs increased with the length of the alkyl chain in the ILs: [C8mim]Cl > [C6mim]Cl > [C4mim]Cl > [C2mim]Cl. The results are the same as those reported by Rodriguez et al.95 On the other hand, they found a very interesting inhibitory effect of [TMG][AA] ILs. [TMG][Ser] strongly inhibited TvL and no oxidation of ABTS took place in the presence of 50 mM [TGM][Ser]. [TMG][Thr] also strongly inhibited the reaction, while both [TMG][Asp] and [TMG][Glu] didn't influence the laccase activity.105 They investigated the origin of the inhibitory action of [TMG][Ser] by MD simulation study and found that the Ser anion was incorporated by the enzyme at a deeper position of T1 Cu2+ than the substrate ABTS and competitively acted to block the ABTS interaction with T1 Cu2+, although [TMG][Ser] didn't influence the protein structure.105
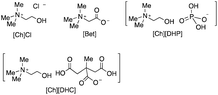
Recently, Chan et al. reported an insight into the effect of cholinium-based ILs on the Trametes versicolor laccase (TvL) activity obtained using experimental and computational approaches.106 They revealed that the laccase activity was enhanced by 21.39% in 0.5 M cholinium dihydrogen citrate ([Ch][DHC]), compared to that in a phosphate buffer medium. On the other hand, two types of cholinium aminoate, i.e., cholinium glycinate ([Ch][Gly]) and cholinium phenylalaninate ([Ch][Phe]), inhibited TvL at concentrations of 0.1 M and 0.5 M, respectively. A molecular dynamics (MD) simulation study revealed that the enhancement of the laccase activity in [Ch][DHC] might be attributed to the highly stabilized and compact structure of laccase, facilitating a better internal electron transfer during the laccase–substrate interactions.106 As shown in Fig. 12, the MD simulation suggests that the laccase structure enlarged in the presence of [Ch][Gly], while no significant size enlargement was obtained for [Ch][DHC]. The authors theorized that the enhancement of the laccase activity in [Ch][DHC] might be attributed to the highly stabilized and compact structure of laccase, facilitating a better internal electron transfer during the laccase–substrate interactions. The MD simulation also suggested that the high accumulation of the [DHC] anion on the surface of the laccase could be attributed to the higher interaction between the [DHC] anion and amino acid residues on the laccase surface. Based on these results the authors hypothesized that this intermolecular interaction could potentially enhance the catalytic activity of laccase in [Ch][DHC], where the abundant presence of the [DHC] anion at the hydration layer on the laccase surface may shield the laccase against the neighbouring water molecules. It is hypothesized that the shorter distances of the T1Cu-tripeptide in [Ch][DHC] could be the reason for the laccase activity enhancement.106
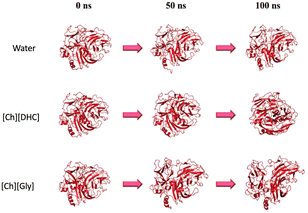 | ||
| Fig. 12 Structural evolution of laccase in different solvent environments based on the MD simulation study.106 Copyright Elsevier, 2024. | ||
In order to discuss the interaction of ILs towards an enzyme, the Hofmeister effect, that was originally observed during the salting-out process of protein aqueous solutions, has been frequently used (Fig. 13).68,75,107–109 Yan et al. proposed that kosmotropic anions, which have strong hydration properties, generally enhance protein stabilization, while hydrophobic chaotropic anions cause a significant destabilization of proteins. In contrast, chaotropic cations could enhance the stabilization of proteins.108 Zhao emphasized the importance of the Hoffmeister effect for designing suitable ILs for biotransformations.109 However, Kim and co-workers suggested that the effect of ions on the activity and stability of enzymes is not exactly the same as the Hoffmeister order.110 In the case of lipases, it has been established that ILs, which consist of a combination of chaotropic anions and chaotropic cations, are generally useful for biocatalytic reactions as solvents.50,51,109 On the other hand, in the case of laccases, ILs that consist of a chaotropic cation with a kosmotropic anion tended to enhance the stability of the enzyme when the IL was used as an additive or co-solvent in a buffer aqueous solution system. For example, [P6,6,6,14] is a highly chaotropic cation and, in fact, [P6,6,6,14][NTf2] increased the activity of laccase.96 As previously mentioned, Rodríguez et al.95 reported that the alkyl chain length of the imidazolium ring influenced the activity of laccase and the inactivation ratio of laccase increases with the length of the alkyl chain in the ILs; i.e., [C10mim]Cl > [C8mim]Cl > [C4mim]Cl.95 Later, Patel et al. also reported similar results.105 Since [C10mim] is supposed to be the most chaotropic cation among these three imidazolium cations, the results do not follow the Hoffmeister rule. On the other hand, Dabirmanesh et al. reported that the thermal stability of the laccase towards ILs was enhanced in the order of C2mim]Cl > [C4mim]Cl > [C6mim]Cl.149 These results are also the opposite trend to the Hoffmeister rule. However, adding ILs, which have [EtSO4] anions, caused a higher stabilizing effect compared to the [OAc] anion (reported by Stevens and Das et al.).175 Since the [EtSO4] anion is known to be more kosmotropic than the [OAc] anion (Fig. 13), the results are in line with that predicted using the Hoffmeister rule. The results of the MD simulation and docking test suggest that laccase's activity and stability are dependent on both the conformation and surface state of the enzyme (the details are described in chapter 3.4). Due to the space allowance of the laccase, component ions of the IL and DESs can bind near the active site and the entrance part of the enzyme and cause both modification of the loop structure and total conformation of the enzyme. This seems to provide these confusing results regarding the Hoffmeister effect.
 | ||
| Fig. 13 The Hoffmeister series of the kosmotropic and chaotropic ions.68,75,107–109 | ||
3.2 Reactivity of laccase-catalysed reactions in DESs
Deep eutectic solvents (DESs)53,54 are now widely acknowledged as a new class of ionic liquid (IL) analogues. Khodaverdian et al. demonstrated the Bacillus HR03 laccase-catalysed oxidation of ABTS in DESs and found that betaine-based DESs facilitated laccase stability in comparison to those of an aqueous buffer and choline chloride eutectics. The highest activity of the enzyme was observed in 20% (v/v) DES (glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (2
betaine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)).111
1)).111
Toledo and co-workers investigated a laccase-catalysed reaction in the presence of 16 types of DES aqueous solutions using the laccase-mediated degradation of dyes as a model reaction; the enzymatic reaction strongly depended on the DESs. Furthermore, the laccase activity also depended on the DES constituents, molar ratio, and concentration. They found that [Ch][DHC]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl (1
Xyl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES at 10 and 25 wt% led to the best results and the laccase activity was enhanced up to 200% in the presence of 25 wt% of [Ch][DHC]
2) DES at 10 and 25 wt% led to the best results and the laccase activity was enhanced up to 200% in the presence of 25 wt% of [Ch][DHC]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl (1
Xyl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).112 However, a significant drop in the reactivity was attained at the highest concentration (50 wt%). The authors conducted a docking test of DESs with laccase and found that all the polyols only establish hydrogen bonding with enzyme amino acids, particularly with serine, alanine, and histidine. The properties of DESs depend on the combination of hydrogen bond acceptor (HBA) properties and hydrogen bond donor (HBD) properties (Fig. 14). The absolute values of the docking affinity energies increase in the order ethylene glycol (EG) < glycerol (Gly) < erythritol (Ery) < xylitol (Xyl), in agreement with the increasing number of hydroxyl groups in the polyol and experimentally verified improvements of the laccase activity. Based on these results, they concluded that the laccase activity is dependent on the number of hydroxyl groups present in the polyols and their ability to form hydrogen bonds with the enzyme amino acids. The absolute docking affinity energies between the ions that compose each HBA and laccase follow the ranking [Ch][DHC] > [Ch][DHP] > betaine [Bet] > [Ch]Cl. The authors also tested the laccase stability in the presence of DESs at extreme storage temperatures (60 °C and −80 °C) and found that an enhanced laccase activity was accomplished when the enzyme was stored at low temperatures, at least up to 20 days, though no significant protection at high temperatures was recorded.112
2).112 However, a significant drop in the reactivity was attained at the highest concentration (50 wt%). The authors conducted a docking test of DESs with laccase and found that all the polyols only establish hydrogen bonding with enzyme amino acids, particularly with serine, alanine, and histidine. The properties of DESs depend on the combination of hydrogen bond acceptor (HBA) properties and hydrogen bond donor (HBD) properties (Fig. 14). The absolute values of the docking affinity energies increase in the order ethylene glycol (EG) < glycerol (Gly) < erythritol (Ery) < xylitol (Xyl), in agreement with the increasing number of hydroxyl groups in the polyol and experimentally verified improvements of the laccase activity. Based on these results, they concluded that the laccase activity is dependent on the number of hydroxyl groups present in the polyols and their ability to form hydrogen bonds with the enzyme amino acids. The absolute docking affinity energies between the ions that compose each HBA and laccase follow the ranking [Ch][DHC] > [Ch][DHP] > betaine [Bet] > [Ch]Cl. The authors also tested the laccase stability in the presence of DESs at extreme storage temperatures (60 °C and −80 °C) and found that an enhanced laccase activity was accomplished when the enzyme was stored at low temperatures, at least up to 20 days, though no significant protection at high temperatures was recorded.112
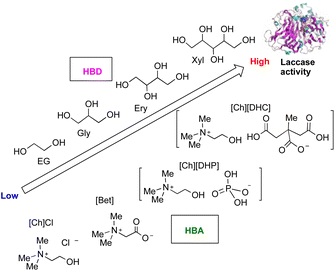 | ||
| Fig. 14 Laccase activity in the presence of DESs.112 | ||
Delorme et al. found that incubation of TvL with 25% (wt) of an aqueous solution of DES (betaine–xylitol = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 70 °C for 15 min resulted in markedly enhanced activity (10-fold greater than that of the control).113
1) at 70 °C for 15 min resulted in markedly enhanced activity (10-fold greater than that of the control).113
Varriale et al. reported an amazing increased thermal stability of laccase in the presence of four types of betaine-based DESs using six types of laccases. The authors evaluated the stabilizing effect of DESs (Bet–Et (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Bet–G (2
1), Bet–G (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Bet–Xyl (2
1), Bet–Xyl (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and Bet–Sor (2
1), and Bet–Sor (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and found that Bet–Sor (2
1)) and found that Bet–Sor (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) most effectively stabilized the enzyme; all enzymes retained ca. 40% of the original activity even after 2 min. at 90 °C in the presence of 25 wt% Bet–Sor (2
1) most effectively stabilized the enzyme; all enzymes retained ca. 40% of the original activity even after 2 min. at 90 °C in the presence of 25 wt% Bet–Sor (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), while the enzymes completely and quickly lost their activity within only 1 min in the absence of Bet–Sor (2
1), while the enzymes completely and quickly lost their activity within only 1 min in the absence of Bet–Sor (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).114
1).114
Vasiléva et al. reported the laccase-catalysed polymerization of aniline and 3-aminobenzoic acid in the presence of DESs and revealed that 1.4-fold enhanced activity was recorded when the reaction was carried out in the presence of 10% (v/v) betaine based-DES (betaine–glycerol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).115
2).115
Mojtabavi and co-workers reported the enhanced stability of laccase using betaine at high tempetratures.116 They found that betaine at high temperatures exhibited a protective effect on the a-helical contents of the enzyme, while no significant change was detected in the enzyme secondary structure contents. The MD simulation study suggested that betaine and ion molecules could be excluded from the enzyme surface and contributed to maintaining its stability and activity.116 Lys377 was recognized as the most critical residue for the electrostatic interactions with the osmolyte at low and high temperatures. Since the higher temperature could not affect the electrostatic interaction between the carboxyl group of betaine and the basic residues, the results indicated that the negatively charged amino acids could strengthen the electrostatic interactions with betaine at high temperature.116
Freitas et al. also reported the results of evaluation of seven types of DESs for an Ascomycete M. thermophila laccase (AmtL)-catalysed reaction in the polymerization of catechol as a model reaction.117 The authors found that the AmtL catalysed synthesis of polymers exhibited high degrees of polymerization (more than 20 monomeric units) in the DESs composed of DL-lactic acid or sodium DL-lactate and glycerol.117
Recently, strong interests have been paid for the origin of the stabilizing effects of DESs on the laccase activity. It is well known that water molecules play an important role in maintaining a suitable conformation and flexibility of the enzymes in ILs, in particular lipases.51 Although lipases exhibited a high activity even in pure ILs, they lost their activity if the essential water was lost.51 Zhou et al. very carefully discussed the so-called water effect on the influence of DESs against the laccase activity.75 As previously mentioned, it was suggested that both the cations and anions of the ILs can bind to the enzymes and the anion modified the three-dimensional structure of this enzyme.90 Laccase-catalysed reactions are more easily influenced by the solvent system compared to hydrolases, because the active site of the laccase is located nearer to the surface of the enzyme. It is thus reasonable that laccases lose their activity under highly concentrated conditions of ILs and DESs. On the other hand, ILs and DESs exist in a completely solvated state at 25 to 10 wt%; since the water molecule is small, the enzyme is mostly surrounded by water under such conditions. However, ILs and DESs have a certain impact on the activity of laccase. The properties of DESs depend on the combination of the HBA and HBD, and each component individually affects the enzyme structure. There needs to be more results in order to obtain a deep insight into this phenomenon.118,119
Yu et al. investigated the catalytic performance of laccase in a hydrophobic ionic liquid-based bicontinuous microemulsion in microemulsion systems using the laccase-catalysed oxidation of 2,6-dimethoxyphenol (DMP) as the model reaction and revealed that a bicontinuous microemulsion consisting of [C8mim][NTf2]/buffer/polyethylene alkyl ether (CnEm)/n-hexanol is a suitable medium for the laccase-catalysed reaction.120 Wang and Huang investigated reactivity of the Trametes versicolor laccase (TvL) in the oxidation of ABTS in an anionic surfactant-stabilized hydrophobic ionic liquid-based bicontinuous microemulsion under different temperature conditions; the [C8mim][PF6]-based bicontinuous microemulsion system has a moderate phase inversion temperature and high surfactant efficiency. The authors concluded that the optimal catalytic efficiency of TvL in the microemulsion was estimated to be ca. 50 °C, which was significantly higher than that (40 °C) in the aqueous medium system, indicating that the bicontinuous microstructure indeed improved the thermal stability of TvL.121
3.3 Enhanced activity of laccase by immobilization engineering
It is well known that the immobilization of an enzyme is an effective means to enhance the thermal stabilization and improve the tolerance against solvents. Rogers et al. reported the improved stability of laccase entrapped by a cellulose film; the authors prepared IL-coated laccase cellulose films and investigated their activity using the colour change of a diazo type dye, syringaldazine (Fig. 15).61 Among the three tested ILs, i.e., [C1mim]Cl, [HPmim]Cl, and [C4mim][NTf2], the laccase immobilized on [C4mim][NTf2]-coated cellulose displayed an ca. 2-fold higher activity compared to that on the non-IL-immobilized cellulose.61 Various types of immobilized materials have been employed for laccase: ILs,122,123 carbon nanotubes−ionic liquid nanocomposites,117 sol–gel-silica,124,125 modified silica,126 glyoxyl–agarose beads,127 organic–inorganic nanocomposites,131 magnetic nanoparticles,128,129,133,136,137 carbon nanotube (CNT)-IL composites, polymer included ILs,130,145 porous wood,134 sponge like cellulose composite polymers,132 mesoporous silica,133 DESs,135 hydrogels,138 lignocellulosic residues from bioethanol production,139 IL-substituted MOFs,140,141 IL-carbon nanotube composites,142 magnetic graphene oxide,143 and magnetic polymers.144 Among the examples, several recent examples were chosen among which were those reported in papers that were published after 2020, because our previous review didn't cover these examples.68 | ||
| Fig. 15 IL-cellulose-immobilized laccase mediated colour-change reaction.61 | ||
Gu et al. reported preparation of cellulose composite polymer (GDC) beads which were fabricated by initiating the polymerization of dopamine, glycidylmethacrylate and N,N-methylene bisacrylamide in a cellulose solution dissolved in an IL. The laccase was covalently immobilized by the reaction between the amino groups of the enzyme and the epoxy groups and quinone groups and phenolic hydroxyls on the beads as illustrated in Fig. 16.132 The authors evaluated activity of their immobilized laccase using the dye degradation reaction of indole and carbazole in the presence of ATBS as a mediator. The immobilized enzyme (Lac/GDC) exhibited high activity and recyclable use was accomplished.132 The authors evaluated their immobilized laccase by the dye degradation reaction of indole and carbazole in the presence of ATBS as a mediator. Magnetic particles are well known as useful supporting materials for enzymes due to the ease of removal of the immobilized enzyme from the reaction mixture.
