Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemical and solvent-free assembly of zirconium-based metal–organic frameworks†
Krunoslav
Užarević‡
b,
Timothy C.
Wang
c,
Su-Young
Moon
c,
Athena M.
Fidelli
a,
Joseph T.
Hupp
c,
Omar K.
Farha
*cd and
Tomislav
Friščić
*a
aDepartment of Chemistry, McGill University, 801 Sherbrooke St. W., H3A 0B8 Montreal, Canada. E-mail: tomislav.friscic@mcgill.ca
bInstitute Ruder Boskovic, Zagreb, Croatia
cDepartment of Chemistry and International Institute for Nanotechnology, Northwestern University, Evanston, IL 60208, USA. E-mail: o-farha@northwestern.edu
dDepartment of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
First published on 8th December 2015
Abstract
We develop the first mechanochemical and solvent-free routes for zirconium metal–organic frameworks, making the frameworks UiO-66 and UiO-66-NH2 accessible on the gram scale without strong acids, high temperatures or excess reactants. The frameworks form either by milling, or spontaneous self-assembly by simply exposing solid mixtures of reactants to organic vapour. The generated frameworks exhibit high porosity and catalytic activity in the hydrolysis of model nerve agents, on par with their solvothermally generated counterparts.
Metal–organic frameworks (MOFs)1 are one of the most active areas of materials science, with proof-of-concept applications in gas storage and separation,2,3 catalysis,4 chemical defense,5 sensing,6 light harvesting7 and more. The growing impact of MOFs is reflected by their recent commercialisation, which made a small number of MOFs based on Mg, Al, Fe, Cu and Zn commercially available on the laboratory research scale.8 However, chemical stability to strong acids or bases, and retention of microporosity upon extended exposure to different atmospheres remain central problems for most MOFs, including those currently being manufactured commercially.9
MOFs based on carboxylate linkers and 12-coordinate cationic Zr6O4(OH)412+ clusters (Fig. 1),10 exemplified by terephthalate-based UiO-6611 and derivatives, have great potential as catalysts,12 given a high concentration of well-dispersed metal-based nodes, exceptional aqueous stability over a range of pH values, high surface areas, and tunable chemical structures.13 Despite continuous efforts and improvements,14 however, the syntheses of UiO-66 and analogues remain encumbered by challenging procedures involving aggressive reagents.11a,15 So far, the most reliable syntheses of UiO-66 and related MOFs rely on hydrochloric acid in hot organic solvents, achieving reproducibility at the expense of structural defects.16 Thus, the exploitation and potential commercialisation of excellent material properties of UiO-66 and analogues remain hindered by adverse synthesis.
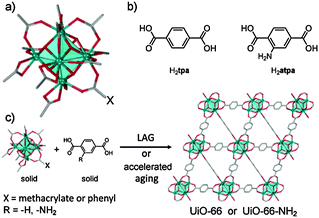 | ||
| Fig. 1 (a) A carboxylate-capped Zr6O4(OH)412+ cluster; (b) terephthalic and 2-aminoterephthalic acids; (c) herein developed syntheses of UiO-type MOFs. | ||
Mechanochemistry, i.e. reactivity induced or sustained by mechanical force,17 has been recently introduced as an alternative to conventional MOF syntheses.18 Mechanochemical techniques, such as liquid-assisted grinding (LAG),19 can enable the synthesis of MOFs20 without bulk solvents, aggressive conditions and/or corrosive reagents frequently employed in solution syntheses.21 Importantly, varying the liquid additive (given as η, ratio of liquid volume to weight of reactants22) in LAG provides a unique opportunity to optimise and direct mechanochemical reactions without large modifications in the milling procedures. Recent applications of LAG enabled the synthesis of MOFs from poorly soluble metal oxides and discovery of novel phases.23
We now describe the first implementation of mechanochemistry and solvent-free ‘accelerated aging’ (AA)24 for the synthesis of zirconium-based MOFs, providing new, surprisingly simple routes to gram amounts of UiO-66 and its amino-analogue UiO-66-NH211,25 without using bulk solvents, aggressive reagents or high temperatures.
