Terahertz spectroscopy: a powerful new tool for the chemical sciences?
Alexander I.
McIntosh
ab,
Bin
Yang
a,
Stephen M.
Goldup
b,
Michael
Watkinson
b and
Robert S.
Donnan
*a
aQueen Mary University of London, School of Electrical Engineering & Computer Science, Mile End Road, London, E1 4NS, UK. E-mail: robert.donnan@eecs.qmul.ac.uk
bQueen Mary University of London, School of Biological & Chemical Sciences, Joseph Priestley Building, Mile End Road, London, E1 4NS, UK
First published on 5th December 2011
Abstract
Terahertz spectroscopy is only now beginning to make its transition from initial development by physicists and engineers to broader use by chemists, materials scientists and biologists, thanks to the increasing availability of commercial terahertz spectrometers. With the unique insights that terahertz spectroscopy can provide into intermolecular bonding and crystalline matter, it could prove to be an invaluable addition to the chemist's analytical toolset. This tutorial review aims to give an introduction to terahertz spectroscopy, its techniques, equipment, current applications and potential for the chemical sciences to a broad readership.
 Left to right: Bin Yang, Stephen Goldup, Robert Donnan, Michael Watkinson and Alex McIntosh | Alex McIntosh earned a MChem from the University of Oxford in 2003 and a PhD (with Prof. R.M. Lambert) from the University of Cambridge in 2007. After his PhD he worked in support of pre-clinical drug formulation as a Research Chemist in the Analytical Sciences division of Merck & Co. He is currently a post-doctoral research assistant at Queen Mary, University of London engaged in the application of THz systems and spectroscopy to the chemical sciences. |
Bin Yang graduated from Beijing University of Posts and Telecommunications, China, in 2001. He received his MSc and PhD degrees in Electronic Engineering in 2004 and 2008 respectively, in the Antenna and Electromagnetics Group of the Department of Electronic Engineering, Queen Mary, University of London, UK, where he is currently a post-doctoral assistant engaged in the development of mm/submm-wave and THz measurement systems, and their applications in biology and chemistry science. |
Stephen Goldup received a degree in chemistry from the University of Oxford (2000) and a PhD in Organic Chemistry under the supervision of Prof. Tony Barrett from Imperial College London (2005). He undertook post doctoral studies with Prof. David Leigh at the University of Edinburgh (2005) researching rotaxane and catenane molecular machines before moving to QMUL as a Leverhulme Trust Early Career Fellow (2008). In 2009 he was awarded a Royal Society University Research Fellowship to support his work on synthesising molecular machines. Other research interests include novel methods for rotaxane synthesis, mechanically interlocked materials, biosensors and the application of unusual physical techniques to organic chemistry. |
Michael Watkinson was an undergraduate chemistry student at the University of St Andrews before moving to UMIST for his PhD to work with the late Professor Noel McAuliffe in manganese Schiff base chemistry. After a year as Royal Society post-doctoral research fellow at The Unversidad de Santiago de Compostela, Spain he returned to the UK to a post-doctoral position with Professor Andy Whiting (then at UMIST) before being appointed to his current position at Queen Mary, University of London in 1998, where he was promoted to Senior Lecturer 2003, Reader in Synthetic Chemistry in 2007 and professor in Synthetic Chemistry 2010. His current research interests focus on ligand design to provide functional complexes in both catalysis and sensing. |
Robert S Donnan received a PhD (Solid State Physics) in 2000 from the University of Wollongong, NSW, Australia and became a post-doctoral research assistant in the Department of Electronic Engineering at Queen Mary, University of London, UK in 2001, developing diffracted Gaussian beam optics for the design-verification of quasi-optical systems. In 2003 he was appointed to a Lectureship in the Department. He is presently leading a group exploring the application of alternative coherent THz sources to actively control chemical systems. Early exploratory work is seeking to direct the output from a Vector Network Analyser (VNA) into quasi-optical circuitry in which a THz beam is coupled into a chemical specimen. These VNA studies have been conducted in tandem with THz Time Domain Spectroscopy (THz-TDS), in order to investigate the characterization and control of supramolecular chemistry and crystallization. |
Introduction
The terahertz (THz) region of the electromagnetic spectrum can be described as being from 0.1 to 10 THz (3.3 cm−1 to 333.6 cm−1), bridging the microwave region and the far infrared. The general term THzspectroscopy refers to techniques which employ coherent sources as opposed to the incoherent sources historically used in far infrared spectroscopes. Most coherent work in this region has traditionally been in the lower frequency end of this spectrum by astronomers studying rotational spectra of gases.1 Coherent sources present many advantages, such as improved signal to noise, and in recent years the technology for generating and detecting coherent THz radiation has developed rapidly meaning that the whole of this largely unexplored region of the electromagnetic spectrum is now much more accessible. As a result this technology is now starting to make the transition from its beginnings in the early 1990s as a preserve for physicists and engineers to a useful tool for those working in the chemical sciences. In fact commercial spectrometers are now starting to become readily available. However due to the relative infancy of THz spectroscopy, especially in the chemical sciences, work in this area is still limited and the literature is underdeveloped and disparate. This is also the case with regard to THz metrology where further developments are required to standardise measurements and methodologies in order to allow THz spectroscopy to become a routine tool for the chemical sciences.2THz spectroscopy has the potential to provide a powerful and informative link between infrared spectroscopy and microwave spectroscopy, with potential in supramolecular chemistry, protein analysis and studies of molecular solids; while in the solid state it can potentially be a useful alternative to X-ray diffraction (XRD), solid state NMR (ssNMR) and Differential Scanning Calorimetry (DSC). This review aims to introduce the broad chemical readership to this new analytical tool and to discuss the current uses and strengths of THz spectroscopy and highlight its potential impact on the chemical sciences.