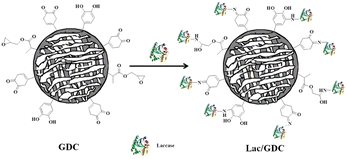 | ||
| Fig. 16 Schematic illustration of immobilization of laccase on GDC beads.132 Copyright Springer, 2020. | ||
Liu et al. (Liu and Zhang) reported the preparation of magnetic supporting materials for laccase that consist of the co-immobilization of an electron mediator (ABTS) and laccase onto dialdehyde starch (DAS) cross-linked magnetic chitosan nanomaterials (MACS-NIL-DAS@lac);137 the preparation process of this immobilized enzyme is illustrated in Fig. 17.137 MACS-NIL-DAS@lac was an excellent catalyst for the organic pollutant removal performance; the removal rate of 2,4-dichlorophenol (10 mg L−1) as a model phenolic pollutant by MACS-NIL-DAS@lac (1 U) reached 100% within 6 h, and the removal efficiency remained at 88.6% after six catalytic runs.137
 | ||
| Fig. 17 Preparation of MACS-NIL-Das@laccase.137 | ||
Liu et al. (H. Liu and Q. Li) further synthesized an amino acid (proline)-based ionic liquid (IL) modified magnetic composite material (IL-magnetic gelatin) and prepared IL-magnetic gelatin-immobilized laccase (IL-magnetic gelatin@laccase). They found that the affinity between the IL-magnetic gelatin@laccase and the substrate was enhanced due to decreased α-helices and increased β-sheets compared to the free laccase; the enzyme exhibited excellent reusability in degrading 2,4-DCP, maintaining over 55% degradation efficiency after 10 repeated reactions.138
Zhang and Lu et al. demonstrated the preparation of a metal–organic framework (MOF) immobilised laccase (Fig. 18).140 The authors prepared an IL-connected metal–organic framework (ILs-NH2-MIL-101) and used it for immobilization of laccase. The removal efficiency of this enzyme reached 95.4%, 91.4%, 90.3%, and 89.8% for 50 mg L−1 of 2,4-dichlorophenol, 4-chlorophenol, bisphenol A, and indole, respectively. The authors also investigated catalytic properties of this immobilised enzyme by a MD simulation study; it was suggested that the immobilized laccase had a rigid structure and stronger hydrogen bonding interactions with the substrate compared to the native laccase. This facilitated the stability of the laccase and the degradation efficiency of these polyphenol compounds.140
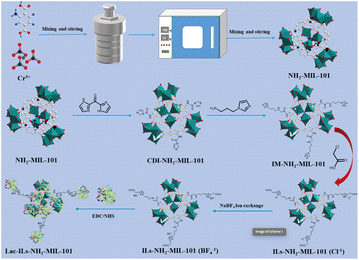 | ||
| Fig. 18 A schematic diagram illustrating the covalent immobilization of laccase on ILs-NH2-MIL-101.140 Copyright Elsevier, 2023. | ||
The same team (W. Zhang and Q. Wang et al.) recently reported preparation of immobilized TvL using an updated version of a magnetic metal–organic framework (laccase-ILs-MIL-100-Fe3O4) which has imidazolium-based ionic liquids as surface modifiers. The resulting enzyme exhibited high activity for degradation of phenolic pollutant, 2,4-DCP.141
Zhang and co-workers (W. Zhang and Y. Zhang) reported the preparation of magnetic graphite oxide (GO) immobilized laccase.143 They modified GO using an imidazolium type IL which has a terminal carboxylic acid moiety. Using this IL-GO, they prepared the immobilized enzyme, laccase-ILs-MGO, as shown in Fig. 19. The enzyme exhibited a remarkable decomposition efficiency of 95.5% towards 2,4-dichlorophenol (2,4-DCP) within 12 h and maintained over a 70.0% removal efficiency after seven reaction cycles.143 The authors conducted a molecular docking study and revealed that immobilized laccase had a higher structural rigidity and stronger hydrogen bond interactions with substrates compared to the free laccase. They suggested that this might contribute to the increasing stability of both the laccase and substrate degradation efficiency.143
 | ||
| Fig. 19 Preparation of laccase-ILs-MGO.143 Copyright Elsevier, 2023. | ||
Zhang et al. (W. Zhang and M. Zhang) prepared magnetic polydopamine immobilized laccase and the enzyme exhibited a broad pH and temperature stability. The authors reported that the activity of the immobilized laccase remained at approximately 80% of its activity even after incubation at 50 °C and over a 65% activity after storage for 30 days, which is 1.6 times and 2.9 times higher than that of the free laccase, respectively.144
Liang et al. prepared a mesoporous polymeric IL and used it for laccase immobilization; the resulting immobilized laccase was proved to be an efficient catalyst for the degradation of phenolic pollutants.145
3.4 Improved compatibility of laccase in ILs and DESs by protein engineering
Recently, the direct evolution technology of enzymes has remarkably progressed.146,147 ILs have been successfully used to dissolve lignin. However, the low activity of laccases in the presence of ILs sometimes becomes a serious limitation when using laccases for lignin degradation. Liu and Schwaneberg et al. investigated the molecular mechanism of the inhibitory effect of imidazolium ILs towards laccase and this was derived from binding of the imidazolium ILs to a certain site of the enzyme.148 They found that [C2mim][EtSO4] can dissolve lignin and its anion does not inhibit laccase. Furthermore, the ABTS radical cation was not affected by [C2mim][EtSO4]. Thus the authors developed a directed evolution protocol using the ABTS-screening assay in a 96-well microtiter plate and succeeded in improving the resistance of laccase in [C2mim][EtSO4]. Four laccases (lcc1_2005, lcc1_1997, lcc2 and CVLG1) from Trametes versicolor were expressed in Saccharomyces cerevisiae and finally lcc2 was selected as the starting point due to its superior resistance and activity in the presence of [C2mim][EtSO4].After two rounds of directed evolution, the authors obtained the mutant laccase lcc2 variant M3 (Phe265Ser/Ala318Val) which displayed an ca. 4.5-fold higher activity than the lcc2 wild type (WT) in the presence of 15% (v/v) [C2mim][EtSO4] and a 3.5-fold higher activity than lcc2 WT in a buffer. Fig. 20 shows the results of a docking test with ABTS and laccase.148 The docking test indicated that the mediator ABTS binds at Ser264 in the wild type laccase, while a hydrogen bonding network with Ser264 and Ser265 was observed for M3. As mentioned in chapter 2, laccase has a large entrance area for accessing the active site, and it is suggested that cations of the ILs might be incorporated into the pocket and influence the catalytic activity.148 The authors proposed that there might be a more sufficient space for ABTS binding to the T1 Cu site in the lcc2 variant M3 compared to that of the native lcc2 when the [C2mim] cation was incorporated into the active pocket. This may be the origin of the improved stability of M3 by mutation.145
 | ||
| Fig. 20 Results of the docking test with ABTS and laccases. The left figure shows the ABTS binding mode of lcc2 WT (ABTS forms a hydrogen bond with Ser264 in lcc2 WT) and the right figure shows the ABTS binding mode of M3 (ABTS forms hydrogen bonds with Ser264 and Ser265 in lcc2 M3).148 Copyright RSC, 2013. | ||
Dabirmanesh et al. succeeded in obtaining an ionic liquid tolerant laccase by gene engineering by evaluating 450 clones of the laccase from Bacillus HR03.149 The authors found that a mutation at Glu188Tyr and Glu188Phe critically influenced the thermal stability at 80 °C and a significant modified thermal stability was observed in the presence of 200 mM of three chloride types of ionic liquids, [C2mim]Cl, [C4mim]Cl or [C6mim]Cl, in two mutants. These ILs caused destabilization of both the mutants and the wild type enzyme. However, the thermal tolerance of the mutants was higher than that of the wild type laccase even in the presence of these ILs and the enhanced thermal activity is in the order of C2 > C4 > C6. Since these three ILs contain the same anion (Cl−), it was revealed that the side substituent on the cationic part of the ILs critically influenced the thermal stability of this laccase. The authors suggested Glu188 as a critical amino acid moiety that could influence the activity by mutation (Fig. 21(B)).149
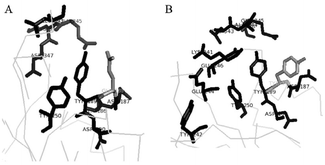 | ||
| Fig. 21 Local protein structures of two laccases. The position of Glu188 and neighboring residues in the wild-type (A) and Glu188Tyr variant (B).149 Copyright Elsevier, 2015. | ||
Pardo et al. succeeded in creating a new laccase with a high-redox potential by swapping its second redox in the copper domain with that from another fungal laccase, which introduced a pool of neutral mutations in the protein sequence without affecting the enzyme functionality.150 The new laccase150 showed an outstanding stability towards temperature, pH (2–9) and organic solvents, such as ethanol and methanol, while maintaining the ability to oxidize high-redox potential substrates. They changed the amino acid residue on domain 2 and found that both the thermal stability and tolerance against [C4mim][OTf] were increased for the mutant. Since this part was different from that of Schwaneberg's work, it is anticipated that there might be more key parts to improve the laccase activity by mutation. The authors further confirmed that treatment of a kraft lignin with [C4mim][OTf] was successful at high temperature and contributed to shortening the reaction time.150
Chauhan and co-workers reported success in creating a thermo-alkali stable laccase (S1-20LAC) from Staphylococcus which led to a 16-fold enhancement in the enzyme yield. This enzyme exhibited a very high temperature tolerance and the optimum temperature and pH for S1-20LAC were 85 °C and pH 9.0, respectively. S1-20 retained a significant amount of activity even in the presence of 20% (v/v) ILs, [C2mim][OAc], [C2mim]Br, [C4mim]Cl, and [C10mim]Cl.151
Wallraf et al. developed a loop engineering strategy for improving the lcc2 activity in ILs and an aqueous solution.152 The authors attempted to increase the activity by the computer-assisted protein engineering of lcc2 from Trametes versicolor in the presence of the ILs. They found that the loop L1 (amino acid residues 284–320) was critical for improving its activity in [C2mim][EtSO4] and an aqueous solution (Fig. 22).152 The authors postulated that the amino acid residues at positions 285, 310, 312, and 318 play a significant role in maintaining the loop flexibility of the enzyme and thus influence the reactivity; the loop of OM3 has 14 hydrogen bonds in the loop and neighboring domains, and this might lead to the rigid and stable structure of the enzyme. On the other hand, it was found that those of the wild lcc2 had only 9 hydrogen bondings.152
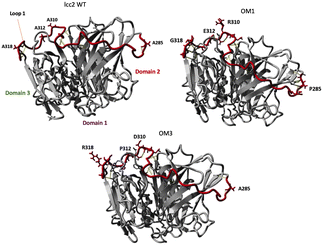 | ||
| Fig. 22 Hydrogen bonds formed with the four identified beneficial amino acid positions (285, 310, 312, and 318) in the domain-connecting loop. Hydrogen bonds are depicted in yellow.152 Copyright RSC, 2018. | ||
Stevens et al. prepared mutants of the hyperthermophilic laccase and examined the relationship between the mutation point and its reactivity against [C2mim][OAc] which is known as a good cellulose dissolving agent and an inhibitor of the laccase using ABTS oxidation as a model reaction.153 The authors focused on the L1 loop as the mutation point and found that, in particular, on changing glutamic acid 170 to tyrosine, the mutant E170Y displayed a higher activity compared to wild type enzyme in a buffer solution. They created a total of 8 single amino acid mutants, but unfortunately, no mutant was found which exhibited a high reactivity in the presence of 20% (v/v) of [C2mim][OAc].153
Su et al. reported direct evolution of laccase via droplet-based microfluidic screening (DMFS) technology (Fig. 23).154 In this study, a DMFS system including a heating step and pico-injection was used to sort large laccase libraries. From this method, they obtained 12 variants of laccase with improved thermotolerance. Recombination of three identified substitutions of Asp to Asn on the surface resulted in the best variant M20, which displayed 24.0-fold higher remaining activity at 58.8 °C and 1.9–3.4-fold higher remaining activity after incubation in organic solvent solution (20% (v/v) methanol and ethanol) and IL solution (20% (v/v) [C2mim][EtSO4]) for 12 h. The authors revealed that the recombination of the three beneficial substitutions, Asp98Asn, Asp474Asn, and Asp340Asn on the surface introduced more hydrogen bonds compared to the wild type, which made M20 more thermostable.154 The authors applied their developed laccase to the decoloration of a synthetic dye, Amplex Red.154
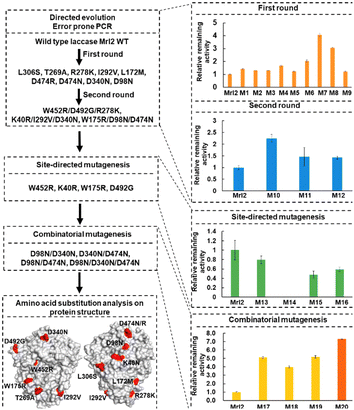 | ||
| Fig. 23 Schematic diagram of laccase engineering through FADS-facilitated directed evolution.154 Amino acid substitutions are shown in red colour. Copyright ACS, 2025. | ||
Another method to improve the activity of laccases is the surface engineering of the enzyme. Chan et al. found that the conformation of Myceliophthora thermophila laccase (MtL) was changed to a more flexible state in the presence of DES; the random coil content of MtL was slightly increased by the addition of DES ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol].155 Varriale et al. reported that the activity of laccase depends on the binding energy with DESs, particularly under high temperature conditions.156 Toledo et al. proposed that the DES component interacts with an amino acid residue of the laccase to cause rearrangement of its conformation, thus allowing an improved accessibility of the substrate to its catalyst domain.112 Based on these findings, surface charge engineering and substrate binding cleft engineering have been proposed.72 Pramanik et al. proposed that the interaction of the enzyme hydration shell between the electrostatic repulsion of ions of the ILs might be the primary driving factor that allows an enhanced tolerance of the enzyme toward ILs by a computer analysis, though this was proposed using the results using lipase as a model enzyme.157 Vicente et al. reported that the theromostability of Bsidiomycete PM1 laccase improved 2-fold by modifying the flexible surface loops of the enzyme compared to that of the native one.158 It was reported that the interaction region of laccase with DESs was located in the surface loop 1 (see, Fig. 22) and the substrate binding site.112,159–162 This region was believed to be responsible for the tolerance of laccase towards a solvent.112
glycerol].155 Varriale et al. reported that the activity of laccase depends on the binding energy with DESs, particularly under high temperature conditions.156 Toledo et al. proposed that the DES component interacts with an amino acid residue of the laccase to cause rearrangement of its conformation, thus allowing an improved accessibility of the substrate to its catalyst domain.112 Based on these findings, surface charge engineering and substrate binding cleft engineering have been proposed.72 Pramanik et al. proposed that the interaction of the enzyme hydration shell between the electrostatic repulsion of ions of the ILs might be the primary driving factor that allows an enhanced tolerance of the enzyme toward ILs by a computer analysis, though this was proposed using the results using lipase as a model enzyme.157 Vicente et al. reported that the theromostability of Bsidiomycete PM1 laccase improved 2-fold by modifying the flexible surface loops of the enzyme compared to that of the native one.158 It was reported that the interaction region of laccase with DESs was located in the surface loop 1 (see, Fig. 22) and the substrate binding site.112,159–162 This region was believed to be responsible for the tolerance of laccase towards a solvent.112
Pham et al. reported preparation of an engineered laccase from Fomitiporia mediterranea.159 They prepared a surface modified laccase which has a carbohydrate binding module (Fom CBM) and investigated its activity compared to that of the native laccase (Form lac). The authors used the CBM from the Trichoderma reesei exoglucanase 1 (Uniprok protein ID: P62694) and fused it to the C-terminus of the laccase (Fom_Lac) (Fig. 24). Fig. 24 shows the results of an MD simulation study of a model of laccase which has a carbohydrate binding module.159 CBM-bind laccase (Fom_CBM) and the original laccase (Fom_Lac) catalyze the β-O-4′ ether and C–C bond breaking, and Fom_CBM displayed a high activity under acidic conditions (pH < 6); the saccharification yields were enhanced compared to the native laccase. It was further found that adding Fom_CBM to mixtures of cellulases and hemicellulases improved the sugar yields by 140% compared to the untreated pine and 32% compared to the cholinium lysinate ([Ch][Lys])-pretreated pine, respectively.159
 | ||
| Fig. 24 Three-dimensional visualization of a Fom_CBM molecule in the solvent box (A).159 MDPI, 2024, Open Access. | ||
Nordwald et al. reported an interesting activation method of an enzyme in the presence of ILs;160 they prepared a surface modified enzyme by chemical succinylation and acetylation and found that the stability of the enzyme in aqueous-IL mixtures was improved; there exists a clear connection between the ratio of the enzyme-containing positive-to-negative sites and enzyme stability in the ILs; the stability of chymotrypsin to imidazolium ILs depended on the ratio of the positively charged amine-to-negatively charged acid groups. At a ratio of 0.39, the half-life of chymotrypsin was increased 1.6- and 4.3-fold relative to the wild-type chymotrypsin in [C4mim]Cl and [C2mim][EtSO4], respectively.160 Although the authors have not yet applied this method to laccase, it is anticipated that this may be useful for improving the laccase stability towards ILs.