The principal requirement for the synthesis of UiO-66 and related MOFs is the formation of Zr6O4(OH)4 nodes. We considered a mechanochemical strategy similar to that recently used for IRMOFs,26i.e. reaction of a pre-assembled benzoate cluster Zr6O4(OH)4(C6H5CO2)12 (1) with terephthalic acid (H2tpa, Fig. 1). Precursor 1 was readily obtained from zirconium propoxide, Zr(OPr)4 (see ESI†).27 Dry milling of 1 and H2tpa in the correct stoichiometric ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 did not lead to a chemical reaction, according to powder X-ray diffraction (PXRD) analysis of the reaction mixture after 90 min milling. Switching to LAG (η = 0.66 μL g−1
6 did not lead to a chemical reaction, according to powder X-ray diffraction (PXRD) analysis of the reaction mixture after 90 min milling. Switching to LAG (η = 0.66 μL g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22) with N,N-dimethylformamide (DMF) gave a new product exhibiting a broad PXRD feature consistent with the (111) reflection of UiO-66 (Fig. 2a–f, also see the ESI†). Product formation was accompanied by the complete disappearance of reactant X-ray reflections.
22) with N,N-dimethylformamide (DMF) gave a new product exhibiting a broad PXRD feature consistent with the (111) reflection of UiO-66 (Fig. 2a–f, also see the ESI†). Product formation was accompanied by the complete disappearance of reactant X-ray reflections.
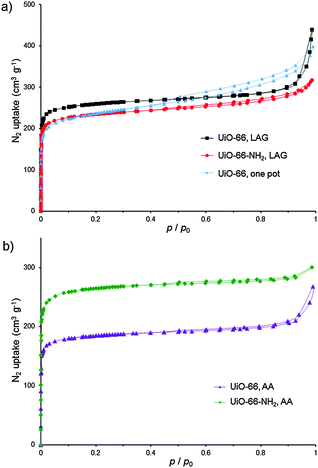
PXRD analysis suggests a successful exchange of benzoate ligands on 1, leading to UiO-66 with poor crystallinity. An alternative precursor based on methacrylic acid, Zr6O4(OH)4(C2H3CO2)12 (2),14c,28 was used as well, without much improvement. At this point, we turned to varying the milling liquid. Switching from DMF to methanol (MeOH) led to little improvement in the crystallinity of product from 1 (Fig. 2g). However, LAG of 2 and H2tpa with MeOH (η = 0.66 μL mg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22) gave a product whose PXRD pattern exhibited sharp, well-defined reflections, with positions consistent with the UiO-66 structure (Fig. 2h). Washing was performed with MeOH only, allowing for the first time the complete exclusion of HCl and DMF from the synthesis of a UiO-type MOF. Using a Spex 8000 mill, the synthesis of UiO-66 by MeOH LAG was accomplished on the 3 g scale in 75 min (Fig. 2i and Fig. S1 and S2, ESI†). The BET surface area for the product, calculated from the N2 sorption isotherm at 77 K, was 1020 m2 g−1 (Table 1 and Fig. 3).29 Next, we targeted UiO-66-NH2, based on 2-aminoterephthalic acid (H2atpa),25 recently established as an excellent catalyst for the hydrolytic degradation of nerve agent simulants.30 Based on the optimised mechanosynthesis of UiO-66, we milled 2 and H2atpa in the presence of MeOH. After 90 min, PXRD revealed the formation of UiO-66-NH2, isostructural to UiO-66. Using a Spex 8000 mill, the mechanosynthesis of UiO-66-NH2 was performed on a 1.5 g scale in 45 min (Fig. 2j and Fig. S1–S3, ESI†). Based on a N2 isotherm at 77 K, the BET surface of UiO-66-NH2 after washing and activation was 945 m2 g−1 (Table 1 and Fig. 3a), consistent with the literature.11e,16a,25,31