Terahertz spectrum and spectroscopic activity
The THz region lies between the microwave and infrared regions of the electromagnetic spectrum and information from THz spectroscopy reflects the interface of these different spectroscopies. Thus, the energy of photons in the THz region allows THz spectroscopy to investigate vibrational activity, which appears outside the range of infrared spectrometers while rotational and torsional modes of gaseous molecules of higher energy than those detected by microwave spectroscopy can be observed in the low frequency region of the THz spectrum. An approximate description of activity in this region is shown in Fig. 1. Rotational activity in the THz region is qualitatively the same as that observed in microwave spectroscopy with the obvious difference being at higher energy and thus generally observed for transitions between higher J levels or for rotations with smaller moments of inertia. The types of vibrational activity observed in the THz region are however qualitatively different to that observed by infrared spectroscopy. | ||
| Fig. 1 Molecular modes and activity in the terahertz region of the electromagnetic spectrum. | ||
Vibrational terahertz spectroscopy
By examining the simple treatment of a harmonic oscillator given in eqn (1), it is clear vibrations from bonds with smaller force constants and/or larger reduced masses than observed in infrared spectroscopy can be expected to present themselves in the THz region. Thus for small molecules in the gas phase typically the only observed vibrational THz activity is from bending modes and even these mostly lie in the far infrared (Fig. 1). | (1) |
In contrast, vibrational modes involving the motion of large subunits in large molecules, particularly biomolecules (proteins, DNA, lipidsetc.), which have very large reduced masses and whose force constants are mostly influenced by weak intermolecular forces are very active in the THz region. Given the unique insight into the tertiary structure of such important molecules the analysis of these modes can provide, it is perhaps unsurprising that the investigation of biomolecules is a rapidly growing area of study within THz spectroscopy and constitutes a large proportion of the literature.3
For the same reasons (large reduced mass and low force constant) it is also possible to observe vibrational modes of supramolecular complexes of small molecules which are bound together by intermolecular forces, i.e.hydrogen bonding, halogen bonding and van der Waals interactions. This is most commonly observed in the condensed phase and provides a rich source of activity for THz spectroscopy. The combination of such intermolecular vibrations and crystalline phonon modes in the solid state allows rapid and non-destructive access to information regarding the exact state of a solid sample. Indeed, distinct THz spectra are observed for crystalline and amorphous samples, different crystalline polymorphs, and due to the presence or absence of solvent in the lattice allowing the hydration state of the sample to be probed directly. Thus, THz spectroscopy can be used in place of DSC for the study of phase transitions or as a complementary technique to ssNMR and XRD. Given the importance of the characterisation of solids, often in real time, in the pharmaceutical and security sectors, the identification and analysis of solid samples using THz radiation is an important area of research.
Since intermolecular bonding has such an influence on THz spectra the physical form of any system being studied has a huge impact on the appearance of these spectra. Thus, the gas phase, where intermolecular interactions have minimal influence, can be thought of as being distinct from the study of condensed phases, where intermolecular interactions have significant influence.
Gas phase terahertz spectroscopy
The study of gases within the THz region is probably the most mature area due to its similarities with gas phase microwave spectroscopy. As a result of the minimal influence of intermolecular interactions in the gas phase, spectra are dominated by the rotational and torsional activity of molecules. In fact sub-THz radiation (commonly defined as 0.1–1 THz) has long been a valuable tool for astronomers in characterising astronomical systems due to the ability of microwave and THz spectra to identify gases through the fingerprint of their rotational spectra. High resolution rotational spectroscopy is also invaluable in providing very precise structural information regarding molecular species. Examples of gases that absorb in the THz region include carbon monoxide,4nitrous oxide,5 and propane.6 However it is also important to note that water exhibits a large number of rotational transitions in the THz region.7,8 Thus, the large amount of water vapour in the natural atmosphere is an important consideration in any THz spectroscopy measurements.Due to the maturity of microwave spectroscopy, rotational spectroscopy is a well-developed and documented area. Extensive libraries of rotational spectra and texts on the subject exist; THz spectroscopy for the study of gas phase rotational spectra will therefore not be discussed further here. The interested reader is directed elsewhere.9
Condensed phase terahertz spectroscopy
As outlined above, intermolecular interactions dominate the appearance of spectra in the THz region and can therefore provide us with valuable information regarding these interactions in the condensed phase. Key to the appearance of THz spectra in the condensed phase is the long range order present in a sample and the microenvironment of the intermolecular bonds. For sharp spectral features to exist good long range order and well defined intermolecular bonding needs to be present. Since this is absent in amorphous solids and liquids such samples tend to exhibit fewer spectral features and if present they tend to be broad in nature.By comparison, crystalline materials exhibit more spectral features which are also much more defined than those observed in the amorphous phase and liquids. This difference between amorphous and crystalline materials is exhibited clearly in the absorbance spectra of indomethacin10 where, upon undergoing a transition from an amorphous phase to a crystalline structure a broad, featureless THz absorption spectrum is replaced with a spectrum in which clear transitions are observed (Fig. 2).
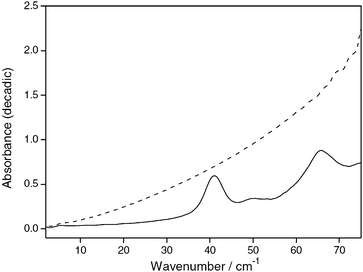 | ||
| Fig. 2 Absorbance spectra of indomethacin crystalline (solid line) and amorphous (dashed line) 75% in polyethylene. Reproduced with permission of Elsevier from ref. 10. | ||
Although the study of crystalline materials is by far the most well developed region of THz spectroscopy and the richest source of literature within the field, the resulting spectra cannot be readily assigned to isolated functional groups (cf.infrared spectroscopy) as spectral features can be attributed to a mix of the phonon modes and isolated intermolecular vibrations in the crystal. Thus computational methods are essential in gaining an understanding of terahertz spectra. A range of different computational approaches has been used, including isolated molecule calculations,11,12 rigid molecule atom-atom potentials,13 and solid state DFT.15–18Table 1 shows calculations using an isolated molecule approach for assigning the spectra of 2,4-dinitrotoluene.12 Assignment of these features gives us an understanding of the types of modes active in the THz region and where they are active in the spectrum. It can also be seen in Table 1 that this isolated DFT approach is effective at replicating THz spectra at higher frequencies where bending modes appear. However, at low and sub-THz frequencies spectral features are not as easily assigned as these features result from phonons and intermolecular vibrational modes within the crystal lattice. Thus the use of computationally more detailed approaches, which treat both the molecule and the lattice, are required for a full understanding of THz spectra.
| Experimental | B3LYP/6-311+G** | Assignment | ||
|---|---|---|---|---|
| Frequency (THz) | Intensity | Frequency (THz) | Intensity | |
| 1.08 | Phonon or intermolecular mode | |||
| 2.52 | Phonon or intermolecular mode | |||
| 5.01 | s | 4.92 | s | 2,4 C–NO2 in plane bend |
| 8.88 | s | 8.61 | s | Ring out of plane bend |
| 10.56 | s | 10.53 | s | Ring in plane bend |
| 11.58 | w | 11.82 | w | Methyl deformation, ring out of plane bend |
| 12.81 | w | 13.08 | w | C–CH3 out of plane wagging |
| 14.34 | s | 14.58 | s | 4 C–N out of plane wagging |
| 15.81 | m | 15.96 | m | 4 C–N in plane bending, ring torsion |
| 19.05 | s | 19.38 | s | Ring torsion |
The use of rigid molecules and atom-atom potentials, where the geometry of a molecule is fixed and placed within a lattice is a computationally cheap method of achieving this. This method has been shown to be effective for use with small rigid molecular structures, such as benzoic acid,13 but is expected to be unsatisfactory for larger, more dynamic molecules, where modes resulting from the mixing of intra and intermolecular vibrations are present. Although computationally demanding, solid state DFT is a more thorough theoretical treatment which incorporates these intra and intermolecular contributions, resulting in a better fit between theory and experiment.