Bae and co-workers reported a unique method for improving the stability of a laccase. They used a copper-based IL in order to enhance the refolding efficiency of laccase from Trametes versicolor.161 The authors found that the laccase refolding yield was improved more than 2.7 times and contributed to the increasing stability of this laccase compared to the conventional refolding state in a buffer which contains urea as a refolding agent. 1-Ethyl-3-methylimidazolium trichlorocuprate ([C2mim][CuCl3]) was added to a refolding buffer instead of urea; the highest refolding yield was attained at 25 °C. They concluded that this copper-based IL, [C2mim][CuCl3], was exclusively effective for the refolding process of a copper-containing protein like laccases.161
4 Laccase-catalysed reactions for biomass valorization
4.1 Depolymerization of lignocellulosic materials by the laccase-catalysed reaction
It is well known that a lignocellulosic material is hampered by biomass recalcitrance towards enzymatic degradation. In order to achieve degradation of lignocellulosic materials, the combination of ILs and DESs with laccases is an attractive system, because ILs and DESs could dissolve lignocellulosic compounds. It was reported that laccases played an important role in the lignin degradation in nature.162 Therefore, numerous review papers in this field have been published.6–26,30–58,165 In this chapter, recent examples of the degradation of lignocellulosic compounds are presented focusing on the combined use of ILs or DESs with laccases.Moniruzzaman and Ono demonstrated IL assisted delignification via a laccase-catalysed reaction.63,163 It was reported that the IL allowed partial delignification (20–30%) of wood.63,163 After IL treatment of lignin at high temperature (over 100 °C), cellulose rich materials can readily be precipitated with an anti-solvent, such as acetone and ethanol.164–169 Their protocol for preparing cellulose is depicted in Fig. 25;63 the wood sample was initially treated with [C2mim][OAc] at 80 °C for 1 h, causing swelling of the wood cell wall due to the ability of ILs to disrupt the hydrogen bond network between cellulose and lignin. After cooling the wood–IL mixture to RT, an acetate buffer (100 mM, pH 4.5) containing laccase was added to the flask in the presence of 1-hydroxybenzotriazole (HBT) as a mediator. The laccase-mediated delignification was then conducted with the supply of O2 bubbles at 50 °C. After cooling the reaction mixture to RT, 0.1 M NaOH was used to wash the ILs and lignin away to afford the cellulosic fibers (Fig. 25).63
 | ||
| Fig. 25 Preparation of cellulose fibre using an IL and a subsequent laccase-catalysed reaction.63 Copyright Elsevier, 2012. | ||
Ninomiya et al. reported the oxidative depolymerization of lignin by a different technique;169 the IL-pretreated/enzyme lignin was prepared by [C2mim][OAc] pretreatment and subsequent enzymatic saccharification. Upon the oxidative depolymerization of the lignin sample from eucalyptus by alkaline nitrobenzene oxidation, the total yield of vanillin and syringaldehyde was increased up to 48% compared to the untreated wood sample (36.6%). The yield was enhanced up to 48.0% for the IL-pretreated/enzyme lignin.169
Liu and co-workers accomplished the two-step chemo-enzymatic depolymerisation of the lignin model compound (1)62,170 by the IL/laccase reaction and subsequent alkali hydrolysis reaction protocol (Fig. 26).170 It is proposed that the most important step of the lignin degradation was oxidative cleavage of the β-O-4 bonds in the lignin molecules.171,172 To achieve this hypothesis, the β-O-4 model compound (1)62,173,174 was treated with the laccase variant lcc2M3 in the presence of 10% (v/v) [C2mim][EtSO4] in an aqueous sodium acetate buffer (pH 4) at rt for 3 days under ambient air conditions (Fig. 26).170 As a result, the α-oxidized product 2 was obtained in 81% yield, which was then treated with a NaOH solution of a mixed solvent of D2O and CD3OD and its change was investigated by NMR spectroscopy. The allyl ether product 3 was obtained in 86% yield; then this underwent degradation by the alkali treatment (NaOH in EtOH) to afford 3,4-dimethoxybenzoic acid (4) and 2-methoxyphenol (5) in 52% and 39% yield, respectively (Fig. 26, upper scheme A).170 The authors further demonstrated the lignin (beech wood) depolymerization under the same reaction conditions and confirmed that the laccase-mediated lignin depolymerization proceeded through the same pathway as that in the model reaction by the 2D-NMR spectroscopic analysis (Fig. 26, lower scheme B).170
 | ||
| Fig. 26 Chemo-enzymatic depolymerisation of lignin using IL/laccase and subsequent alkali esterification.170 | ||
Stevens et al. accomplished the depolymerisation of lignin using a laccase-IL system.175 They investigated the influence of three ILs, i.e., [DEA][HSO4], [Ch][Lys], and [C2mim][OAc] ([C2C1Im][OAc]), on the activity of Trametes velsicolor laccase (TvL). TvL exhibited a minimal loss of activity in the presence of 10% [DEA][HSO4] (Fig. 27).175 The authors further found that [DEA][HSO4] is a noncompetitive inhibitor, while [Ch][Lys]) and [C2mim][OAc] are mixed inhibitors by kinetic experiments. A docking simulation study suggested that [Ch][Lys] and [C2mim][OAc] disrupt residues leading to the active site. Stevens et al. also established that TvL oxidized the β-O-4′ linkage of a model dimer in the presence of ABTS as a mediator in [C2mim][OAc] or [Ch][Lys]. Alkaline lignin was thus converted to depolymerization products, i.e., vanillin, acetosyringone, syringaldehyde, and acetovanillone as the major products.175
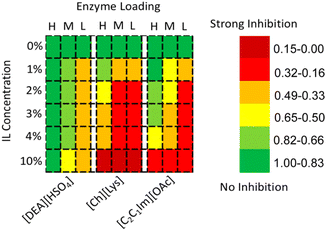 | ||
| Fig. 27 Influence of three ILs on laccase (TvL) activity.177 Copyright ACS, 2019. | ||
Chauhan et al. reported the results of an investigation of the organic solvent and IL compatibility of three lignolytic enzymes, i.e., lignin peroxidase (LiP), manganese peroxidase (MnP) and laccase (Lac), produced from the H. aswanensis strain ABC_IITR obtained by the Solid State Fermentation (SSF) of wheat bran. They used 40% (v/v) pyridine along with 1.5 M NaCl and 0.15 mM cholinium laurate ([Ch][LA]) based ionic liquid as the model reaction system.176 The authors discovered that the addition of metal ions such as Fe increased the LiP and MnP activities, while Cu enhanced only the laccase activity. Furthermore, a higher degradation of the Kalson lignin was achieved in [Ch][LA] as compared to that in the presence of 40% (v/v) pyridine.176
Chang and co-workers attempted to enhance the bio-delignification pretreatment of rice straw using laccase in the presence of the IL, [Amim]Cl or surfactant (TritonX-100). The addition of 750 mg L−1 [Amim]Cl and 500 mg L−1 TritonX-100 increased the lignin removal ratio by 18.49% and 31.79%, which is higher than that of only laccase (11.97%).177 The enzymatic saccharification process was next carried out and the highest cellulose conversions, 40.96%, 38.24%, and 37.91% were obtained after 72 h of enzymatic saccharification when the substrate was washed with distilled water after pretreatment of the rice straw with laccase + TritonX-100, laccase + [Amim]Cl, and laccase only, respectively.177
Stevens et al. accomplished lignin depolymerization using thermophilic laccase with an IL.178 The authors found that the thermophilic fungal laccase from Myceliophthora thermophila retained high levels of activity in the IL [C2mim][EtSO4], making it the laccase to be used for lignin valorization. On the other hand, the laccase activity markedly dropped in 15% (v/v) [C2mim][OAc]. Docking simulations suggested that [C2mim][OAc] disrupted residues leading to the active site and inhibited the enzyme activity.178
Pena et al. reported lignin depolymerization using a combination of an IL with the Myceliophthora thermophila laccase-mediated reaction;179 the treatment of the technical lignin Indulin AT, a pine kraft lignin commercialized by Meadwestvaco, with a recombinant laccase, or with a combination of this enzyme with the mediator, ABTS, has been carried out at 25 °C in a mildly acidic buffered aqueous medium in the presence of [C2mim][OAc]. Due to the limited solubility of Indulin AT in water, only partial dissolution occurred. However, when the reaction was carried out in the presence of [C2mim][OAc], the yields of the phenolic degradation products, i.e., catechin hydrate, gallic acid, and 4-hydroxybenzoic acid, were significantly improved. The authors suggested that this was provided due to the increased solubility of Indulin AT in [C2mim][OAc].179
Quesada-Salas et al. reported that the pretreatment of Miscanthus giganteus with [C2mim][OAc], followed by enzymatic hydrolysis and oxidative depolymerization, resulted in the efficient production of monomeric sugars and phenolic intermediates; they found that the phenolic monomers were released during the [C2mim][OAc]-pretreatment process at 110 °C for 40 min.180
Sawhney et al. reported an increased sugar production yield by the combinatorial pretreatment method of laccase and cellulase/xylanase using DESs.181 They selected formic acid (FA) as the best hydrogen bond donor (HBD) with [Ch]Cl as the hydrogen acceptor (HBA) after evaluation of eight types of HBDs, i.e., formic acid (FA), acetic acid (AA), acetamide (A), urea (U), glycerol (G), imidazole (I), urea (U), and L-lactic acid (LAC). The authors thus demonstrated the combinatorial method in the sugar production process using [Ch]Cl–FA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) as follows: the rice straw was first treated with 10% (w/v) in acetate buffer, pH 5.0, with [Ch]Cl–FA, under microwave irradiation at 130 °C for 10 min, ramped for 2 min and held for 8 min. The addition of deionized water then provided the DES-pretreated RS. The resulting RS was treated with laccase at 50 °C for 24 h and then finally subjected to cellulase/xylase at the same temperature for 24 h. The yield of the reducing sugar was increased ca. 10-fold compared to the reaction without DES pretreatment and laccase (Fig. 28).181 As shown in Fig. 28, microwave treatment gave better results than ultrasonication.
2 molar ratio) as follows: the rice straw was first treated with 10% (w/v) in acetate buffer, pH 5.0, with [Ch]Cl–FA, under microwave irradiation at 130 °C for 10 min, ramped for 2 min and held for 8 min. The addition of deionized water then provided the DES-pretreated RS. The resulting RS was treated with laccase at 50 °C for 24 h and then finally subjected to cellulase/xylase at the same temperature for 24 h. The yield of the reducing sugar was increased ca. 10-fold compared to the reaction without DES pretreatment and laccase (Fig. 28).181 As shown in Fig. 28, microwave treatment gave better results than ultrasonication.
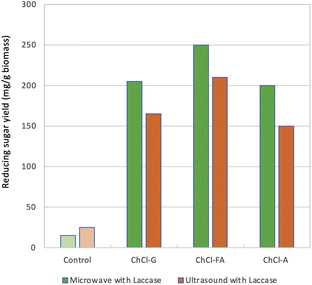 | ||
| Fig. 28 Increased saccharification efficiency in the combinatorial method of pretreatment of laccase and DES.181 Copyright Elsevier, 2023. | ||
In order to decompose rubber waste, Chittella et al. reported that a rubber glove was initially treated with a DES ([Ch]Cl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) at 140 °C and then conducted subsequent oxidation at 37 °C using Klebsiella aerogenes which included laccase and manganese peroxide, to achieve decomposition of the rubber waste with a maximum weight loss of 43%.182
2)) at 140 °C and then conducted subsequent oxidation at 37 °C using Klebsiella aerogenes which included laccase and manganese peroxide, to achieve decomposition of the rubber waste with a maximum weight loss of 43%.182
The DES assisted laccase-pretreatment of biomass was effective for lignin removal and allowed an increase in the reduced-sugar yield. Lin reported that a corn stover sample was initially treated with laccase (Bacillus amyloliquefaciens LC02) in a mixed solvent of buffer (pH 7) and 25% (v/v) DES in the presence of ABTS. After the treatment process, the insoluble part was obtained by centrifugation. The lignin content was reduced in this process, and subsequent hydrolysis of the insoluble residue was achieved using cellulase to afford sugars in good yield (ca. 2-fold increased yield was attained compared to that of untreated corn stover); the best DES for this process was Lac-BHX (betaine–H2O–xylitol, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).183
1).183
It was reported that laccases oxidized polymeric compounds, such as polyurethane,184 humic acid,185 and polyethylene,186,187 with the help of UV irradiation187 and Nylon-66,187 to achieve depolymerization of these polymers. Although the reaction upon adding neither ILs nor DESs has not yet been examined,184–187 it is expected that the efficiency of these reactions may be improved using ILs or DESs in the future.