22) gave a product whose PXRD pattern exhibited sharp, well-defined reflections, with positions consistent with the UiO-66 structure (Fig. 2h). Washing was performed with MeOH only, allowing for the first time the complete exclusion of HCl and DMF from the synthesis of a UiO-type MOF. Using a Spex 8000 mill, the synthesis of UiO-66 by MeOH LAG was accomplished on the 3 g scale in 75 min (Fig. 2i and Fig. S1 and S2, ESI†). The BET surface area for the product, calculated from the N2 sorption isotherm at 77 K, was 1020 m2 g−1 (Table 1 and Fig. 3).29 Next, we targeted UiO-66-NH2, based on 2-aminoterephthalic acid (H2atpa),25 recently established as an excellent catalyst for the hydrolytic degradation of nerve agent simulants.30 Based on the optimised mechanosynthesis of UiO-66, we milled 2 and H2atpa in the presence of MeOH. After 90 min, PXRD revealed the formation of UiO-66-NH2, isostructural to UiO-66. Using a Spex 8000 mill, the mechanosynthesis of UiO-66-NH2 was performed on a 1.5 g scale in 45 min (Fig. 2j and Fig. S1–S3, ESI†). Based on a N2 isotherm at 77 K, the BET surface of UiO-66-NH2 after washing and activation was 945 m2 g−1 (Table 1 and Fig. 3a), consistent with the literature.11e,16a,25,31
| MOF | Precursor | BET surface area (m2 g−1) |
|---|---|---|
| a Details of activation are given in the ESI. b All reactions were performed by LAG, using MeOH as the milling liquid. c One-pot synthesis from Zr(OPr)4, acrylic acid and H2tpa using MeOH as the grinding liquid. | ||
| UiO-6629 | 2 | 1020 |
| UiO-66-NH2 | 1 | 925 |
| UiO-66-NH2 | 2 | 945 |
| UiO-6629 | Zr(OPr)4c | 890 |
UiO-66-NH2 was also accessible by LAG from 1, giving a BET surface area of 925 m2 g−1 (Table 1 and Fig. S4, ESI†). The synthesis of UiO-66-NH2 involved only MeOH as the milling and washing liquid, again eliminating the need for DMF. Finally, we attempted a one-pot synthesis of UiO-66 directly from commercial zirconium propoxide, methacrylic acid and stoichiometric H2tpa. After 90 min, one-pot milling in the presence of MeOH gave UiO-66 with a high surface area (Table 1, Fig. 3a and Fig. S5, ESI†), providing the first one-step route to UiO-66 from zirconium propoxide, an attractive replacement for ZrCl4 and ZrOCl2.
We next explored AA, an operationally simple technique that can achieve the solvent-free, low-energy synthesis of metal–organic materials24 by exposing a physical mixture of reactants to mild temperature and a suitable atmosphere. AA was previously applied to prepare salts, cocrystals, metal oxalate MOFs, zeolitic imidazolate frameworks and, recently, HKUST-1.24 Short grinding (<1 min in an agate mortar) of a physical mixture of 2 with H2tpa or H2atpa, followed by exposure to MeOH vapor at 45 °C led to the spontaneous assembly of UiO-66 and UiO-66-NH2, respectively (see ESI†). Throughout aging, the samples remained solid and the products were highly crystalline, as evidenced by PXRD, which revealed the complete disappearance of the reactants after 3 days (UiO-66-NH2) and 1 week (UiO-66) (Fig. S6 and S7, ESI†). MOFs, synthesised at the >1 g scale, exhibited high BET areas after activation (Table 2 and Fig. 3b).