As would be expected, due to the low-energy nature of systems in this region (kT at 298 K corresponds to 6.2 THz), THz spectra are strongly temperature dependent with significant broadening observed with increasing temperature. This strong spectral temperature-dependence however, can be potentially advantageous. Accurate, remote and local temperature measurement should be possible through the use of multi-wavelength (spectrum) pyrometry, enabling sensitive temperature measurement at low temperatures (<500 K).19,20
Current and future applications
Using the physical principles and ideas previously described THz spectroscopy has been demonstrated in a very wide range of areas relevant to the chemical sciences. In addition to academic research, THz spectroscopy is of interest to a number of industries that have strong overlap with the chemical sciences, including the pharmaceutical, security and manufacturing sectors. Some of the current uses of THz spectroscopy and its potential are described in the following sections.Fingerprinting and chemical identification
The ability to measure rotational constants in the THz region means different gases can be identified readily and this has been applied in atmospheric and astrochemistry. In particular, developments in instrumentation allow the rapid, real-time identification of isotopologues,21 which are challenging to analyse using conventional techniques. This approach may find future application in a number of areas including the identification of isotopically labelled species generated in metabolic studies.There is growing interest in using THz spectroscopy to ‘fingerprint’ and identify unknown compounds in the condensed phase. Since radiation at THz frequencies can pass relatively unhindered through non-polar and non-metallic packaging (e.g. polyethylene and PTFE), the security sector has shown significant interest in using THz spectroscopy to identify unknown substances hidden in dry packaging. As a result there is a significant proportion of THz spectroscopy literature devoted to small molecules and materials related to illegal drugs and explosives. Examples of recorded spectra for crystalline solids include ketamine,22 ecstasy,23 and dinitrotoluene.12THz spectroscopy has also been used to identify a range of flammable liquids.24
As with all spectroscopic techniques the identification of mixtures with THz spectroscopy is challenging but examples of its use in the study of mixtures are known.25,26
Studies of liquid dynamics
Although the literature is dominated by studies in the solid phase (a result of the more defined spectra), work on liquid systems has been demonstrated and THz spectroscopy can offer many unique insights even in these disordered systems. Work with liquid systems in the THz region as with other spectroscopic techniques can be challenging and requires specific experimental considerations due to the high attenuation by many liquid phases especially water. Use of narrow liquid transmission cells or ATR systems enables the effective study of such systems. Although liquids exhibit little long-range order and THz spectral features are broad, it has been shown through combining molecular dynamics calculations and THz spectroscopy measurements, that it is possible to observe hydrogen bonding networks in liquid water.27 Since THz spectroscopy is able to detect these hydrogen bond networks and can be time resolved on the sub-picosecond scale it has allowed a number of groups to study the dynamics of solvation. For example, by using THz spectroscopy Haventith and co-workers were able to study changes in the solvation of lactose on the femtosecond to picosecond timescale and determine the size of the hydration layer to be 5.13 ± 0.24 Å.28 This ability to observe changes in hydration structure of water has been used most extensively in studying biological systems and shall be discussed in the next section.Additionally, in one of its earliest uses, pulsed THz spectroscopy was used to study the molecular dynamics of water and lower alcohols (methanol, ethanol and propan-1-ol) through the direct measurement of complex permittivity.29 This showed that femtosecond pulsed THz spectroscopy could be used to successfully test existing models of dielectric relaxation in a previously unexplored region of the spectrum and one that is ideally suited for studying time dependent phenomena. More recently THz spectroscopy has been used to study the dielectrics of pentanol isomers through measurement of the complex permittivity, showing spectral sensitivity to structural changes in these isomers.30
Studies of biomolecules in the solid state
The study of chemical systems relevant to biology is one of the most active areas of THz spectroscopy. Due to the large mass of biomolecules, and the conformations of these molecules being strongly influenced by weak interactions, they can provide a rich source of activity in the THz spectrum in the solid state. Work on DNA in the THz region was undertaken as far back as 1986.31 Here highly orientated films of DNA salts were studied from 5 to 300 K and a number of vibrational modes were detected which were sensitive to the hydration state of the sample. More recently, THz spectroscopy was used to study calf thymus DNA at differing humidities allowing conformational changes upon hydration to be observed.32Proteins, including type I collagen and bovine serum albumin, were also studied and shown to exhibit broadband adsorption suggesting a large density of low frequency collective modes. Changes in spectra with humidity and hydration suggest the technique is also sensitive to conformational change for these proteins. In addition to DNA and proteins the polysaccharide biomolecules cellulose and chitin have also been studied. Here features present at low temperatures (10 K), but absent at high temperatures (300 K), were found and were assigned to one-dimensional phonon modes along the backbone of the polymer.33In order to fully analyse the information gained from studying large biomolecules in the solid state it is necessary to combine experimental observations with theoretical studies. Solid state DFT has been used in the study of α-lactose monohydrate which exhibits clear and well defined sub-THz spectra, with a sharp, well-defined peak at 0.53 THz (Fig. 3). Through modelling, this feature has been shown to correspond to a hindered rotation of the whole lactose molecule along the b-axis of the crystal within the hydrogen bond network.34 This peak can be fitted extremely well with a Lorentzian function, showing that the vibrational mode undergoes homogenous dampening, while the fitting also shows that the lifetime of the excited vibrational state is 14.6 ps, leading to spectral broadening which is exacerbated at higher temperatures due to the inherent properties of THz spectroscopy as discussed in the introduction.35
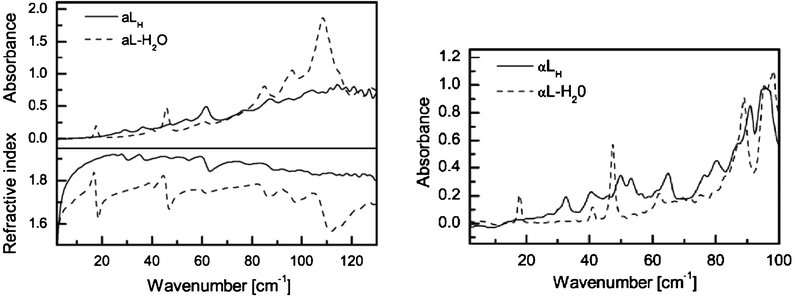 | ||
| Fig. 3 Terahertz absorption spectra and frequency dependent refractive indices of α-lactose anhydrate (aLH) and α-lactose monohydrate (aL-H2O) at 293 K (left) and 90 K (right). (Reproduced with permission of Elsevier from ref. 14). | ||
Studies of biomolecules in aqueous solution