4.2 Decomposition of phenolic compounds and dyes using the laccase-catalysed reaction
There are many types of artificial persistent aromatic chemicals in wastewater and they sometimes cause serious trouble. Such typical chemicals are synthetic dyes. The development of methods that allow the decomposition of synthetic dyes under environmentally benign conditions is thus strongly desired.188Chang et al. applied the laccase-catalysed reaction for the decomposition of bisphenol A.189 The authors found that SiO2-immobilisation markedly enhanced the stability of laccase in water and an 80% initial reactivity was recorded after 30 reaction cycles of continuous use. The activity of the SiO2-immobilized laccase was influenced by the surfactant additive and the activity was increased by the addition of Triton X-100 (5 mM) to 1.4-fold higher than that under no additive conditions. The ILs also affected the reactivity, but the addition of both [C4mim]Cl and [Amim]Cl slightly reduced activity.189
On the other hand, Jia et al. accomplished the efficient degradation of phytotoxic 2,4,5-trichlorophenol (2,4,5-TCP) using the immobilized laccase (La-AS@BC/HMA-DA) (Fig. 29); this immobilized enzyme exhibited the perfect degradation of 2,4,5-TCP, while the degradation efficiency of the native laccase was only 38.2% under the same reaction conditions (Fig. 29).190 The laccase-catalysed reaction was also applicable to the decomposition of poly-halogenated-phenols and numerous synthetic dyes. The decomposition of these aromatic pollutants involved in wastewater is an awakened problem. It was reported that laccases could effectively remove various dyes in wastewater (Fig. 30).131,138,141,145,154,190–199
 | ||
| Fig. 29 Degradation of 2,4,5-TCP by the immoblized laccase.190 | ||
 | ||
| Fig. 30 List of synthetic dyes and phenolic pollutants decomposed by the laccase-mediated reaction.131,138,141,145,154,190–199 | ||
Bento et al. reported the decolorization of indigo carmine by laccase in the presence of a 20 mM [N1,1,1,10]Br aqueous solution.197 By switching the IL to the water miscible IL, [Ch][H2PO4], the highest decomposition rate was attained (ca 4.5-fold compared to the control reaction), though 300 mM IL was required to achieve the result.197 The authors suggested that the IL might cause modification of the a-helix structure of the enzyme, and this contributed to activation of the enzyme.197
HajKacem and co-workers produced a membrane type bioreactor (laccase-PILM) which involved laccase with an IL and the cross linking agent, PVC. In order to optimize the IL, they examined four types of ILs, i.e., [C8mim][NTf2], [Ch][NTf2], [Ch][H2PO4], and [HEA][HCO2].198 The reactivity depended on the ILs, and [Ch][H2PO4] was proved to be the best IL with a high reusability, though the decolorization rate was reduced 75% compared to the batch system.198
Zhou et al. reported the production of cellobionic acid from lignin using a mixed enzyme system that involved cellulases, cellobiose dehydrogenase (CDH) derived from N. crassa HL10, and laccase;200 the system converted Avicel (cellulose) to cellobionic acid (cellobionate) in the absence of any redox mediator. The authors hypothesized that lignin and the lignin degradation products were able to serve as redox mediators for the CDH-laccase conversion system. The authors also found that a similar level of cellobionate production was attained to that with the ABTS addition when N. crassa HL10 was grown on Avicel. The formation of lignin radicals and the quenching of lignin radicals by the reduced CDH were verified by an electron paramagnetic resonance (EPR) experiment, providing further evidence that lignin radicals can serve as electron acceptors of the reduced CDH. The results indicated that lignocellulosic biomass is a self-sufficient substrate for the production of cellobionate.200
4.3 Further applications of laccase-catalysed reactions
The laccase-catalysed reaction is also applicable to the polymerization of several aromatic compounds; Fodor et al. reported the radical polymerization of vinylimidazole.201 Zhang et al. demonstrated polyaniline (PANI) synthesis using the laccase-catalysed oxidation of aniline.202 The authors conducted a laccase-catalysed reaction in a sodium dodecyl benzene sulfonate micellar solution and found that the presence of a low level of [TMA][TfO] was beneficial to increase the synthesis of PANI, probably due to the extension of the lifetime of the aniline cation radicals.202Bassanini et al. reported the synthesis of phenylpropanoid glycoside by the laccase-catalysed oxidative radical coupling of lignols, coniferyl and p-coumaryl alcohols as substrates.203 Lahtinena et al. investigated the laccase-catalysed radical coupling of coniferyl alcohol in the presence of an IL, [Amim]Cl, using laccase from the ascomycete Melanocarpus albomyces.204 Although the laccase activity was reduced by the addition of [Amim]Cl, the authors obtained an interesting result that the dehydropolymer product pattern of the coupling reaction of 2,4-dimethoxyphenol was modified by the addition of [Amim]Cl compared with those of the control reaction. Furthermore, the molecular weights of the products were significantly increased when the reaction was conducted in the presence of an IL. They supposed that this might be due to the increasing stability of the radical species in the IL.204
Khlupova and Lisitskaya et al. reported the polymerization of dihydroquercetin (DHQ) by the laccase-catalysed oxidation using an IL-immobilized laccase system.205 The synthesized oligomers have a number average molecular weight of 1050 g mol−1 with a polydispersity index of 1.41. Although the physicochemical characteristics of the DHQ oligomers synthesized using LC/IL did not differ from the characteristics of the oligomers obtained with the native laccase, they accomplished the recyclable use of the catalyst using their IL-immobilized laccase.205
Khlupova and Vasil'eva et al. next reported the oligomerization of DHQ in the presence of TEMPO as a mediator in a DES-buffer solvent system.206 The authors performed the DHQ oxidation using Trametes hirsuta laccase in a 60% (v/v) betaine–glycerol DES (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) buffer aqueous solution in the presence of the redox mediator TEMPO. The average molecular weight of the DHQ oligomers (oligoDHQ) reached 1800 g mol−1 with a polydispersity index of 1.09. NMR spectroscopy suggested that the produced oligo DHQ had a linear structure with an average chain length of 6 monomers (Fig. 31).206 The same team (M. E. Khlupova and O. V. Morozova et al.) also reported the preparation of a (+)-catechin oligomer in a DES–buffer mixture (betaine/glycerol 60% (v/v) and 40% (v/v) buffer pH 4.5) using Trametes hirsute laccase.207
2) buffer aqueous solution in the presence of the redox mediator TEMPO. The average molecular weight of the DHQ oligomers (oligoDHQ) reached 1800 g mol−1 with a polydispersity index of 1.09. NMR spectroscopy suggested that the produced oligo DHQ had a linear structure with an average chain length of 6 monomers (Fig. 31).206 The same team (M. E. Khlupova and O. V. Morozova et al.) also reported the preparation of a (+)-catechin oligomer in a DES–buffer mixture (betaine/glycerol 60% (v/v) and 40% (v/v) buffer pH 4.5) using Trametes hirsute laccase.207
 | ||
| Fig. 31 Polymerization of dihydroquercetin (DHQ) by laccase-catalysed oxidation.206 | ||
Muñiz-Mouro and Tavares et al. reported the laccase-catalysed oligomerization of rutin in the presence of ILs.208 Rutin is known as an antioxidant compound that displays a broad range of biological activities and health-related benefits, but presents a low water solubility that suppresses its polymerization process. This difficulty was solved using biocompatible aqueous biphasic systems composed of the IL ([CH][DHP]) and polyethylene glycol (PEG 600). The authors accomplished three recycles of the reaction system using their system as shown in Fig. 32. The yield of the rutin oligomerization product reached 95% in the first cycle, 91% in the second cycle, and 89% in the last cycle.208 The same team (Magalhães and Pereira et al.) further reported the production of polydopamine (PDAi) using the laccase-catalysed oxidation in an IL solvent system;210 the highest enzymatic polymerization of dopamine was achieved at pH 5.5, 30 °C and 2 mg ml−1 of dopamine in the presence of ABTS as a mediator. ABTS and the ILs caused laccase confinement in one phase while PDAi was recovered in the opposite phase. The system allowed easy separation of laccase from the reaction product and the polymerization of dopamine in ABTS led to a remarkable improvement in the polymerization rate, i.e., 3.9-fold higher than those of the conventional chemical PDA polymerization.210
 | ||
| Fig. 32 Laccase-catalysed system for the dimerization of rutin.208 | ||
It should be emphasized that laccase-mediated reactions need neither harsh reaction conditions nor environmentally toxic oxidants. There have been several further interesting laccase-catalysed reactions reported. As examples, laccase-catalysed oxidative coupling of estrogens,209 the laccase-catalysed domino reaction between hydroquinone and cyclic 1,3-dicarbonyls,211 and the laccase-mediated oxidation/aldol sequential reaction212 are particularly interesting ones, though neither ILs nor DESs were employed in these reactions. However, it is expected that applying ILs or DESs to these reactions may provide better results in the future. The application of laccase for a biosensor was also not mentioned. However, since laccases have been proved to be useful for the detection of polyphenol compounds213 and ILs can stabilize the enzyme, numerous biosensor systems have been developed using laccase-catalysed reactions with ILs. The recent excellent review papers about this topic were cited here.214,215
5 Conclusion
The use of ILs and DEEs has been even involved in the lignocellulosic industry process. Borregaard in Norway uses the IL engineering for the production of a lignin-based polymer.216 Ioncell® in Finland is using ILs for a textile manufacturing process.217 Futamura Chemical in Japan is now manufacturing a nonwoven fabric using IL engineering.218 However, the combination of laccase and ILs or DESs has not yet been employed on an industrial scale.It has been confirmed that laccase-mediated reaction systems allow the depolymerization of lignocellulosic compounds and the decomposition of aromatic pollutants in wastewater with the help of ILs or DESs. Table 1 shows the list of ILs and DESs that were investigated for their effects against laccases. Due to the short distance between the active site and the surface of the laccase, laccases can accept a wide variety of large molecules. However, as shown in this article, ILs and DESs can modify the laccase's activity and stability. ILs and DESs should be regarded as not the reaction media but as controlling agents of laccase-catalysed reactions.
Using ILs and DESs is beneficial for laccase-catalysed reactions, i.e., by resulting in easy separation of products and enzyme, increasing the stability of the enzyme, and enhancing the reactivity. At present, ILs which have cations with a short alkyl chain and hydrophilic anions, such as [EtSO4]− or dihydrogen citrate [DHC], seem to be the suitable ILs for the activation or stabilization of laccase. On the other hand, it seems that the best combination of DESs has not yet been optimized. Many researchers evaluated DESs which contained [Ch]Cl or betaine as the [HBA] part because these cations are safe compounds. At present, as mentioned in chapter 3.2, [Ch][DHC] or [Ch][DHP] seems to be a good [HBA] moiety and polyhydroxylated compounds, such as xylitol (Xyl), seem to be good [HBD] moieties. However, since each component of the DES individually affects the enzyme structure, it is difficult to optimize the best components of DESs.
As described in chapter 3, three strategies seem to be considered for improving the activity or increasing the tolerance of laccases against ILs, i.e., (1) direct evolution of the enzyme that is a really powerful tool for tailoring the enzyme, (2) design of supporting materials including ILs for the immobilization of a laccase, and (3) modification of the surface of a laccase protein by chemical methods or protein engineering. It is expected that a more rational design of laccases for increased tolerance towards ILs and DESs would be proposed in the future.
Laccases have a wide applicability and the reaction proceeds under very mild conditions. As described in chapter 4, laccase can cleave the C–O bond and C–C bond in lignin by a radical process in an ambient atmosphere or in oxygen. Selective oxidative depolymerization of a lignocellulosic biomass mediated by laccases is the first step of their valorisation process. Laccases are also regarded as useful catalysts for achieving the decomposition of artificial and persistent aromatic chemicals in wastewater. Since the reactions were accomplished under hazardous chemical reagent-free conditions, we expect that further investigation in the field of laccase-mediated oxidation might become even more important for sustainable chemistry in the future.
Abbreviations
| [C1mim] | 1,3-Dimethylimidazolium |
| [C2mim] | 1-Ethyl-3-methylimidazolium |
| [C4mim] | 1-Butyl-3-methylimidazolium |
| [C6mim] | 1-n-Hexyl-3-methylimidazolium |
| [C8mim] | 1-Methyl-3-octylimidazolium |
| [HPmim] | 1-(3-Hydroxypropyl)-3-methylimidazolium |
| [TMA] | Tetramethylammonium |
| [4-MBP] | 1-Butyl-4-methylpyridinium |
| [C6py] | 1-Butylpyridinium |
| [Amim] | 1-Allyl-3-methylimidazolium |
| [N1,1,1,10] | N-Decyl-N,N,N-trimethylammonium |
| [N4,4,4,4] | Tetrabutylammonium |
| [N1,8,8,8] | N-Methyl-N,N,N-trioctylamin-1-ium |
| [N1(2OH)3] | Tris(2-hydroxyethyl)methylammonium |
| [P4,4,4,4] | Tetrabutylphosphonium |
| [P6,6,6,14] | Trihexyltetradecylphosphonium |
| [Ch] | 2-Hydroxyethyl-N,N,N-trimethylammonium (Cholinium) |
| [HCO2] | Formate |
| [OAc] | Acetate |
| [Dec] | Decanoate |
| [DHC] | Dihydrogen citrate |
| [LA] | Laurate |
| [Gly] | Glycinate |
| [Phe] | Phenylalanate |
| [Lys] | Lysinate |
| [AOT] | 1,4-bis(2-Ethoxyhexyl)sulfosuccinate |
| [BF4] | Tetrafluoroborate |
| [PF6] | Hexafluorophosphate |
| [Sac] | Saccharinate |
| [DCA] | Dicyanamide |
| [OTf] | Trifluoromethanesulfonate |
| [HSO4] | Hydrogen sulfate |
| [C1OSO3] | Methyl sulfate |
| [C3(2-C1)OSO3] | 2-Methylpropyl sulfate |
| [EtSO4] | Ethyl sulfate |
| [C2OC2OSO3] | 2-Ethoxyethylsulfate |
| [C1(OC2)2OSO3] | 2-(2-Methoxyethoxy)ethylsulfate |
| [NTf2] | Bis(trifluoromethylsulfonyl)amide |
| [DHP] | Dihydrogen phosphate |
| [CuCl3] | Trichlorocuprate |
| [N5,5,5C3S] | N,N,N-Tripentyl-3-sulfonyl-1-propaneammonium |
| [Bet] | Betaine |
| [DHC] | Dihydrogen citrate |
| G | Glycerol |
| EG | Ethylene glycol |
| Ery | Erythritol |
| Xyl | Xylitol |
| Sor | Sorbitol |
| FA | Formic acid |
| AA | Acetic acid |
| A | Acetamide |
| CA | Citric acid |
| LA | Lactic acid |
| I | Imidazole |
| U | Urea |
Data availability
This is a review article. All the data reported here can be found in the cited papers in the reference section.Author contributions
YT and TI equally contributed to preparing this manuscript.Conflicts of interest
The authors declare no conflict interest.References
- J. J. Stewart, T. Akiyama, C. Chapple, J. Ralph and S. D. Mansfield, The Effects on Lignin Structure of Overexpression of Ferulate 5-Hydroxylase in Hybrid Poplar, Plant Physiol., 2009, 150, 621–635, DOI:10.1104/pp.109.137059.