With the materials in hand, we examined the catalytic activity of UiO-66-NH2 made using LAG and AA. Both samples show high hydrolysis activity: 2.5 min and 2 min for an initial half-life for the degradation of the nerve agent simulant dimethyl 4-nitrophenyl phosphate (DMNP, Fig. 4), respectively. The catalytic activity of UiO-66-NH2 made using AA is comparable to that of solvothermally prepared materials (t1/2 = 1 min);30 the difference in half-life is attributed to a difference in the particle size. For mechanochemically made UiO-66-NH2, the average particle size was found to be below 100 nm by dynamic light scattering (DLS) in water (Fig. S8, ESI†). The slower reaction kinetics (t1/2 = 2.5 min) compared to those for UiO-66-NH2 made using AA is likely due to the rapid aggregation and precipitation of nanoparticles during the catalytic reaction. UiO-66 made using LAG or AA also exhibited high catalytic activity, on par with solvothermally made material.5a The samples of UiO-66 and UiO-66-NH2 were characterised by scanning electron microscopy (SEM, see the ESI†) before and after evaluating the catalytic activity. Overall, the catalysis and porosity measurements show that zirconium MOFs made by LAG or AA have properties comparable to solvothermally made materials.
 | ||
| Fig. 4 Hydrolysis of nerve agent simulant DMNP: (a) reaction; (b) hydrolysis profiles in the presence of UiO-66-NH2 made solvothermally (black), by LAG (blue) and AA (red). | ||
We demonstrated that non-conventional synthetic approaches of mechanochemistry and accelerated aging enable a surprising simplification of procedures for making zirconium-based MOFs. The frameworks UiO-66 and UiO-66-NH2, noted for their outstanding stability and catalytic activity, can now be synthesised on the gram scale by a simple, rapid and room-temperature milling procedure which also allows using zirconium propoxide as the starting material.
Surprisingly, gram amounts of the microporous MOFs are obtainable by spontaneous assembly of the organic linker with a readily accessible carboxylate-capped zirconium cluster, simply by exposing a physical mixture of reactants to organic vapour. The herein presented techniques offer a route to highly porous, catalytically active UiO-frameworks under conditions resembling mild processes of small molecular self-assembly, in contrast to the conventional approaches that require acidic reagents (HCl, ZrCl4, ZrOCl2) under solvothermal conditions. These results have the potential to not only improve the wide accessibility of UiO-type MOFs but also to change the way microporous MOFs are synthesised in general.
We acknowledge the NSERC Discovery Grant for funding. KU is supported by the European Commission and the Croatian Ministry of Science, Education and Sports from the Marie Curie FP7-PEOPLE-2011-COFUND program NEWFELPRO, Grant Agreement No. 62. OKF and JTH acknowledge funding by the Army Research Office (project no. W911NF-13-1-0229). We acknowledge Tim Osborne-Jones (Spex SamplePrep LLC) for providing access to a Spex 8000 mill.
Notes and references
- (a) B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5962 CrossRef CAS; (b) S. Kitagawa, M. Munakata and T. Tanimura, Chem. Lett., 1991, 623 CrossRef CAS; (c) O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703 CrossRef CAS; (d) C. J. Kepert and M. J. Rosseinsky, Chem. Commun., 1999, 375 RSC.
- (a) R. B. Getman, Y.-S. Bae, C. E. Wilmer and R. Q. Snurr, Chem. Rev., 2012, 112, 703 CrossRef CAS PubMed; (b) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782 CrossRef CAS PubMed; (c) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed.
- (a) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (b) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- (a) M. Fujita, Y. J. Kwon, S. Washizu and K. Ogura, J. Am. Chem. Soc., 1994, 116, 1151 CrossRef CAS; (b) J. Lee, O. K. Farha, J. Roberts, K. A. Sheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (c) S. Hasegawa, S. Horike, R. Matsuda, S. Furukawa, K. Mochizuki, Y. Kinoshita and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 2607 CrossRef CAS PubMed.