Direct measurement of the solvation layer around proteins has been achieved by measuring changes in the dielectric spectrum of the solution with concentration.36,37 Whilst the direct observation of the low energy vibrational modes of proteins in aqueous media is an attractive application of THz spectroscopy, attenuation by water in the THz region is strong, often resulting in featureless spectra which at first sight yield little information about the actual protein. Despite this, Allen and co-workers have shown that detailed investigation of such systems is possible.38 Studying bovine serum albumin they were able to separate the molar absorption of the solvated protein from the strongly attenuating water, yielding information about the protein structure.In common with other areas of research using THz spectroscopy, such as the study of photoconductive materials (vide infra), the use of time-resolved, pump–probe techniques for biomolecules can provide valuable insights into their dynamic behaviour. This has already been demonstrated by studying the conformational changes of the photoactive yellow protein when excited by blue light.39 Another protein of interest for such studies is rhodopsin, whose response to light has been characterised by THz spectroscopy in both solution and the solid state.40,41
Solid state transformations
Due to the ability of THz spectroscopy to clearly distinguish between samples with good and poor long range order and other changes in intermolecular bonding networks, it is a powerful technique for the study of phase changes in condensed systems, be this a change in polymorph or a crystallisation processes. Work in this area has already demonstrated the potential for THz spectroscopy as a useful addition to other solid state techniques such as DSC, ssNMR and XRD.THz spectroscopy has the advantage of being able to monitor such changes in situ, in real time. Monitoring of the THz spectrum of carbamazepine as the temperature was varied allowed the direct observation of transitions between amorphous, rubbery and crystalline (both forms III and I) states (Fig. 4).42 Using this approach it was possible to clearly distinguish the glass transition and other phase changes in this system. The distinct THz spectra for carbamazepine form III and I polymorphs (Fig. 5) also enabled the investigation of the inter-conversion of these polymorphs in comparison to DSC showing the complimentarity of these techniques.43
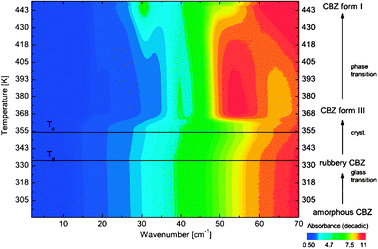 | ||
| Fig. 4 Contour plot of the non-isothermal heating of amorphous carbamazepine (CBZ) from room temperature. The sample was heated from 293 K until its melting point at a heating rate of 1 K min−1. (Reproduced with permission of John Wiley & Sons, Inc. from ref. 42.) | ||
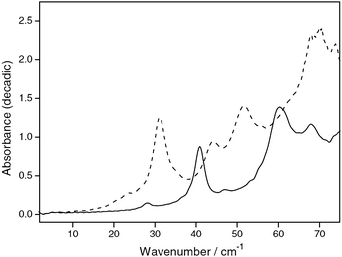 | ||
| Fig. 5 Absorbance spectra of carbamazepine form III (solid line) and form I (dashed line) 50% in polyethylene. (Reproduced with permission of Elsevier from ref. 10.) | ||
The crystal nucleation of n-alkanes (C23-C26) from melt has also been studied.44 These alkanes demonstrate rotator phases intermediate between the liquid and crystalline solid phases. THz spectroscopy was used to observe the nucleation of crystal formation and also the liquid to rotator phase change through enhancement of specific absorptions in a very narrow temperature range. The observed enhancement in absorption was assigned to the increase in the vibrational density of states during the transition and likened to the boson peak observed in glasses. This change could also be observed by using the refractive index which can be readily obtained using THz time domain spectroscopy (THz-TDS, vide infra).
THz spectroscopy can also be used to study solid state reactions. Co-crystal formation from phenazine and mesaconic acid has been monitored in real time using THz spectroscopy.45Phenazine and mesaconic acid were ground together in an equimolar mixture and, by observing the change in intensity of the peak at 1.2 THz which is characteristic of the phenazine:mesaconic acid co-crystal, the effects of different grinding conditions on co-crystal formation could be determined. Accurate quantification of the rates of co-crystal formation could also be made by constructing a calibration curve from the intensity of the peak at 1.2 THz.
The reaction of p-benzoquinone and p-dihydroxybenzene in the solid-state to form the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex quinhydrone has also been monitored by THz-TDS.46 Using the Beer–Lambert law the progress of the crystal transformation could be observed quantitatively. Further measurements on this system demonstrated that THz spectroscopy is complementary to XRD and solid state FTIR analysis.
1 complex quinhydrone has also been monitored by THz-TDS.46 Using the Beer–Lambert law the progress of the crystal transformation could be observed quantitatively. Further measurements on this system demonstrated that THz spectroscopy is complementary to XRD and solid state FTIR analysis.
THz spectroscopy has also been shown to be a complimentary technique to powder XRD, mid-IR and Raman spectroscopy in the study of the dehydration kinetics of D-glucose monohydrate.47 By heating D-glucose monohydrate and measuring peak area changes (Fig. 6) in the THz spectrum, it was possible to observe conversion in real-time. By measuring reaction rates at five different temperatures, the activation energy of the dehydration could also be calculated. This study also demonstrated the potential of the time-gated and coherent sources used in THz-TDS to help avoid problems due to the noise generated by the heating of the sample.
 | ||
| Fig. 6 THz absorption spectra (with baselines corrected) of D-glucose monohydrate being heated at 45 °C for about 27 min. The numbers mark the positions of absorption peaks. The absorption peaks at 1.80 and 1.96 THz decreased and the absorption peaks at 1.28, 1.43, and 2.08 THz increased over time due to the reduction of D-glucose monohydrate and augment of anhydrous D-glucose during the dehydration. (Reproduced with permission of Elsevier from ref. 47). | ||
Applications in pharmaceutical sciences
It is of paramount importance that all polymorphs (crystalline and amorphous), and hydrated forms of a new active pharmaceutical ingredient (API) are identified in order to optimise the properties with regard to stability, bioavailability and the manufacturing process of the drug, and to ensure that a comprehensive patent is obtained. The ability of THz spectroscopy to identify these different solid forms, including the formation of co-crystals, has the potential to allow efficient and rapid screening.Further, as an API moves through scale-up and the screening process it is important that the solid state form produced and administered is controlled to ensure that results are accurate and representative as polymorphism has a large effect on bioavailability by modifying the solubility. The solid form of a drug has a large impact on stability during storage. Thus it is unsurprising that the pharmaceutical industry has shown great interest in using THz spectroscopy given its ability to rapidly distinguish between the physical form of solids, including polymorphism and hydration.48,49 In addition to the studies on carbamazepine described in the previous section THz spectroscopy has been applied to the study of polymorphism for ranitide hydrochloride,50diclofenic acid,17 and the identification of the hydrate forms of theophylline monohydrate.51
THz spectroscopy's ability to monitor solid state reactions non-invasively also has significant potential for the study of drug product stability during storage, especially as many excipients (pharmacologically inactive components such as tablet coatings) and packaging materials are transparent to THz radiation which enables the in situ, real-time, non-destructive study of tablets. This also allows non-invasive identification of drug products with potential benefits in manufacturing Quality Assurance (QA) and the identification of counterfeit drugs, a growing concern.