- M. Möller and U. Schröder, Hydrothermal production of furfural from xylose and xylan as model compounds for hemicelluloses, RSC Adv., 2013, 3, 22253–22260, 10.1039/c3ra43108h.
- M. Abe, Y. Yashima, M. Seki, T. Miki and M. Nishida, Instrumental analyses of constituent biopolymers in cypress after various chemical treatments for delignification, Ind. Crops Prod., 2024, 212, 118327, DOI:10.1016/j.indcrop.2024.118327.
- A. Corma, S. Iborra and A. Velty, Chemical Routes for the Transformation of Biomass into Chemicals, Chem. Rev., 2007, 107, 2411–2502, DOI:10.1021/cr050989d.
- J. Zakzeski, P. C. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, The catalytic valorization of lignin for the production of renewable chemicals, Chem. Rev., 2010, 110, 3552–3599, DOI:10.1021/cr900354u.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Deconstruction of lignocellulosic biomass with ionic liquids, Green Chem., 2013, 15, 550–583, 10.1039/C2GC36364J.
- G. Chatel and R. D. Rogers, Review: Oxidation of Lignin Using Ionic Liquids-An Innovative Strategy To Produce Renewable Chemicals, ACS Sustainable Chem. Eng., 2014, 2, 322–339, DOI:10.1021/sc4004086.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Lignin Valorization: Improving Lignin Processing in the Biorefinery, Science, 2014, 344, 1246843, DOI:10.1126/science.1246843.
- Z. Armstrong, K. Mewis, C. Strachan and S. J. Hallam, Biocatalysts for biomass deconstruction from environmental genomics, Curr. Opin. Chem. Biol., 2015, 29, 18–25, DOI:10.1016/j.cbpa.2015.06.032.
- O. Y. Abdelaziz, D. P. Brink, J. Prothmann, K. Ravi, M. Sun, J. Garcia-Hidalgo, M. Sandahl, C. P. Hulteberg, C. Turner, G. Liden and M. F. Gorwa-Grauslund, Biological valorization of low molecular weight lignin, Biotechnol. Adv., 2016, 34, 1318–1346, DOI:10.1016/j.biotechadv.2016.10.001.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis, Angew. Chem., Int. Ed., 2016, 55, 8164–8215, DOI:10.1002/anie.201510351.
- H. Zhu, W. Luo, P. N. Ciesielski, Z. Fang, J. Y. Zhu, G. Henriksson, M. E. Himmel and L. Hu, Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications, Chem. Rev., 2016, 116, 9305–9374, DOI:10.1021/acs.chemrev.6b00225.
- P. Sudarsanam, R. Zhong, S. Van den Bosch, S. M. Coman, V. I. Parvulescu and B. F. Sels, Functionalised heterogeneous catalysts for sustainable biomass valorisation, Chem. Soc. Rev., 2018, 47, 8349–8402, 10.1039/C8CS00410B.
- L. A. Z. Torres, A. L. Woiciechowski, V. O. de Andrade Tanobe, S. G. Karp, L. C. G. Lorenci, C. Faulds and C. R. Soccol, Lignin as a potential source of high-added value compounds: a review, J. Clean. Prod., 2020, 263, 121499, DOI:10.1016/j.jclepro.2020.121499.
- J. S. Mahajan, R. M. O'Dea, J. B. Norris, L. T. J. Korley and T. H. Epps III, Aromatics from Lignocellulosic Biomass: A Platform for High-Performance Thermosets, ACS Sustainable Chem. Eng., 2020, 8, 15072–15096, DOI:10.1021/acssuschemeng.0c04817.
- G. Olivieri, R. H. Wijffels, A. Marzocchella and M. E. Russo, Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass, Catalysts, 2021, 11, 680, DOI:10.3390/catal11060680.
- S. K. Singh, Biological treatment of plant biomass and factors affecting bioactivity, J. Clean. Prod., 2021, 279, 123546, DOI:10.1016/j.jclepro.2020.123546.
- M. Zhou, O. A. Fakayode, A. E. A. Yagoub, Q. Ji and C. Zhou, Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization, Renew. Sustain. Energy Rev., 2022, 156, 111986, DOI:10.1016/j.rser.2021.111986.
- R. R. Singhania, A. K. Patel, T. Raj, C.-W. Chen, V. K. Ponnusamy, N. Tahir, S.-H. Kim and C.-D. Dong, Lignin valorisation via enzymes: a sustainable approach, Fuel, 2022, 311, 122608, DOI:10.1016/j.fuel.2021.122608.
- A. do Espirito Santo Pereira, J. L. de Oliveira, S. M. Savassa, C. B. Roǵerio, G. A. de Medeiros and L. F. Fraceto, Lignin nanoparticles: new insights for a sustainable agriculture, J. Clean. Prod., 2022, 345, 131145, DOI:10.1016/j.jclepro.2022.131145.
- R. de Moraes Dutenkefer, P. G. Machado and C. de Oliveira Ribeiro, Lignin chemical derivatives in Brazilian sugarcane sector: an alternative to make 2G ethanol viable?, J. Clean. Prod., 2022, 369, 133286, DOI:10.1016/j.jclepro.2022.133286.
- O. Y. Abdelaziz, I. Clemmensen, S. Meier, C. A. E. Costa, A. E. Rodrigues, C. P. Hulteberg and A. Riisager, On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges, ChemSusChem, 2022, 15, e202201232, DOI:10.1002/cssc.202201232.
- S. Wang, A. Cheng, F. Liu, J. Zhang, T. Xia, X. Zeng, W. Fan and Y. Zhang, Catalytic conversion network for lignocellulosic biomass valorization: a panoramic view, Ind. Chem. Mater., 2023, 1, 188–206, 10.1039/D2IM00054G.
- A. Devi, S. Bajar, Z. U. D. Sheikh, A. Singh, N. Kotwal, A. Bharti, S. Raina, R. Kouser and R. Kothari, A perspective towards sustainable and economically viable approach of waste biorefineries through lignin valorization, Biomass Convers. Biorefin., 2024 DOI:10.1007/s13399-024-05793-x.
- E. Liu, M. I. V. Mercado, F. Segato and M. R. Wilkins, A green pathway for lignin valorization: enzymatic lignin depolymerization in biocompatible ionic liquids and deep eutectic solvents, Enzyme Microb. Technol., 2024, 174, 110392, DOI:10.1016/j.enzmictec.2023.110392.
- I. P. da Paixão Cansado, P. A. M. Mourão, J. E. Castanheiro, P. F. Geraldo, S. R. Suero and B. L. Cano, A Review of the Biomass Valorization Hierarchy, Sustainability, 2025, 17, 335, DOI:10.3390/su17010335.
- P. Chowdhary, N. More and A. Yadav, Ram Naresh Bharagava, Chapter 12-Ligninolytic Enzymes: An Introduction and Applications in the Food Industry, Enzymes in Food Biotechnology, 2019, 181–195, DOI:10.1016/B978-0-12-813280-7.00012-8.
- E. I. Solomon, U. M. Sundaram and T. E. Machonkin, Multicopper oxidases and oxygenases, Chem. Rev., 1996, 96, 2563–2605, DOI:10.1021/cr950046o.
- S. Riva, Laccases: blue enzymes for green Chemistry, Trends Biotechnol., 2006, 24, 219–226, DOI:10.1016/j.tibtech.2006.03.006.
- D. M. Mate and M. Alcalde, Laccase: a multi-purpose biocatalyst at the forefront of biotechnology, Microb. Biotechnol., 2017, 10, 1457–1467, DOI:10.1111/1751-7915.12422.
- I. Bassanini, E. E. Ferrandi, S. Riva and D. Monti, Biocatalysis with Laccases: An Updated Overview, Catalysts, 2021, 11, 26, DOI:10.3390/catal11010026.
- L. T. Nguyen, D.-P. Phan, A. Sarwar, M. H. Tran, O. K. Lee and E. Y. Lee, Valorization of industrial lignin to value-added chemicals by chemical depolymerization and biological conversion, Ind. Crops Prod., 2021, 161, 113219, DOI:10.1016/j.indcrop.2020.113219.
- S. R. L. Zhao, J. Zhang, D. Zhao, L. Jia, B. Qin, X. Cao, L. Zang, F. Lu and F. Liu, Biological degradation of lignin: a critical review on progress and perspectives, Ind. Crops Prod., 2022, 188, 115715, DOI:10.1016/j.indcrop.2022.115715.
- J. Viña-Gonzalez and M. Alcalde, Directed evolution of the aryl-alcohol oxidase: beyond the lab bench, Comput. Struct. Biotechnol. J., 2020, 18, 1800–1810, DOI:10.1016/j.csbj.2020.06.037.
- S. R. Couto and J. L. T. Herrera, Industrial and biotechnological applications of laccases: a review, Biotechnol. Adv., 2006, 24, 500–513, DOI:10.1016/j.biotechadv.2006.04.003.
- O. V. Morozova, G. P. Shumakovich, S. V. Shleev and Ya. I. Yaropolov, Laccase–Mediator Systems and Their Applications: A Review, Appl. Biochem. Microbiol., 2007, 43, 523–535, DOI:10.1134/S0003683807050055.
- F. Hollmann and I. W. C. E. Arends, Enzyme Initiated Radical Polymerizations, Polymers, 2012, 4, 759–793, DOI:10.3390/polym4010759.
- G. S. Nyanhongo, E. N. Prasetyo, E. H. Acero and G. M. Guebitz, Advances in the Application of Oxidative Enzymes in Biopolymer Chemistry and Biomaterial Research, ACS Symp. Ser., 2012, 1107(18), 329–349, DOI:10.1021/bk-2012-1107.ch018.
- J.-R. Jeon, P. Baldrian, K. Murugesan and Y.-S. Chang, Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications, Microb. Biotechnol., 2012, 5, 318–332, DOI:10.1111/j.1751-7915.2011.00273.x.
- M. Mogharabi and A. M. Faramarzi, Laccase and Laccase-Mediated Systems in the Synthesis of Organic Compounds, Adv. Synth. Catal., 2014, 356, 897–927, DOI:10.1002/adsc.201300960.
- C. Hautphenne, M. Penninckx and F. Debaste, Product formation from phenolic compounds removal by laccases: a review, Environ. Technol. Innov., 2016, 5, 250–266, DOI:10.1016/j.eti.2016.04.001.
- E. Mitsou, A. Xenakis and M. Zoumpanioti, Oxidation Catalysis by Enzymes in Microemulsions, Catalysts, 2017, 7, 52, DOI:10.3390/catal7020052.
- J. M. Gomes, S. S. Silva and R. L. Reis, Biocompatible ionic liquids: fundamental behaviours and applications, Chem. Soc. Rev., 2019, 48, 4317–4335, 10.1039/C9CS00016J.
- A. D. Moreno, D. Ibarra, M. E. Eugenio and E. Tomas-PeJó, Laccases as versatile enzymes: from industrial uses to novel applications, J. Chem. Technol. Biotechnol., 2020, 95, 481–494, DOI:10.1002/jctb.6224.
- T. Brugnari, D. M. Braga, C. S. A. dos Santos, B. H. C. Torres, T. A. Modkovski, C. W. I. Haminiuk and G. M. Maciel, Laccases as green and versatile biocatalysts: from lab to enzyme market—an overview, Bioresour. Bioprocess., 2021, 8, 131, DOI:10.1186/s40643-021-00484-1.
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Verlag GmbH, 2002, ISBNs: 3-527-30515-7 (Hardback); 3-527-60070-1 Search PubMed.
- N. V. Plechkova and K. R. Seddon, Applications of ionic liquids in the chemical Industry, Chem. Soc. Rev., 2008, 37, 123–150, 10.1039/B006677J.
- J. P. Hallett and T. Welton, Room-temperature ionic liquids: solvents for synthesis and catalysis. 2, Chem. Rev., 2011, 111, 3508–3576, DOI:10.1021/cr1003248.
- C. Dai, Ji. Zhang, C. Huang and Z. Lei, Ionic Liquids in Selective Oxidation: Catalysts and Solvents, Chem. Rev., 2017, 117, 6929–6983, DOI:10.1021/acs.chemrev.7b00030.
- Application of Ionic Liquids in Biotechnology. Advances in Biochemical Engineering/Biotechnology, ed. T. Itoh and Y.-M. Koo, Springer/Nature, 2018, DOI:10.1007/10_2018_62, ISBN: 978-3-030-23080-7.
- T. Itoh, Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis, Chem. Rev., 2017, 117, 10567–10607 CrossRef CAS PubMed.
- T. Nishihara, A. Shiomi, S. Kadotani, T. Nokami and T. Itoh, Enhanced activity and remarkable improved stability of a Burkholderia cepacia lipase by the coating with a triazolium alkyl-PEG sulfate ionic liquid, Green Chem., 2017, 19, 5250–5256, 10.1039/C7GC02319G.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chem. Commun., 2003, 70–71, 10.1039/B210714G.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids, J. Am. Chem. Soc., 2004, 126, 9142–9147, DOI:10.1021/ja048266j.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114, 11060–11082, DOI:10.1021/cr300162p.
- V. Gotor-Fernández and C. E. Paul, Deep eutectic solvents for redox biocatalysis, J. Biotechnol., 2019, 293, 24–35, DOI:10.1016/j.jbiotec.2018.12.018.
- S. M. Taklimi, A. Divsalar, B. Ghalandari, X. Ding, M. L. Di Gioia, K. A. Omar and A. A. Saboury, Effects of deep eutectic solvents on the activity and stability of enzymes, J. Mol. Liq., 2023, 377, 121562, DOI:10.1016/j.molliq.2023.121562.
- N. Zhang, P. D. de Maria and S. Kara, Biocatalysis for the Synthesis of Active Pharmaceutical Ingredients in Deep Eutectic Solvents: State-of-the-Art and Prospects, Catalysts, 2024, 14, 84, DOI:10.3390/catal14010084.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, Dissolution of Cellose with Ionic Liquids, J. Am. Chem. Soc., 2002, 124, 4974–4975, DOI:10.1021/ja025790m.
- G. Hinckley, V. V. Mozhaev, C. Budde and Y. L. Khmelnitsky, Oxidative enzymes possess catalytic activity in systems with ionic liquids, Biotechnol. Lett., 2002, 24, 2083–2087, DOI:10.1023/A:1021305229969.
- M. B. Turner, S. K. Spear, J. D. Holbrey and R. D. Rogers, Production of Bioactive Cellulose Films Reconstituted from Ionic Liquids, Biomacromolecules, 2004, 5, 1379–1384, DOI:10.1021/bm049748q.
- A. M. Barreca, M. Fabbrini, C. Galli, P. Gentili and S. Ljunggren, Laccase/mediated oxidation of a lignin model for improved delignification procedures, J. Mol. Catal. B: Enzym., 2003, 26, 105–110, DOI:10.1016/j.molcatb.2003.08.001.