- (a) M. J. Katz, J. E. Mondloch, R. K. Totten, J. K. Park, S. T. Nguyen, O. K. Farha and J. T. Hupp, Angew. Chem., Int. Ed., 2013, 53, 497 CrossRef PubMed; (b) J. E. Mondloch, M. J. Katz, W. C. Isley, P. Ghosh, P. Liao, W. Bury, G. W. Wagner, M. G. Hall, J. B. DeCoste, G. W. Peterson, R. Q. Snurr, C. J. Cramer, J. T. Hupp and O. K. Farha, Nat. Mater., 2015, 14, 512 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed.
- (a) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982 RSC; (b) C. Y. Lee, O. K. Farha, B. J. Hong, A. A. Sarjeant, S. T. Nguyen and J. T. Hupp, J. Am. Chem. Soc., 2011, 133, 15858 CrossRef CAS PubMed; (c) A. Fateeva, P. A. Chater, C. P. Ireland, A. A. Tahir, Y. Z. Khimyak, P. V. Wiper, J. R. Darwent and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2012, 51, 7440 CrossRef CAS PubMed.
- (a) A. U. Czaja, N. Trukhan and U. Müller, Chem. Soc. Rev., 2009, 38, 1284 RSC; (b) U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626 RSC.
- (a) C. Mottillo and T. Friščić, Angew. Chem., Int. Ed., 2014, 53, 7471 CrossRef CAS PubMed; (b) N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575 CrossRef CAS PubMed; (c) P. Küsgens, M. Rose, I. Senkovska, H. Fröde, A. Henschel, S. Siegle and S. Kaskel, Microporous Mesoporous Mater., 2008, 120, 325 CrossRef.
- (a) T. C. Wang, W. Bury, D. A. Gómez-Gualdrón, N. A. Vermeulen, J. E. Mondloch, P. Deria, K. Zhang, P. Z. Moghadam, A. A. Sarjeant, R. Q. Snurr, J. F. Stoddart, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2015, 137, 3585 CrossRef CAS PubMed; (b) D. A. Gómez-Gualdrón, O. V. Gutov, V. Krungleviciute, B. Borah, J. E. Mondloch, J. T. Hupp, T. Yildirim, O. K. Farha and R. Q. Snurr, Chem. Mater., 2014, 26, 5632 CrossRef; (c) S. B. Kalidindi, S. Nayak, M. E. Briggs, S. Jansat, A. P. Katsoulidis, G. J. Miller, J. E. Warren, D. Antypov, F. Corà, B. Slater, M. R. Prestly, C. Martí-Gastaldo and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2015, 54, 221 CrossRef CAS PubMed; (d) G. Nickerl, I. Senkovska and S. Kaskel, Chem. Commun., 2015, 51, 2280 RSC; (e) H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369 CrossRef CAS PubMed; (f) V. Bon, I. Senkovska, I. A. Baburin and S. Kaskel, Cryst. Growth Des., 2013, 13, 1231 CrossRef CAS; (g) S.-Y. Moon, Y. Liu, J. T. Hupp and O. K. Farha, Angew. Chem., Int. Ed., 2015, 54, 6795 CrossRef CAS PubMed.
- (a) J. Hafizovic Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef CAS PubMed; (b) K. M. Choi, K. Na, G. A. Somorjai and O. M. Yaghi, J. Am. Chem. Soc., 2015, 137, 7810 CrossRef CAS PubMed; (c) J. Long, S. Wang, Z. Ding, S. Wang, Y. Zhou, L. Huang and X. Wang, Chem. Commun., 2012, 48, 11656 RSC; (d) M. Kandiah, M. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. Quadrelli, F. Bonino and K. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS.
- (a) F. Vermoortele, R. Ameloot, A. Vimont, C. Serre and D. De Vos, Chem. Commun., 2011, 47, 1521 RSC; (b) F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465 CrossRef CAS PubMed; (c) L. Shen, W. Wu, R. Liang, R. Lin and L. Wu, Nanoscale, 2013, 5, 9374 RSC; (d) L. Shen, S. Liang, W. Wu, R. Liand and L. Wu, Dalton Trans., 2013, 42, 13649 RSC.