In addition to using THz radiation to examine the physical form of the active ingredient, changes in refractive index between layers and coatings due to fractures mean it is also possible to examine the physical features of tablets non-destructively. Thus THz examination of pharmaceutical products can be combined with 2D and 3D imaging, resulting in an extremely powerful technique. In addition to potential applications in QA during manufacturing and storage, THz spectroscopy has been shown to be effective for assessing tablet coating and dissolution rates.52–56
Materials chemistry
THz spectroscopy has the capability to be an extremely important technique for studying the chemistry of materials, particularly those relating to electronics due to its ability to study low energy phenomena. This has been demonstrated for the characterisation of low energy molecular vibrations in phenylene oligomers.57 These conjugated polymers are of use in optoelectronics where it is critical to gain an understanding of these low energy molecular vibrations as they are believed to have a significant effect on charge carrier diffusion in these materials.An additional advantage of THz spectroscopy is that dynamic processes (such as photo-excited carriers) can be studied with pump–probe THz-TDS due to the time-gated THz generation. This use of pump–probe techniques to study carrier dynamics was demonstrated for photoconductivity of the functionalised pentacene organic semiconductor.58
Another active area in materials science for THz spectroscopy is in the study of high-temperature superconductors due to the importance of low energy phenomena in these materials. Superconductors, such as MgB2 have been characterised using THz-TDS.59 Parameters such as superconducting energy gap and magnetic penetration depth can be measured and the study of superconductors through their emission of THz radiation can also been undertaken.60
As well as being useful in the study of materials a significant body of work in materials chemistry is in the development of new sources and detectors for THz spectroscopy.61
Coherent control and excitation
As with all spectroscopy, in addition to passive observation of phenomena there is also the potential to influence molecules, reactions and processes by coherent excitation. The area of vibrational excitation has been growing and a significant body of experimental and theoretical work has been published.62–67 Similar work carried out in the infrared has benefited from the introduction and use of lasers, a coherent and bright light source. Since the THz region also benefits from such coherent and bright sources, similar applications should be possible. In fact the excitation of systems with THz radiation has already been demonstrated: the excitation of acoustic phonons in the inorganic system AlGaAs68 and the excitation of crystalline L-arginine69 have both been studied. The first study is analogous to the time-resolved, pump–probe experiments described in the previous section for the study of dynamics in semiconductors. Here the pump consists of THz radiation and the probe is optical. Acoustic phonons in the un-doped semiconductor AlGaAs were excited using intense coherent and single-cycle THz pulses. The subsequent relaxation of these could then be monitored by using time delayed optical pulses tuned to the interband transition of the AlGaAs semiconductor.For the L-arginine study, a crystalline sample was excited using an intense THz pulse and changes to the observed peak shape in its THz absorption spectrum were monitored. The pump pulse was shown to lead to excitation of the intermolecular vibrational mode at 1.1 THz. Modelling of the peak at 1.1 THz and the transformation of its shape and position demonstrated that excitation led to an increase of 20 levels in the anharmonic intermolecular potential well, taking the system far from thermal equilibrium. The coherent control of the ferroelectric PbTiO3 has also been investigated in a theoretical study,70 Indicating that through the use of properly shaped THz fields it should be possible to initiate a structural change of the ferroelectric crystal through movement of the ions within the lattice.
Terahertz spectroscopy techniques
Compared to FTIR spectroscopy, where these instruments are now ubiquitous in chemistry laboratories, THz spectroscopy is much more expensive and less accessible. These limitations can however be explained by the relative age of the techniques. Infrared spectroscopy is much more mature and commercial spectrometers have been available for over fifty years. Since reliable sources of THz radiation have only emerged in the last twenty years THz spectroscopy in comparison is still very much in its infancy. However, these sources compared to infrared sources have the advantage of being bright body, more stable and coherent. These advantages coupled with sensitive photon detectors results in superior dynamic range and signal to noise ratios being possible. Additionally, detection methods in THz spectroscopy, allow for the possibility of measuring phase and amplitude instead of just intensity. This is in comparison to commercial infrared systems which are predominantly FTS (power spectrometry) rather than dispersion FTS (i.e. cross-correlation FTS) which records complex amplitude. Since amplitude and phase are directly related to the absorption co-efficient and refractive index this means complex permittivity can be easily obtained from THz spectroscopy. A number of THz radiation sources and systems are now available for use in spectroscopy. These can be broadly split into the older incoherent techniques familiar to far-infrared spectroscopic measurements and the more recent coherent THz techniques.Incoherent techniques
Fourier Transform Infrared ( FTIR ) interferometry is a mature methodology used for probing molecular resonances. It uses thermal (or ‘noise’) sources such as arc lamps or an incandescent SiC globar to generate extremely wide bandwidth of radiation (from THz to the visible). A helium-cooled bolometer is normally coupled to this to record the interferogram. For such systems the limit of spectral resolution is nominally 0.5 cm−1 (at the penalty of a long scan-time). Thermal-source interferometry is inherently characterised by the principal trade-off between resolution and signal to noise ratio (S/N). The S/N is typically around 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 3 THz. It deteriorates rapidly below this and can be enhanced by averaging over multiple scanning events. An averaged interferogram may thus take minutes to several tens of minutes to record. The power from such sources is low and the peak output power is typically around 100 nW and this can become significantly worse in the sub-THz band (near to the noise-floor).71
1 at 3 THz. It deteriorates rapidly below this and can be enhanced by averaging over multiple scanning events. An averaged interferogram may thus take minutes to several tens of minutes to record. The power from such sources is low and the peak output power is typically around 100 nW and this can become significantly worse in the sub-THz band (near to the noise-floor).71
Coherent techniques
The Vector Network Analyzer (VNA) is a highly sophisticated and versatile microwave diagnostic instrument which directly applies the principle of the conservation of energy. A signal of known absolute amplitude and phase is propagated into a ‘system’ and the VNA records the division of signal (or scattering) among different paths, i.e. transmission, reflection and absorption. The VNA is a generator and receiver of ultra-phase-stable, coherent, fine- and widely-tunable electromagnetic radiation. At present such instruments can produce and detect, with the aid of frequency multipliers, the amplitude and phase of radiation having wavelengths from infinity to 0.3 mm (i.e. 33 cm−1 or 1012 Hz). This can be done with a high precision energy bandwidth (6.25 × 10−5 cm−1 or 1.875 MHz) that is of the order of a million-fold higher in resolution than spectrometries currently used routinely by chemists.The quantum cascade laser (QCL) is another instrument for generating coherent THz signals. It is assembled from repeating units. Each unit has an active region for creating the required population inversion for which relaxation yields THz photons (but over a narrow tuneable band). The electrons continuously escape to the subsequent injector band to induce other same-frequency photons. The cascade nature implies that the produced photons in each period couple together to achieve a higher energy level. The first THz QCL was demonstrated in 2001 at 4.4 THz72 and subsequent intensive research has produced rapid improvements. The spectral coverage has been extended to the sub-THz with working temperatures at around 170 K for pulsed signals and around 120 K for continuous wave signals. The output power is on the order of hundreds of mW. Like FTIR, the QCL has difficulties for achieving high-power sub-THz emission and this is due to small sub-band energy separations and higher free-carrier losses. Room-temperature operation is also as yet unavailable and this significantly limits its scope for practical applications.