- M. Moniruzzaman and T. Ono, Ionic liquid assisted enzymatic delignification of wood biomass: a new ‘green’ and efficient approach for isolating of cellulose fibers, Biochem. Eng. J., 2012, 60, 156–160, DOI:10.1016/j.bej.2011.11.001.
- Z. Zhang, J. Song and B. Han, Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids, Chem. Rev., 2017, 117, 6834–6880, DOI:10.1021/acs.chemrev.6b00457.
- P. Sudarsanam, R. Zhong, S. Van den Bosch, S. M. Coman, V. I. Parvulescu and B. F. Sels, Functionalised heterogeneous catalysts for sustainable biomass valorisation, Chem. Soc. Rev., 2018, 47, 8349–8402, 10.1039/C8CS00410B.
- J. M. Gomes, S. S. Silva and R. L. Reis, Biocompatible ionic liquids: fundamental behaviours and applications, Chem. Soc. Rev., 2019, 48, 4317–4335, 10.1039/C9CS00016J.
- A. Y. Patel, K. S. Jonnalagadda, N. Paradis, T. D. Vaden, C. Wu and Gr. A. Caputo, Effects of Ionic Liquids on Metalloproteins, Molecules, 2021, 26, 514, DOI:10.3390/molecules26020514.
- T. Itoh and Y. Takagi, Laccase-Catalyzed Reactions in Ionic Liquids for Green Sustainable Chemistry, ACS Sustainable Chem. Eng., 2021, 9, 1443–1458, DOI:10.1021/acssuschemeng.0c07097.
- Biocatalysis in Green Solvents, ed. P. Lozano, Academic Press, 2022, DOI:10.1016/B978-0-323-91306-5.00014-5, ISBN: 978-0-323-91306-5, eBook ISBN: 978-0-323-91425-3.
- J. S. Almeida, E. V. Capela, A. M. Loueiro, A. P. M. Tavares and M. G. Freire, An Overview on the Recent Advances in Alternative Solvents as Stabilizers of Proteins and Enzymes, Chem. Eng., 2022, 6, 51, DOI:10.3390/chemengineering6040051.
- M. Hatimuria, J. Das, Kr. Gavvala, S. Bag and A. Pabbathi, Recent advances in the use of laccase enzyme in deep eutectic solvents, Sustain. Chem. Pharm., 2023, 33, 101148, DOI:10.1016/j.scp.2023.101148.
- T. Chen, W. Xiao, Z. Wang, T. Xie, C. Yi and Z. Xu, Design and engineering of heterogeneous nitroxide-mediated catalytic systems for selective oxidation: efficiency and sustainability, Mater. Today Chem., 2022, 24, 100872, DOI:10.1016/j.mtchem.2022.100872.
- E. Erdem and J. M. Woodley, Industrially useful enzymology: translating biocatalysis from laboratory to process, Chem Catal., 2022, 2, 2499–2505, DOI:10.1016/j.checat.2022.09.037.
- L. M. C. L. F. Curran, L. T. M. Pham, K. L. Sale and B. A. Simmons, Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts, Biotechnol. Adv., 2022, 54, 107809, DOI:10.1016/j.biotechadv.2021.107809.
- M. Zhou, O. A. Fakayode, M. Ren, H. li, J. Liang, A. E. A. Yagoub, Z. Fan and C. Zhou, Laccase-catalyzed lignin depolymerization in deep eutectic solvents: challenges and prospects, Bioresour. Bioprocess., 2023, 10, 21, DOI:10.1186/s40643-023-00640-9.
- J. Jayakumar, D. Priyadarshini, A. Parthasarathy and S. R. Reddy, Recent Advances in Molecular Oxygen Assisted Laccase Catalyzed Sustainable Organic Transformations, Asian J. Org. Chem., 2023, e202200564, DOI:10.1002/ajoc.202200564.
- R. A. Sheldon, M. L. Bode and N. Mathebula, Green and sustainable solvents for biocatalytic oxidations, Curr. Opin. Green Sustain. Chem., 2023, 39, 100741, DOI:10.1016/j.cogsc.2022.100741.
- A. Ullah, X. Y. Zhang, C. Liu, Q. Qiao, Q. Shao and J. Shi, Process intensification strategies for green solvent mediated biomass pretreatment, Bioresour. Technol., 2023, 369, 128394, DOI:10.1016/j.biortech.2022.128394.
- A. Ovejero-Pérez, P. Y. S. Nakasu, C. Hopson, J. M. Costa and J. P. Hallett, Challenges and opportunities on the utilisation of ionic liquid for biomass pretreatment and valorisation, npj Mater. Sustain., 2024, 2, 7, DOI:10.1038/s44296-024-00015-x.
- H. Yoshida, LXIII.−Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio, J. Chem. Soc. Trans., 1883,(43), 472–486, 10.1039/CT8834300472.
- T. Bertrand, C. Jolivalt, P. Briozzo, E. Caminade, N. Joly, C. Madzak and C. Mougin, Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics, Biochem., 2002, 41, 7325–7333, DOI:10.1021/bi0201318.
- K. Piontek, M. Antorini and T. Choinowski, Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers, J. Biol. Chem., 2002, 277, 37663–37669, DOI:10.1074/jbc.M204571200.
- J. Uppenberg, M. T. Hansen, S. Patkar and T. A. Jones, Structure, 1994, 2, 293–308, DOI:10.1016/S0969-2126(00)00031-9.
- A. V. Lyashenko, O. Belova, A. G. Gabdulkhakov, A. A. Lashkov, A. V. Lisov, A. A. Leontievsky and A. M. Mikhailov, Purification, crystallization and preliminary X-ray structure analysis of the laccase from Ganoderma lucidum, Acta Cryst., 2011, 67, 926–929, DOI:10.1107/S1744309111021129.
- J. P. Kallio, C. Gasparetti, M. Andberg, H. Boer, A. Koivula, K. Kruus, J. Rouvinen and N. Hakulinen, Crystal structure of an ascomycete fungal laccase from Thielavia arenaria- common structural features of asco-laccases, FEBS J., 2011, 278, 2283–2295, DOI:10.1111/j.1742-4658.2011.08146.x.
- S. Roth and A. C. Spiess, Laccases for biorefinery applications: a critical review on challenges and perspectives, Bioprocess Biosyst. Eng., 2015, 38, 2285–2313, DOI:10.1007/s00449-015-1475-7.
- K. Agrawal, V. Chaturvedi and P. Verma, Fungal laccase discovered but yet undiscovered, Bioresour. Bioprocess., 2018, 5, 4, DOI:10.1186/s40643-018-0190-z.
- M. Alcalde, Chapter 26. Laccases: Biological Functions, Molecular Structure and Industrial Applications, Industrial Enzymes, ed. J. Polaina and A. P. MacCabe, Springer, Dordrecht, 2007, pp. 461–476, DOI:10.1007/1-4020-5377-0_26.
- J. Rodakiewicz-Nowak, S. M. Kasture, B. Dudek and J. Haber, Effect of various water-miscible solvents on enzymatic activity of fungal laccases, J. Mol. Catal. B: Enzym., 2000, 11, 1–11, DOI:10.1016/S1381–1177(00)00183-1.
- A. Roohi, M. Reza Housaindokht, M. Reza Bozorgmehr and M. Vakili, Impact of surface-active ionic solutions on the structure and function of laccase from trametes versicolor: insights from molecular dynamics simulations, J. Mol. Graphics Model, 2024, 132, 108844, DOI:10.1016/j.jmgm.2024.108844.
- A. P. M. Tavares, O. Rodriguez and E. A. Macedo, Ionic Liquids as Alternative co-Solvents for Laccase: Study of Enzyme Activity and Stability, Biotechnol. Bioeng., 2008, 101, 201–207, DOI:10.1002/bit.21866.
- R. L. Prior, X. Wu and K. Schaich, Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements, J. Agric. Food Chem., 2005, 53, 4290–4302, DOI:10.1021/jf0502698.
- S. Shipovskov, H. Q. N. Gunaratne, K. R. Seddon and G. Stephens, Catalytic activity of laccases in aqueous solutions of ionic liquids, Green Chem., 2008, 10, 806–810, 10.1039/b716369j.
- A. Domínguez, O. Rodríguez, A. P. M. Tavares, E. A. Macedo, M. A. Longo and M. Á. Sanromán, Studies of laccase from Trametes versicolor in aqueous solutions of several methylimidazolium ionic liquids, Bioresour. Technol., 2011, 102, 7494–7499, DOI:10.1016/j.biortech.2011.05.063.
- O. Rodríguez, R. O. Cristóvão, A. P. M. Tavares and E. A. Macedo, Study of the Alkyl Chain Length on Laccase Stability and Enzymatic Kinetic with Imidazolium Ionic Liquids, Appl. Biochem. Biotechnol., 2011, 164, 524–533, DOI:10.1007/s12010-010-9154-2.
- L. Rehmann, E. Ivanova, J. L. Ferguson, H. Q. N. Gunaratne, K. R. Seddon and G. M. Stephens, Measuring the effect of ionic liquids on laccase activity using a simple, parallel method, Green Chem., 2012, 14, 725–733, 10.1039/C2GC16009A.
- A. P. Tavares, J. A. N. Pereira and A. M. R. B. Xavier, Effect of ionic liquids activation on laccase from Trametes versicolor: Enzymatic stability and activity, Eng. Life Sci., 2012, 648–655, DOI:10.1002/elsc.201100203.
- X. Yu, F. Zou, Y. Li, L. Lu, X. Huanga and Y. Qu, Effect of three trifluoromethanesulfonate ionic liquids on the activity, stability and conformation of laccase, Int. J. Biol. Macromol., 2013, 56, 62–68, DOI:10.1016/j.ijbiomac.2013.02.005.
- J. Feder-Kubis and J. Bryjak, Laccase activity and stability in the presence of menthol-based ionic liquids, Acta Biochim. Pol., 2013, 60, 741–745, DOI:10.18388/abp.2013_2051.
- L. Rehmann, E. Ivanova, H. Q. N. Gunaratne, K. R. Seddon and G. Stephens, Enhanced laccase stability through mediator partitioning into hydrophobic ionic liquids, Green Chem., 2014, 16, 1462–1469, 10.1039/C3GC42189A.
- N. Harwardt, N. Stripling, S. Roth, H. Liu, U. Schwaneberg and A. C. Spiess, Effects of ionic liquids on the reaction kinetics of a laccase–mediator system, RSC Adv., 2014, 4, 17097–17104, 10.1039/C4RA00733F.
- L. Munk, M. L. Andersen and A. S. Meyer, Influence of mediators on laccase catalyzed radical formation in lignin, Enzyme Microb. Technol., 2018, 116, 48–56, DOI:10.1016/j.enzmictec.2018.05.009.
- A. M. Ferreira, H. Passos, A. Okafuji, A. P. M. Tavares, H. Ohno, M. G. Freire and J. A. P. Coutinho, An integrated process for enzymatic catalysis allowing product recovery and enzyme reuse by applying thermoreversible aqueous biphasic systems, Green Chem., 2018, 20, 1218–1223, 10.1039/C7GC03880A.
- J. Sun, H. Liu, W. Yang, S. Chen and S. Fu, Molecular Mechanisms Underlying Inhibitory Binding of Alkylimidazolium Ionic Liquids to Laccase, Molecules, 2017, 22, 1353, DOI:10.3390/molecules22081353.
- A. Y. Patel, A. K. Clark, N. J. Paradis, M. Amin, T. D. Vaden, C. Wu and G. A. Caput, Effects of Ionic Liquids on Laccase from Trametes versicolor, Biophysica, 2021, 1, 429–444, DOI:10.3390/biophysica1040031.
- K. K. Chan, A. F. Pereira, A. I. Valente, A. P. M. Tavares, J. A. P. Coutinho and C. W. Ooi, Investigation of laccase activity in cholinium-based ionic liquids using experimental and molecular dynamics techniques, Int. J. Biol. Macromol., 2024, 277, 134443, DOI:10.1016/j.ijbiomac.2024.134443.
- K. D. Collins and M. W. Washabaugh, The Hoffmeister effect and the behaviour of water at interfaces, Q. Rev. Biophys., 1985, 18, 323–422, DOI:10.1017/S0033583500005369.
- Z. Yang, Hoffmeister effects: an explanation for impact of ionic liquids on biocatalysis, J. Biotechnol., 2009, 144, 12–22, DOI:10.1016/j.jbiotec.2009.04.011.
- H. Zhao, Protein stabilization and enzyme activation in ionic liquids: specific ion effects, J. Chem. Technol. Biotechnol., 2016, 91, 25–50, DOI:10.1002/jctb.4837.
- H. S. Kim, D. Eom, Y.-M. Koo and Y. G. Yingling, The effect of imidazolium cations on the structure and activity of the Candida antarctica lipase B enzyme in ionic liquids, Phys. Chem. Chem. Phys., 2016, 18, 22062–22069, 10.1039/C6CP02355J.
- S. Khodaverdian, B. Dabirmanesh, A. Heydari, E. Dashtban-moghadam, K. Khajeh and F. Ghazi, Activity, stability and structure of laccase in betaine based natural deep eutectic solvents, Int. J. Biol. Macromol., 2018, 107, 2574–2579, DOI:10.1016/j.ijbiomac.2017.10.144.
- M. L. Toledo, M. M. Pereira, M. G. Freire, J. P. A. Silva, J. A. P. Coutinho and A. P. M. Tavares, Laccase Activation in Deep Eutectic Solvents, ACS Sustainable Chem. Eng., 2019, 7, 11806–11814, DOI:10.1021/acssuschemeng.9b02179.
- A. E. Delorme, J.-M. Andanson and V. Verney, Improving laccase thermostability with aqueous natural deep eutectic solvents, Int. J. Biol. Macromol., 2020, 163, 919–926, DOI:10.1016/j.ijbiomac.2020.07.022.
- S. Varriale, A. E. Delorme, J. M. Andanson, J. Devemy, P. Malfreyt, V. Verney and C. Pezzella, Enhancing the Thermostability of Engineered Laccases in Aqueous Betaine-Based Natural Deep Eutectic Solvents, ACS Sustainable Chem. Eng., 2022, 10, 572–581, DOI:10.1021/acssuschemeng.1c07104.
- I. Vasiléva, O. Morozova, G. Shumakovich and A. Yaropolov, Betaine-Based Deep Eutectic Solvent as a New Media for Laccase-Catalyzed Template-Guided Polymerization/Copolymerization of Aniline and 3-Aminobenzoic Acid, Int. J. Mol. Sci., 2022, 23, 11409, DOI:10.3390/ijms231911409.
- S. Mojtabavi, M. Jafari, N. Samadi, Fa. Mehrnejad and M. A. Faramarzi, Insights into the Molecular-Level details of betaine interactions with Laccase under various thermal conditions, J. Mol. Liq., 2021, 339, 116832, DOI:10.1016/j.molliq.2021.116832.
- D. S. Freitas, D. Rocha, J. Noro, T. G. Castro, A. Cavaco-Paulo and C. Silva, Eutectic Mixtures As Green Solvents for Laccase-Catalyzed Reactions, ACS Sustainable Chem. Eng., 2023, 11, 16594–16607, DOI:10.1021/acssuschemeng.3c04944.
- D. S. Freitas, A. Cavaco-Paulo and C. Silva, Enhancing insights into the phenomena of deep eutectic solvents, Sus. Mat. Technol., 2024, 41, e01039, DOI:10.1016/j.susmat.2024.e01039.