The most intense THz radiation source available is the free-electron laser (FEL). This source generates a THz signal by intense magnetic-field-induced bunching of high-velocity electrons propagating in a vacuum flight-tube. The output power can be further increased by an energy recovery linear accelerator (linac) to achieve average broadband THz beam powers of 100 W. However, as a result of its size and cost, FELs are high-capital facilities. Operating on a similar principle to the FEL, but compact at lab-bench scale is the Backward Wave Oscillator (BWO). A heated-cathode is the source of an electron beam that interacts with a periodic-cavity (slow-wave) structure. The retarding effect generates an oscillating electric field that propagates against the direction of the electron beam. The output signal beam frequency is coupled out near the electron gun and delivers typical powers in the range of 50 mW at the lower sub-THz band to near 2 mW at 2 THz.
THz
time domain
spectroscopy
(THz-TDS) is probably the most commonly found and accessible technique for chemists and chemistry applications. As a result it is probably the most widely described technique in the literature pertaining to the chemical sciences. A typical THz-TDS set up is shown in Fig. 7. At the heart of this system is the generation of THz radiation, which is facilitated by producing an ultrafast electro-optical frequency rectification of an optical laser input signal to a THz signal. This is undertaken by excitation of a non-linear crystal (typically GaAs) by a pulsed femtosecond laser beam (commonly from a Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sapphire laser). The laser path used for generation is one which has been split just after the source into a pump and probe beam. The pump beam passes through a delay stage prior to excitation of the non-linear crystal. Following generation, the THz radiation is propagated along a beam path to the sample.
Sapphire laser). The laser path used for generation is one which has been split just after the source into a pump and probe beam. The pump beam passes through a delay stage prior to excitation of the non-linear crystal. Following generation, the THz radiation is propagated along a beam path to the sample.
 | ||
| Fig. 7 Schematic representation of a common Terahertz Time Domain Spectroscopy System. | ||
After passing through the sample the resulting spectra can be detected using a number of methods.73 However, electro-optical sampling is the most commonly used. Here the THz radiation from the sample is directed to a crystal which can become birefringent when exposed to the electric field of the THz radiation. Typically a 〈110〉 ZnTe crystal is used. A portion of the optical beam from the laser is also directed through this crystal which results in its polarisation rotating in proportion to the magnitude of the THz electric field. By using a Wollaston prism the beam is then split into two orthogonal pulses (probe and measurement) which are passed on to the balanced photo-diode detectors for measurement. By measuring the signal as a function of time-delay between the THz radiation and the probe beam, the electric field of the THz pulse in the time domain can be built up. As with FTIR a Fourier Transform of the raw data is required to yield the equivalent frequency domain spectrum which most chemists are familiar with. The THz-TDS described above is how a system would typically be found in an academic research setting. The first commercial systems were introduced in 2003 and a handful of companies are now selling commercial systems based upon these principles.
In addition it is possible, as with Raman, mid and near-infrared spectroscopy, to undertake chemical imaging with THz radiation. By using a focussed beam and moving the specimen of interest through the focused beam it is possible to build up a spatial map of chemical distribution and physical features in two dimensions and even in three dimensions. These imaging techniques have been developed out of interest from the security and manufacturing sectors. For more information regarding these techniques the reader is directed elsewhere.74
Experimental considerations
As with all spectroscopic techniques certain considerations have to be made when performing THz spectroscopy to achieve good quality spectra. As is the case for infrared spectroscopy, water can have a detrimental effect on THz spectral quality. Whereas vibrational modes of water complicate infrared spectra, rotational modes of water are observed in the THz region, spanning the whole THz range up to 19.5 THz.75,76 It is thus important moisture is eliminated from any regions where the THz radiation is present. This can typically be achieved by purging of the spectrometer with dry gas. It is also important to note that although this absorption by water can have a detrimental effect on spectral quality these absorptions can also be beneficial by providing a means of calibrating an instrument.Solid state samples, like samples for transmission infrared measurement, are commonly produced by mixing the material of interest with an inert and transparent matrix and compressing the blend into pellets. Powdered polyethylene and PTFE, due to their chemically inert nature and transparency to THz radiation, are suitable matrix materials for sample preparation. For studies involving other sample types, measurement-cells can be constructed from a range of solid materials with good transparency in the THz region. These include sapphire, z-cut quartz, high density polyethylene (HDPE) and polymethylpentane (TPX®).
Conclusions
Due to developments in the last twenty years spectroscopy in the THz region of the electromagnetic spectrum is now becoming a reality. This technology is now in the process of transitioning from its initial development by physicists and engineers to being a useful tool for chemists. With a maturing of this technology an increasing number of researchers are working in this area and the technique is becoming more accessible. However, as a result of its relative infancy, the THz spectrum still remains relatively unexplored compared to other regions of the electromagnetic spectrum. The brightness and coherence of THz sources allows spectroscopy to be undertaken with very good signal to noise and dynamic range compared to current infrared techniques. Further to this, the availability of time-gated sources on a femtosecond timescale, allows time-resolved measurements of important dynamic physical phenomena to be studied. In addition detection of amplitude and phase as opposed to just intensity allows THz spectroscopy to easily measure important parameters such as refractive index and complex permittivity, enabling the investigation of molecular dynamics in liquids. In the solid state, THz spectroscopy has already shown itself to be a useful technique and is emerging as a powerful addition to other solid state spectroscopies, XRD and DSC. It has also shown that it can provide insights into the low energy dynamics of large biomolecules. This ability of THz spectroscopy to study low energy vibrational modes allows valuable insights into the little studied and understood area of intermolecular interactions. Increased understanding of such interactions and systems could have implications for a number of important topics in the chemical sciences, such as molecular crystallisation, solvation, biochemistry, surface science and supramolecular chemistry.Notes and references
- J. M. Payne, Proc. IEEE, 1989, 77, 993–1017 CrossRef CAS.
- Z. Popovic and E. N. Grossman, IEEE Transactions on Terahertz Science and Technology, 2011, 1, 133–144 CrossRef.
- D. F. Plusquellic, K. Siegrist, E. J. Heilweil and O. Esenturk, ChemPhysChem, 2007, 8, 2412–2431 CrossRef CAS.
- T. D. Varberg and K. M. Evenson, IEEE Trans. Instrum. Meas., 1993, 42(2), 412–414 CrossRef.
- B. J. Drouin and F. W. Maiwald, J. Mol. Spectrosc., 2006, 236(2), 260–262 CrossRef CAS.
- B. J. Drouin, J. C. Pearson, A. Walters and V. Lattanzi, J. Mol. Spectrosc., 2006, 240(2), 227–237 CrossRef CAS.
- R. T. Hall, D. Vrabec and J. M. Dowling, Appl. Opt., 1966, 5, 1147–1158 CrossRef CAS.
- R. T. Hall and J. M. Dowling, J. Chem. Phys., 1970, 52, 1161–1165 CrossRef CAS.
- J. M. Brown and A. Carrington, Rotational Spectroscopy of Diatomic Molecules, Cambridge University Press, Cambridge, 1st edn, 2003 Search PubMed.
- C. J. Strachan, T. Rades, D. A. Newnham, K. C. Gordon, M. Pepper and P. F. Taday, Chem. Phys. Lett., 2004, 390, 20–24 CrossRef CAS.
- L. Ning, J. L. Shen, J. H. Sun, L. S. Liang, X. Y. Xu, M. H. Lu and J. Yan, Opt. Express, 2005, 13, 6750–6755 CrossRef.
- Y. Q. Chen, H. B. Liu, Y. Q. Deng, D. Schauki, M. J. Fitch, R. Osiander, C. Dodson, J. B. Spicer, M. Shur and X. C. Zhang, Chem. Phys. Lett., 2004, 400, 357–361 CrossRef CAS.
- R. Y. Li, J. A. Zeitler, D. Tomerini, E. P. J. Parrott, L. F. Gladden and G. M. Day, Phys. Chem. Chem. Phys., 2010, 12(20), 5329–5340 RSC.
- J. A. Zeitler, K. Kogermann, J. Rantanen, T. Rades, P. F. Taday, M. Pepper, J. Aaltonen and C. J. Strachan, Int. J. Pharm., 2007, 334, 78–84 CrossRef CAS.
- D. G. Allis, A. M. Fedor, T. M. Korter, J. E. Bjarnason and E. R. Brown, Chem. Phys. Lett., 2007, 440, 203–209 CrossRef CAS.
- P. M. Hakey, M. R. Hudson, D. G. Allis, W. Ouellette and T. M. Korter, J. Phys. Chem. A, 2009, 113, 13013–13022 CrossRef CAS.
- M. D. King, W. D. Buchanan and T. M. Korter, Anal. Chem., 2011, 83, 3786–3792 CrossRef CAS.
- M. D. King, W. D. Buchanan and T. M. Korter, J. Pharm. Sci., 2011, 100, 1116–1129 CrossRef CAS.
- R. S. Donnan, PhD Thesis, University of Wollongong, 2000.
- G. B. Hunter, C. D. Allemand and T. W. Eagar, Opt. Eng., 1986, 25, 1222–1231 CAS.
- D. M. Mittleman, R. H. Jacobsen, R. Neelamani, R. G. Baraniuk and M. C. Nuss, Appl. Phys. B: Lasers Opt., 1998, 67(3), 379–390 CrossRef CAS.
- G. Q. Wang, J. L. Shen and Y. Jia, J. Appl. Phys., 2007, 102(1), 013106 CrossRef.
- D. G. Allis, P. M. Hakey and T. M. Korter, Chem. Phys. Lett., 2008, 463(4–6), 353–356 CrossRef CAS.
- T. Ikeda, A. Matsushita, M. Tatsuno, Y. Minami, M. Yamaguchi, K. Yamamoto, M. Tani and M. Hangyo, Appl. Phys. Lett., 2005, 87(3), 034105 CrossRef.
- Y. Watanabe, K. Kawase, T. Ikari, H. Ito, Y. Ishikawa and H. Minamide, Opt. Commun., 2004, 234, 125–129 CrossRef CAS.
- Z. Zhang, X. Yu, H. Zhao, T. Xiao, Z. Xi and H. Xu, Opt. Commun., 2007, 277, 273–276 CrossRef CAS.
- H. Yada, M. Nagai and K. Tanaka, Chem. Phys. Lett., 2009, 473, 279–283 CrossRef CAS.
- U. Heugen, G. Schwaab, E. Brundermann, M. Heyden, X. Yu, D. M. Leitner and M. Havenith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12301–12306 CrossRef CAS.
- J. T. Kindt and C. A. Schmuttenmaer, J. Phys. Chem., 1996, 100, 10373–10379 CrossRef CAS.
- Y. Yomogida, Y. Sato, K. Yamakawa, R. Nozaki, T. Mishina and J. Nakahara, J. Mol. Struct., 2010, 970(1–3), 171–176 CrossRef CAS.
- A. Wittlin, L. Genzel, F. Kremer, S. Haseler, A. Poglitsch and A. Rupprecht, Phys. Rev. A: At., Mol., Opt. Phys., 1986, 34, 493–500 CrossRef CAS.
- A. G. Markelz, A. Roitberg and E. J. Heilweil, Chem. Phys. Lett., 2000, 320, 42–48 CrossRef CAS.
- B. Fischer, M. Hoffmann, H. Helm, G. Modjesch and P. U. Jepsen, Semicond. Sci. Technol., 2005, 20, S246–S253 CrossRef CAS.
- D. G. Allis, A. M. Fedor, T. M. Korter, J. E. Bjarnason and E. R. Brown, Chem. Phys. Lett., 2007, 440(4–6), 203–209 CrossRef CAS.
- E. R. Brown, J. E. Bjarnason, A. M. Fedor and T. M. Korter, Appl. Phys. Lett., 2007, 90(6), 061908 CrossRef.
- Y. W. Sun, Y. T. Zhang and E. Pickwell-MacPherson, Biophys. J., 2011, 100, 225–231 CrossRef CAS.
- M. Heyden and M. Havenith, Methods, 2010, 52, 74–83 CrossRef CAS.
- J. Xu, K. W. Plaxco and S. J. Allen, Protein Sci., 2006, 15, 1175–1181 CrossRef CAS.
- E. Castro-Camus and M. B. Johnston, Chem. Phys. Lett., 2008, 455, 289–292 CrossRef CAS.
- R. Balu, H. Zhang, E. Zukowski, J. Y. Chen, A. G. Markelz and S. K. Gregurick, Biophys. J., 2008, 94, 3217–3226 CrossRef CAS.