- D. Zhou, X. Chen, G. Li, M. Zhao and D. Li, Effect of deep eutectic solvents on activity, stability, and selectivity of enzymes: novel insights and further prospects, Int. J. Biol. Macromol., 2025, 284, 138148, DOI:10.1016/j.ijbiomac.2024.138148.
- X. Yu, Qi. Li, M. Wang, N. Du and X. Huang, Study on the catalytic performance of laccase in the hydrophobic ionic liquid-based bicontinuous microemulsion stabilized by polyoxyethylene-type nonionic surfactants, Soft Matter, 2016, 12, 1713–1720, 10.1039/C5SM02704G.
- R. Wang and X. Huang, Anionic surfactant-stabilized hydrophobic ionic liquid-based bicontinuous microemulsion: formulation, microstructure and laccase kinetics, J. Mol. Liq., 2019, 292, 111404, DOI:10.1016/j.molliq.2019.111404.
- Y. Liu, L. Huang and S. Dong, Electrochemical Catalysis and Thermal Stability Characterization of Laccase−Carbon Nanotubes−Ionic Liquid Nanocomposite Modified Graphite Electrode, Biosens. Bioelectron., 2007, 23, 35–41, DOI:10.1016/j.bios.2007.03.009.
- A. P. M. Tavares, O. Rodriguez and E. A. Macedo, Ionic liquids as alternative co-solvents for laccase: study of enzyme activity and stability, Biotechnol. Bioeng., 2008, 101, 201–207, DOI:10.1002/bit.21866.
- N. A. Mohidem and H. Mat, The Catalytic Activity of Laccase Immobilized in Sol-Gel-Silica, J. Appl. Sci., 2009, 9, 3141–3145, DOI:10.3923/jas.2009.3141.3145.
- N. A. Mohidem and H. B. Mat, The catalytic activity enhancement and biodegradation potential of free laccase and novel sol–gel laccase in non-conventional solvents, Bioresour. Technol., 2012, 114, 472–477, DOI:10.1016/j.biortech.2012.02.138.
- A. P. M. Tavares, O. Rodríguez, M. Fernández-Fernández, A. Domínguez, D. Moldes, M. A. Sanromán and E. A. Macedo, Immobilization of laccase on modified silica: stabilization, thermal inactivation and kinetic behaviour in 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid, Bioresour. Technol., 2013, 131, 405–412, DOI:10.1016/j.biortech.2012.12.139.
- M. Fernández-Fernández, D. Moldes, A. Domínguez, M. Á. Sanromán, A. P. M. Tavares, O. Rodríguez and E. A. Macedo, Stability and kinetic behavior of immobilized laccase from Myceliophthora thermophila in the presence of the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate, Biotechnol. Progress, 2014, 30, 790–796, DOI:10.1002/btpr.1910.
- J. Lin, Q. Wen, S. Chen, X. Le, X. Zhou and L. Huang, Synthesis of amine-functionalized Fe3O4@C nanoparticles for laccaseimmobilization, Int. J. Biol. Macromol., 2017, 96, 377–383, DOI:10.1016/j.ijbiomac.2016.12.051.
- R. Amin, A. Khorshidi, A. F. Shojaei, S. Rezaei and M. A. Faramarzi, Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste, Int. J. Biol. Macromol., 2018, 114, 106–113, DOI:10.1016/j.ijbiomac.2018.03.086.
- S. H. Kacem, S. Galai, A. P. de los Ríos, F. J. Fernández-Fernández and I. Smaali, New efficient laccase immobilization strategy using ionic liquids for biocatalysis and microbial fuel cells applications, J. Chem. Technol. Biotechnol., 2018, 93, 174–183, DOI:10.1002/jctb.5337.
- X. Qiu, J. Qin, M. Xu, L. Kang and Y. Hu, Organic-inorganic nanocomposites fabricated via functional ionic liquid as the bridging agent for Laccase immobilization and its application in 2,4-dichlorophenol removal, Colloids Surf. B Biointerfaces, 2019, 179, 260–269, DOI:10.1016/j.colsurfb.2019.04.002.
- Y. Gu, P. Xue and K. Shi, A novel support of sponge-like cellulose composite polymer for immobilizing laccase and its application in nitrogenous organics biodegradation, J. Porous Mater., 2020, 27, 73–82, DOI:10.1007/s10934-019-00786-y.
- X. Qiu, Y. Wang, Y. Xue, W. Li and Y. Hu, Laccase immobilized on magnetic nanoparticles modified by aminofunctionalized ionic liquid via dialdehyde starch for phenolic compounds biodegradation, Chem. Eng. J., 2020, 391, 123564, DOI:10.1016/j.cej.2019.123564.
- C. Goldhahn, J. A. Taut, M. Schubert, I. Burgert and M. Chanana, Enzyme immobilization inside the porous wood structure: a natural scaffold for continuous-flow biocatalysis, RSC Adv., 2020, 10, 20608–20619, 10.1039/C9RA10633B.
- A. M. Ferreira, A. I. Valente, L. S. Castro, J. A. P. Coutinho, M. G. Freire and A. P. M. Tavares, Sustainable liquid supports for laccase immobilization and reuse: degradation of dyes in aqueous biphasic systems, Biotechnol. Bioeng., 2021, 118, 2514–2523, DOI:10.1002/bit.27764.
- R. Liu, S. Wang, M. Han, W. Zhang, H. Xu and Y. Hu, Co-immobilization of electron mediator and laccase onto dialdehyde starch cross-linked magnetic chitosan nanomaterials for organic pollutants' removal, Bioprocess Biosyst. Eng., 2022, 45, 1955–1966, DOI:10.1007/s00449-022-02799-5.
- R. Liu, W. Zhang, S. Wang, H. Xu and Y. Hu, Magnetic Polyethyleneimine Nanoparticles Fabricated via Ionic Liquid as Bridging Agents for Laccase Immobilization and Its Application in Phenolic Pollutants Removal, Molecules, 2022, 27, 8522, DOI:10.3390/molecules27238522.
- H. Liu, Q. Li, X. Liu, S. Chen, X. Wang, S. Xu, Y. Wang, L. Xu and H. Suo, Introduction of amino acid ionic liquid into the gelatin matrix enhances the performance of immobilized laccase in degrading 2,4-dichlorophenol, J. Water Process Eng., 2024, 66, 106043, DOI:10.1016/j.jwpe.2024.106043.
- V. Vázquez, V. Giorgi, F. Bonfiglio, P. Menéndez, L. Gioia and K. Ovsejevi, Lignocellulosic residues from bioethanol production: a novel source of biopolymers for laccase immobilization, RSC Adv., 2023, 13, 13463–13471, 10.1039/D3RA01520C.
- W. Zhang, Z. Lu, R. Liu, L. Ji, B. Nian and Y. Hu, Ionic liquid modulation of metal-organic framework immobilized laccase and boosted its catalytic performance for organic pollutants removal, J. Environ. Chem. Eng., 2023, 11, 110880, DOI:10.1016/j.jece.2023.110880.
- W. Zhang, Q. Wang, J. Song, M. Zhang and Y. Hu, Ionic liquid interfacial modification of magnetic metal-organic framework enhances laccase stability and catalytic performance in degrading phenolic pollutants, Process Saf. Environ. Prot., 2025, 194, 1081–1091, DOI:10.1016/j.psep.2024.12.083.
- X. Wu and P. Si, Electrochemical detection of lignin from dietary fiber by laccases immobilized on nanocomposite of CNTs and ionic liquid, Int. J. Electrochem. Sci., 2023, 18, 100065, DOI:10.1016/j.ijoes.2023.100065.
- W. Zhang, Y. Zhang, Z. Lu, B. Nian, S. Yang and Y. Hu, Enhanced stability and catalytic performance of laccase immobilized on magnetic graphene oxide modified with ionic liquids, J. Environ. Manage., 2023, 346, 118975, DOI:10.1016/j.jenvman.2023.118975.
- W. Zhang, M. Zhang, J. Song, Q. Wang and Y. Hu, Ionic liquid functionalized magnetic polydopamine enhancing enzyme stability and catalytic efficiency for water pollutant treatment, Surf. Interfaces, 2024, 51, 104625 CrossRef CAS.
- Y. Liang, X. Chen, J. Zeng, J. Ye, B. He, W. Li and J. Sun, Mesoporous Polymeric Ionic Liquid via Confined Polymerization for Laccase Immobilization towards Efficient Degradation of Phenolic Pollutants, Molecules, 2023, 28, 2569, DOI:10.3390/molecules28062569.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Luts, J. C. Moore and K. Robins, Engineering the third wave of biocatalysis, Nature, 2012, 485, 185–194, DOI:10.1038/nature11117.
- D. M. Mate and M. Alcalde, Laccase engineering: from rational design to directed evolution, Biotechnol. Adv., 2015, 33, 25–40, DOI:10.1016/j.biotechadv.2014.12.007.
- H. Liu, L. Zhu, M. Bocola, N. Chen, A. C. Spiess and U. Schwaneberg, Directed Laccase Evolution for Improved Ionic Liquid Resistance, Green Chem., 2013, 15, 1348–1355, 10.1039/c3gc36899h.
- B. Dabirmanesh, K. Khajeh, F. Ghazi, B. Ranjbar and S.-M. Etezad, Semi-rational Approach to Obtain an Ionic Liquid Tolerant Bacterial Laccase through π-type Interactions, Int. J. Biol. Macromol., 2015, 79, 822–829, DOI:10.1016/j.ijbiomac.2015.06.002.
- I. Pardo, D. Rodríguez-Escribano, P. Aza, F. de Salas, A. T. Martínez and S. Camarero, A highly stable laccase obtained by swapping the second cupredoxin domain, Sci. Rep., 2018, 8, 15669, DOI:10.1038/s41598-018-34008-3.
- P. S. Chauhan, B. Goradia and B. Jha, Optimization and up scaling of ionic liquid tolerant and thermo-alkali stable laccase from a marine Staphylococcus arlettae S1-20 using tea waste, J. Taiwan Inst. Chem. Eng., 2018, 86, 1–8, DOI:10.1016/j.jtice.2018.02.032.
- A.-M. Wallraf, H. Liu, L. Zhu, G. Khalfallah, C. Simons and H. Alibiglou, A Loop Engineering Strategy Improves Laccase Lcc2 Activity in Ionic Liquid and Aqueous Solution, Green Chem., 2018, 20, 2801–2812, 10.1039/c7gc03776g.
- J. C. Stevens, D. W. Rodgers, C. Dumon and J. Shi, Characterization and Enzyme Engineering of a Hyperthermophilic Laccase Toward Improving Its Activity in Ionic Liquid, Front. Energy Res., 2020, 8, 158, DOI:10.3389/fenrg.2020.00158.
- X. Su, J. Yang, H. Yuan, C. Liu, R. Tu, P. Liu, Q. Wang and L. Zhu, Directed Evolution of Laccase for Improved Thermal Stability Facilitated by Droplet-Based Microfluidic Screening System, J. Agric. Food Chem., 2022, 70, 13700–13708, DOI:10.1021/acs.jafc.2c05048.
- J. C. Chan, B. Zhang, M. Martinez, B. Kuruba, J. Brozik, C.-H. Kang and X. Zhang, Structural studies of Myceliophthora Thermophila Laccase in the presence of deep eutectic solvents, Enzyme Microb. Technol., 2021, 150, 109890, DOI:10.1016/j.enzmictec.2021.109890.
- S. Varriale, A. E. Delorme, J.-M. Andanson, J. Devemy, P. Malfreyt, V. Verney and C. Pezzella, Enhancing the Thermostability of Engineered Laccases in Aqueous Betaine-Based Natural Deep Eutectic Solvents, ACS Sustainable Chem. Eng., 2022, 10, 572–581, DOI:10.1021/acssuschemeng.1c07104.
- S. Pramanik, H. Cui, G. V. Dhoke, C. B. Yildiz, M. Vedder, K.-E. Jaeger, U. Schwaneberg and M. D. Davari, How Does Surface Charge Engineering of Bacillus subtilis Lipase A Improve Ionic Liquid Resistance? Lessons Learned from Molecular Dynamics Simulations, ACS Sustainable Chem. Eng., 2022, 10, 2689–2698, DOI:10.1021/acssuschemeng.1c07332.
- A. I. Vicente, J. Viña-Gonzalez, I. Mateljak, E. Monza, F. Lucas, V. Guallar and M. Alcalde, Enhancing thermostability by modifying flexible surface loops in an evolved high-redox potential laccase, AIChE J., 2020, 66, e16747, DOI:10.1002/aic.16747.
- L. T. M. Pham, K. Deng, H. Choudhary, T. R. Northen, S. W. Singer, P. D. Adams, B. A. Simmons and K. L. Sale, An Engineered Laccase from Fomitiporia mediterranea Accelerates Lignocellulose Degradation, Biomolecules, 2024, 14, 324, DOI:10.3390/biom14030324.
- E. M. Nordwald and J. L. Kaar, Stabilization of Enzymes in Ionic Liquids via Modification of Enzyme Charge, Biotechnol. Bioeng., 2013, 110, 2352–2360, DOI:10.1002/bit.24910.
- S.-W. Bae, K. Ahn, Y.-M. Koo and S. H. Ha, Refolding of Laccase in Dilution Additive Mode with Copper-Based Ionic Liquid, Appl. Biochem. Biotechnol., 2013, 171, 1289–1298, DOI:10.1007/s12010-013-0422-9.
- H.-D. Youn, Y. C. Hah and S.-O. Kang, Role of Laccase in Lignin Degradation by White-Rot Fungi, FEMS Microbiol. Lett., 1995, 132, 183–188, DOI:10.1111/j.1574-6968.1995.tb07831.x.
- M. Moniruzzaman, T. Ono'Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment, Bioresour. Technol., 2013, 127, 132–137, DOI:10.1016/j.biortech.2012.09.113.
- N. Sun, H. Rodriguez, M. Rahman and R. D. Rogers, Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass?, Chem. Commun., 2011, 47, 1405–1422, 10.1039/C0CC03990J.
- M. Mora-Pale, L. Meli, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass, Biotechnol. Bioeng., 2011, 108, 1229–1245, DOI:10.1002/bit.23108.
- D. A. Fort, R. C. Remsing, R. P. Swatloski, P. Moyna, G. Moyna and R. D. Rogers, Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride, Green Chem., 2007, 9, 63–69, 10.1039/B607614A.
- I. Kilpelainen, H. Xie, A. King, M. Granstrom, S. Heikkinen and D. S. Argyropoulos, Dissolution of wood in ionic liquids, J. Agric. Food Chem., 2007, 55, 9142–9148, DOI:10.1021/jf071692e.
- S. Y. S. S. Y. Tan, D. R. MacFarlane, J. Upfal, L. A. Edye, W. O. S. Doherty, A. F. Patti, J. M. Pringle and J. L. Scott, Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid, Green Chem., 2009, 11, 339–345, 10.1039/B815310H.
- K. Ninomiya, K. Ochiai, M. Eguchi, K. Kuroda, Y. Tsuge, C. Ogino, T. Taima and K. Takahashi, Oxidative depolymerization potential of biorefinery lignin obtained by ionic liquid pretreatment and subsequent enzymatic saccharification of eucalyptus, Ind. Crops Prod., 2018, 111, 457–461, DOI:10.1016/j.indcrop.2017.10.056.