- A. J. Vickers, Y. Ma, R. Dudley, P. J. Reeves, C. A. Reynolds, S. Gadde and Ieee, 2008 33rd International Conference on Infrared, Millimeter and Terahertz Waves, vol. 1 and 2, 2008, 851–852.
- J. A. Zeitler, P. F. Taday, M. Pepper and T. Rades, J. Pharm. Sci., 2007, 96, 2703–2709 CrossRef CAS.
- J. A. Zeitler, D. A. Newnham, P. F. Taday, C. J. Strachan, M. Pepper, K. C. Gordon and T. Rades, Thermochim. Acta, 2005, 436, 71–77 CrossRef CAS.
- J. P. Laib, D. V. Nickel and D. M. Mittleman, Chem. Phys. Lett., 2010, 493, 279–282 CrossRef CAS.
- K. L. Nguyen, T. Friscic, G. M. Day, L. F. Gladden and W. Jones, Nat. Mater., 2007, 6, 206–209 CrossRef CAS.
- M. Ge, W. F. Wang, H. W. Zhao, Z. Y. Zhang, X. H. Yu and W. X. Li, Chem. Phys. Lett., 2007, 444, 355–358 CrossRef CAS.
- H. B. Liu and X. C. Zhang, Chem. Phys. Lett., 2006, 429, 229–233 CrossRef CAS.
- B. Shah, V. K. Kakumanu and A. K. Bansal, J. Pharm. Sci., 2006, 95, 1641–1665 CrossRef CAS.
- J. A. Zeitler, P. F. Taday, D. A. Newnham, M. Pepper, K. C. Gordon and T. Rades, J. Pharm. Pharmacol., 2007, 59, 209–223 CrossRef CAS.
- P. F. Taday, I. V. Bradley, D. D. Arnone and M. Pepper, J. Pharm. Sci., 2003, 92, 831–838 CrossRef CAS.
- P. C. Upadhya, K. L. Nguyen, Y. C. Shen, J. Obradovic, K. Fukushige, R. Griffiths, L. F. Gladden, A. G. Davies and E. H. Linfield, Spectrosc. Lett., 2006, 39, 215–224 CrossRef CAS.
- A. J. Fitzgerald, B. E. Cole and P. F. Taday, J. Pharm. Sci., 2005, 94, 177–183 CrossRef CAS.
- L. Ho, R. Muller, M. Romer, K. C. Gordon, J. Heinamaki, P. Kleinebudde, M. Pepper, T. Rades, Y. C. Shen, C. J. Strachan, P. F. Taday and J. A. Zeitler, J. Controlled Release, 2007, 119, 253–261 CrossRef CAS.
- L. Ho, R. Muller, K. C. Gordon, P. Kleinebudde, M. Pepper, T. Rades, Y. C. Shen, P. F. Taday and J. A. Zeitler, Eur. J. Pharm. Biopharm., 2009, 71, 117–123 CrossRef CAS.
- L. Ho, R. Mueller, K. C. Gordon, P. Kleinebudde, M. Pepper, T. Rades, Y. C. Shen, P. F. Taday and J. A. Zeitler, J. Controlled Release, 2008, 127, 79–87 CrossRef CAS.
- J. A. Spencer, Z. M. Gao, T. Moore, L. F. Buhse, P. F. Taday, D. A. Newnham, Y. C. Shen, A. Portieri and A. Husain, J. Pharm. Sci., 2008, 97, 1543–1550 CrossRef CAS.
- M. B. Johnston, L. M. Herz, A. L. T. Khan, A. Kohler, A. G. Davies and E. H. Linfield, Chem. Phys. Lett., 2003, 377, 256–262 CrossRef CAS.
- F. A. Hegmann, R. R. Tykwinski, K. P. H. Lui, J. E. Bullock and J. E. Anthony, Phys. Rev. Lett., 2002, 89, 227403 CrossRef CAS.
- R. A. Kaindl, M. A. Carnahan, J. Orenstein, D. S. Chemla, H. M. Christen, H. Y. Zhai, M. Paranthaman and D. H. Lowndes, Phys. Rev. Lett., 2002, 88, 027003 CrossRef.
- L. Ozyuzer, A. E. Koshelev, C. Kurter, N. Gopalsami, Q. Li, M. Tachiki, K. Kadowaki, T. Yamamoto, H. Minami, H. Yamaguchi, T. Tachiki, K. E. Gray, W. K. Kwok and U. Welp, Science, 2007, 318, 1291–1293 CrossRef CAS.
- B. Ferguson and X. C. Zhang, Nat. Mater., 2002, 1, 26–33 CrossRef CAS.
- W. S. Warren, H. Rabitz and M. Dahleh, Science, 1993, 259, 1581–1589 CAS.
- R. N. Zare, Science, 1998, 279, 1875–1879 CrossRef CAS.
- F. F. Crim, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12654–12661 CrossRef CAS.
- R. S. Judson and H. Rabitz, Phys. Rev. Lett., 1992, 68, 1500–1503 CrossRef CAS.
- R. J. Levis, G. M. Menkir and H. Rabitz, Science, 2001, 292, 709–713 CrossRef CAS.
- H. Rabitz, R. de Vivie-Riedle, M. Motzkus and K. Kompa, Science, 2000, 288, 824–828 CrossRef CAS.
- J. M. Manceau, P. A. Loukakos and S. Tzortzakis, Appl. Phys. Lett., 2010, 97 CrossRef CAS.
- M. Jewariya, M. Nagai and K. Tanaka, Phys. Rev. Lett., 2010, 105 CrossRef CAS.
- T. T. Qi, Y. H. Shin, K. L. Yeh, K. A. Nelson and A. M. Rappe, Phys. Rev. Lett., 2009, 102, 4 Search PubMed.
- P. Y. Han, M. Tani, M. Usami, S. Kono, R. Kersting and X. C. Zhang, J. Appl. Phys., 2001, 89, 2357–2359 CrossRef CAS.
- R. Kohler, A. Tredicucci, F. Beltram, H. E. Beere, E. H. Linfield, A. G. Davies, D. A. Ritchie, R. C. Iotti and F. Rossi, Nature, 2002, 417, 156–159 CrossRef.
- C. A. Schmuttenmaer, Chem. Rev., 2004, 104, 1759–1779 CrossRef CAS.
- W. L. Chan, J. Deibel and D. M. Mittleman, Rep. Prog. Phys., 2007, 70, 1325–1379 CrossRef.
- W. Withayachumnankul, B. M. Fischer and D. Abbott, Proc. R. Soc. London, Ser. A, 2008, 464, 2435–2456 CrossRef CAS.
- P. F. Bernath, Phys. Chem. Chem. Phys., 2002, 4, 1501–1509 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