- H. Liu, L. Zhu, A. M. Wallraf, C. Räuber, P. M. Grande, N. Anders, C. Gertler, B. Werner, J. Klankermayer, W. Leitner and U. Schwaneberg, Depolymerization of Laccase-Oxidized Lignin in Aqueous Alkaline Solution at 37 °C, ACS Sustainable Chem. Eng., 2019, 7, 11150–11156, DOI:10.1021/acssuschemeng.9b00204.
- S. Kawai, M. Nakagawa and H. Ohashi, Degradation Mechanisms of a Nonphenolic β-O-4 Lignin Model Dimer by Trametes Versicolor Laccase in the Presence of 1-Hydroxybenzotriazole, Enzyme Microb. Technol., 2002, 30, 482–489, DOI:10.1016/S0141-0229(01)00523-3.
- A. Rico, J. Rencoret, J. C. del Río, A. T. Martínez and A. Gutiérrez, Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock, Biotechnol. Biofuels, 2014, 7, 6, DOI:10.1186/1754-6834-7-6.
- F. d'Acunzo, A. M. Barreca and C. Galli, Determination of the activity of laccase, and mediated oxidation of a lignin model compound, in aqueous-organic mixed solvents, J. Mol. Catal. B: Enzym., 2004, 31, 25–30, DOI:10.1016/j.molcatb.2004.07.001.
- J. Buendia, J. Mottweiler and C. Bolm, Preparation of Diastereomerically Pure Dilignol Model Compounds, Chem.–Eur. J., 2011, 17, 13877–13882, DOI:10.1002/chem.201101579.
- J. C. Stevens, L. Das, J. K. Mobley, S. O. Asare, B. C. Lynn, D. W. Rodgers and J. Shi, Understanding Laccase−Ionic Liquid Interactions toward Biocatalytic Lignin Conversion in Aqueous Ionic Liquids, ACS Sustainable Chem. Eng., 2019, 7, 15928–15938, DOI:10.1021/acssuschemeng.9b02151.
- A. K. Chauhan and B. Choudhury, Suitability of organic solvent and cholinium based ionic liquid activated novel lignolytic enzymes of H. aswanensis for enhanced Kalson lignin degradation, Int. J. Biol. Macromol., 2020, 165, 107–117, DOI:10.1016/j.ijbiomac.2020.09.137.
- K.-L. Chang, C.-H. Liu, P. Phitsuwan, K. Ratanakhanokchai, Y.-C. Lin, C.-D. Dong, M.-H. Lin and G. C. C. Yang, Enhancement of Biological Pretreatment on Rice Straw by an Ionic Liquid or Surfactant, Catalysts, 2021, 11, 1274, DOI:10.3390/catal11111274.
- J. C. Stevens and J. Shi, Modifying Surface Charges of a Thermophilic Laccase Toward Improving Activity and Stability in Ionic Liquid, Front. Bioeng. Biotechnol., 2022, 10, 880795, DOI:10.3389/fbioe.2022.880795.
- C. A. Pena, L. F. Ballesteros, H. Rodríguez, E. Rodil, J. A. Teixeira and M. Michelin, Laccase-mediator system for the ionic liquid-assisted treatment of a technical lignin with partial dissolution, Biomass Bioenrgy, 2023, 177, 106928, DOI:10.1016/j.biombioe.2023.106928.
- M. C. Quesada-Salas, M. E. Vuillemin, J. Dillies, R. Dauwe, L. Firdaous, M. Bigan, V. Lambertyn, D. Cailleu, A. Jamali, R. Froidevaux, E. Husson and C. Sarazin, 1-ethyl-3-methyl imidazolium acetate, hemicellulolytic enzymes and laccase-mediator system: toward an integrated co-valorization of polysaccharides and lignin from Miscanthus, Ind. Crops Prod., 2023, 197, 116627, DOI:10.1016/j.indcrop.2023.116627.
- D. Sawhney, S. Vaid, R. Bangotra, S. Sharma, H. C. Dutt, N. Kapoor, R. Mahajan and B. K. Bajaj, Proficient bioconversion of rice straw biomass to bioethanol using a novel combinatorial pretreatment approach based on deep eutectic solvent, microwave irradiation and laccase, Bioresour. Technol., 2023, 375, 128791, DOI:10.1016/j.biortech.2023.128791.
- H. Chittella, L. W. Yoon, S. Ramarad and Z.-W. Lai, Biodegradation of deep eutectic solvent pre-treated natural rubber gloves by Klebsiella aerogenes: a sustainable approach to rubber waste management, Biochem. Eng. J., 2025, 213, 109569, DOI:10.1016/j.bej.2024.109569.
- K. Lin, We. Zhang, Xi. Fan, X. Li, N. Wang, S. Yu and L. Lu, Deep eutectic solvents assisted laccase pretreatment for improving enzymatic hydrolysis of corn stover, Bioprocess Biosyst. Eng., 2025, 48, 209–219, DOI:10.1007/s00449-024-03102-4.
- A. Magnin, L. Entzmann, E. Pollet and L. Averou, Breakthrough in polyurethane bio-recycling: an efficient laccase-mediated system for the degradation of different types of polyurethanes, Waste Manage., 2021, 132, 23–30, DOI:10.1016/j.wasman.2021.07.011.
- A. Lisov, O. Belova, A. Zavarzina, A. Konstantinov and A. Leontievsky, The Role of Laccase from Zygomycetous Fungus Mortierella elasson in Humic Acids Degradation, Agronomy, 2021, 11, 2169, DOI:10.3390/agronomy11112169.
- M. Fujisawa, H. Hirai and T. Nishida, Degradation of Polyethylene and Nylon-66 by the Laccase-Mediator System, J. Polym. Environ., 2002, 9, 103–108, DOI:10.1023/A:1020472426516.
- C. Yao, W. Xia, M. Dou, Y. Du and J. Wu, Oxidative degradation of UV-irradiated polyethylene by laccase-mediator system, J. Hazard. Mater., 2022, 440, 129709, DOI:10.1016/j.jhazmat.2022.129709.
- B. S. Rathi, P. S. Kumar and D.-V. N. Vo, Critical review on hazardous pollutants in water environment: occurrence, monitoring, fate, removal technologies and risk assessment, Sci. Total Environ., 2021, 797, 149134, DOI:10.1016/j.scitotenv.2021.149134.
- K.-L. Chang, T.-C. Teng, C.-K. Fu and C.-H. Liu, Improving biodegradation of Bisphenol A by immobilization and inducer, Process Saf. Environ. Prot., 2019, 128, 128–134, DOI:10.1016/j.psep.2019.05.038.
- J. Jia, P. Xue, L. Ma, K. Shi and R. Li, A novel approach to efficient degradation of pesticide intermediate 2,4,5-trichlorophenol by co-immobilized laccase-acetosyringone biocatalyst, Biochem. Eng. J., 2022, 187, 108607, DOI:10.1016/j.bej.2022.108607.
- R. Campos, A. Kandelbauer, K. H. Robra, A. Cavaco-Paulo and G. M. Gübitz, Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii, J. Biotechnol., 2001, 89, 131–139, DOI:10.1016/S0168-1656(01)00303-0.
- A. Kandelbauer, O. Maute, R. W. Kessler, A. Erlacher and G. M. Gübitz, Study of dye decolorization in an immobilized laccase enzyme-reactor using online spectroscopy, Biotechnol. Bioeng., 2004, 87, 552–563, DOI:10.1002/bit.20162.
- S. Galai, A. P. de los Rios, F. J. Hernandez-Fernandez, S. Haj Kacem and F. Tomas-Alonso, Over-activity and stability of laccase using ionic liquids: screening and application in dye decolorization, RSC Adv., 2015, 5, 16173–16189, 10.1039/C4RA07351G.
- A. L. R. L. Zimbardi, P. F. Camargo, S. Carli, S. A. Neto, L. P. Meleiro, J. C. Rosa, A. R. De Andrade, J. A. Jorge and R. P. M. Furriel, A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization, Int. J. Mol. Sci., 2016, 17, 672, DOI:10.3390/ijms17050672.
- C.-Y. Lai, S.-H. Liu, G.-P. Wu and C.-W. Lin, Enhanced bio-decolorization of acid orange 7 and electricity generation in microbial fuel cells with superabsorbent-containing membrane and laccase-based bio-cathode, J. Clean. Prod., 2017, 166, 381–386, DOI:10.1016/j.jclepro.2017.08.047.
- G. Yaohua and X. Ping, co-immobilized Laccase-mediated Cellulose Microspheres Reconstituted from Ionic Liquid for Indole Degradation, Chem. J. Chinese Univ., 2018, 39, 1846–1852, DOI:10.7503/cjcu20170799.
- R. M. F. Bento, M. R. Almeida, P. Bharmoria, M. G. Freire and A. P. M. Tavares, Improvements in the enzymatic degradation of textile dyes using ionic liquid-based surfactants, Sep. Purif. Technol., 2020, 235, 116191, DOI:10.1016/j.seppur.2019.116191.
- S. HajKacem, S. Galai, F. J. Hernández-Fernandez, A. P. de los Rios, I. Smaali and J. Q. Medina, Bioreactor Membranes for Laccase Immobilization Optimized by Ionic Liquids and Cross-Linking Agents, Appl. Biochem. Biotechnol., 2020, 190, 1–17, DOI:10.1007/s12010-019-03085-z.
- K.-L. Chang, T.-C. Teng, C.-K. Fu and C.-H. Liu, Improving biodegradation of Bisphenol A by immobilization and inducer, Process Saf. Environ. Prot., 2019, 128, 128–134, DOI:10.1016/j.psep.2019.05.038.
- M. Zhou, L. Tao, P. Russell, R. D. Britt, T. Kasuga, X. Lü and Z. Fan, The role of lignin in the conversion of wheat straw to cellobionic acid by Neurospora crassa HL10, Ind. Crops Prod., 2022, 188, 115650, DOI:10.1016/j.indcrop.2022.115650C.
- B. Fodor, O. Gajewska, E. A. Rifaie-Graham, J. Apebende, N. Pollarda and N. Bruns, Laccase-catalyzed controlled radical polymerization of N-vinylimidazole, Polym. Chem., 2016, 7, 6617–6625, 10.1039/C6PY01261B.
- J. Zhang, F. Zou, X. Yu, X. Huang and Y. Qu, Ionic liquid improves the laccase-catalyzed synthesis of water-soluble conducting polyaniline, Colloid Polym. Sci., 2014, 292, 2549–2554, DOI:10.1007/s00396-014-3301-1.
- l. Bassanini, P. Gavezzotti, D. Monti, J. Krejzová, V. Kren and S. Riva, Laccase-catalyzed dimerization of glycosylated lignols, J. Mol. Catal. B: Enzym., 2016, 134, 295–301, DOI:10.1016/j.molcatb.2016.10.019.
- M. Lahtinena, L. Viikari, P. Karhunena, J. Asikkalaa, K. Kruusc and I. Kilpeläinena, On the reactivity of the Melanocarpus albomyces laccase and formation of coniferyl alcohol dehydropolymer (DHP) in the presence of ionic liquid 1-allyl-3methylimidazolium chloride, J. Mol. Catal. B: Enzym., 2013, 86, 169–177, DOI:10.1016/j.molcatb.2012.09.005.
- M. E. Khlupova, K. V. Lisitskaya, A. H. Amandusova, G. P. Shumakovich, I. S. Vasil’eva, E. A. Zaitseva, O. V. Morozova and A. I. Yaropolov, Dihydroquercetin polymerization using laccase immobilized into an ionic liquid, Appl. Biochem. Microbiol., 2016, 52, 452–456, DOI:10.1134/S0003683816040098.
- M. E. Khlupova, I. Vasil’eva, G. Shumakovich, E. Zaitseva, V. Chertkov, A. Shestakova, O. Morozova and A. Yaropolov, Enzymatic Polymerization of Dihydroquercetin (Taxifolin) in Betaine-Based Deep Eutectic Solvent and Product Characterization, Catalysts, 2021, 11, 639, DOI:10.3390/catal11050639.
- M. E. Khlupova, O. V. Morozova, I. S. Vasil’eva, G. P. Shumakovich, E. A. Zaitseva, V. A. Chertkov, A. K. Shestakova and A. I. Yaropolov, Polymerization of (+)-Catechin in a Deep Eutectic Solvent Using a Fungal Laccase: Physicochemical Properties of the Products and Inhibition of α-Glucosidase, Appl. Biochem. Microbiol., 2021, 57, 712–718, DOI:10.1134/S0003683821060065.
- A. Muñiz-Mouro, A. M. Ferreira, J. A. P. Coutinho, M. G. Freire, A. P. M. Tavares, P. Gullón, S. González-García and G. Eibes, Integrated Biocatalytic Platform Based on Aqueous Biphasic Systems for the Sustainable Oligomerization of Rutin, ACS Sustainable Chem. Eng., 2021, 9, 9941–9950, DOI:10.1021/acssuschemeng.1c03399.
- K. Sun, D. Hong, J. Liu, A. Latif, S. Li, G. Chu, W. Qin and Y. Si, Trametes versicolor laccase-assisted oxidative coupling of estrogens: conversion kinetics, linking mechanisms, and practical applications in water purification, Sci. Total Environ., 2021, 782, 146917, DOI:10.1016/j.scitotenv.2021.146917.
- F. F. Magalhães, A. F. Pereira, M. G. Freire and A. P. M. Tavares, New liquid supports in the development of integrated platforms for the reuse of oxidative enzymes and polydopamine production, Front. Bioeng. Biotechnol., 2022, 10, 1037322, DOI:10.3389/fbioe.2022.1037322.
- S. Hajdok, J. Conrad and U. Beifuss, Laccase-Catalyzed Domino Reactions between Hydroquinones and Cyclic 1,3-Dicarbonyls for the Regioselective Synthesis of Substituted p-Benzoquinones, J. Org. Chem., 2012, 77, 445–459, DOI:10.1021/jo202082v.
- Y. Wang, C. Wang, Q. Cheng, Y. Su, H. Li, R. Xiao, C. Tan and G. Liu, A chemo-enzymatic oxidation/aldol sequential process directly converts arylbenzyl alcohols and cyclohexanol into chiral β-hydroxy carbonyls, Green Chem., 2021, 23, 7773–7779, 10.1039/D1GC02831F.
- M. M. Rodríguez-Delgado, G. S. Alemán-Nava, J. M. Rodríguez-Delgado, G. Dieck-Assad, S. O. Martínez-Chapa, D. Barceló and R. Parra, Laccase-based biosensors for detection of phenolic compounds, TrAC, Trends Anal. Chem., 2015, 74, 21–45, DOI:10.1016/j.trac.2015.05.008.
- F. G. Zamani, H. Moulahoum, M. Ak, D. O. Demirkol and S. Timur, Current trends in the development of conducting polymers-based biosensors, TrAC, Trends Anal. Chem., 2019, 118, 264–276, DOI:10.1016/j.trac.2019.05.031.
- M. C. Castrovilli, P. Bolognesi, J. Chiarinelli, L. Avaldi, P. Calandra, A. Antonacci and V. Scognamiglio, The convergence of forefront technologies in the design of laccase based biosensors - An update, TrAC, Trends Anal. Chem., 2019, 119, 115615, DOI:10.1016/j.trac.2019.07.026.
- BORREGAARD (Norway), https://www.borregaard.com/, accessed March 17, 2025.
- IONCELL (Finland), https://ioncell.fi/, accessed March 17, 2025.
- Futamura Chemical CO, LTD (Japan), https://www.futamura.co.jp/english/, accessed March 17, 2025.
| This journal is © The Royal Society of Chemistry 2025 |