- (a) J. E. Mondloch, M. J. Katz, N. Planas, D. Semrouni, L. Gagliardi, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 8944 RSC; (b) C. G. Piscopo, A. Polyzoidis, M. Schwarzer and S. Loebbecke, Microporous Mesoporous Mater., 2015, 208, 30 CrossRef CAS; (c) G. W. Peterson, J. B. DeCoste, F. Fatollahi-Fard and D. K. Britt, Ind. Eng. Chem. Res., 2014, 53, 701 CrossRef CAS.
- (a) J. B. DeCoste, T. J. Demasky, M. J. Katz, O. K. Farha and J. T. Hupp, New J. Chem., 2015, 39, 2396 RSC; (b) A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem. – Eur. J., 2011, 17, 6643 CrossRef CAS PubMed; (c) V. Guillerm, S. Gross, C. Serre, T. Devic, M. Bauer and G. Férey, Chem. Commun., 2010, 46, 767 RSC.
- (a) A. Buragohain and S. Biswas, Eur. J. Inorg. Chem., 2015, 2463 CrossRef CAS; (b) S.-N. Kim, Y.-R. Lee, S.-H. Hong, M.-S. Jang and W.-S. Ahn, Catal. Today, 2015, 245, 54 CrossRef CAS; (c) Y. Tan, W. Zhang, Y. Gao, J. Wu and B. Tang, RSC Adv., 2015, 5, 17601 RSC; (d) F. Ragon, P. Horcajada, H. Chevreau, Y. K. Hwang, U.-H. Lee, S. R. Miller, T. Devic, J.-S. Chang and C. Serre, Inorg. Chem., 2014, 53, 2491 CrossRef CAS PubMed.
- (a) M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449 RSC; (b) G. C. Shearer, S. Chavan, J. Ethiraj, J. G. Vitillo, S. Svelle, U. Olsbye, C. Lamberti, S. Bordiga and K. P. Lillerud, Chem. Mater., 2014, 26, 4068 CrossRef CAS; (c) C. Zlotea, D. Phanon, M. Mazaj, D. Heurtaux, V. Guillerm, C. Serre, P. Horcajada, T. Devic, E. Magnier, F. Cuevas, G. Férey, P. Llewellyn and M. Latroche, Dalton Trans., 2011, 40, 4879 RSC; (d) P. S. Bárcia, D. Guimarães, P. A. P. Mendes, J. A. C. Silva, V. Guillerm, H. Chevreau, C. Serre and A. E. Rodrigues, Microporous Mesoporous Mater., 2011, 139, 67 CrossRef.
- (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC; (b) E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719 RSC.
- P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., Int. Ed., 2010, 49, 9640 CrossRef CAS PubMed.
- (a) T. Friščić, A. V. Trask, W. Jones and W. D. S. Motherwell, Angew. Chem., Int. Ed., 2006, 35, 3523 Search PubMed; (b) D. Braga, S. L. Giaffreda, F. Grepioni, A. Pettersen, L. Maini, M. Curzi and M. Polito, Dalton Trans., 2006, 1249 RSC.
- (a) T. Friščić, J. Mater. Chem., 2010, 20, 7599 RSC; (b) A. Lazuen Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846 RSC.
- A. Czaja, E. Leung, N. Trukhan and U. Müller, Industrial MOF synthesis, in Metal-Organic Frameworks: Applications from Catalysis to Gas Storage, ed. D. Farusseng, Wiley, 1st edn, 2011 Search PubMed.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418 RSC.
- A. D. Katsenis, A. Puškarić, V. Štrukil, C. Mottillo, P. A. Julien, K. Užarević, M.-H. Pham, T.-O. Do, S. A. J. Kimber, P. Lazić, O. Magdysyuk, R. E. Dinnebier, I. Halasz and T. Friščić, Nat. Commun., 2015, 6, 6662 CrossRef CAS PubMed.
- (a) M. J. Cliffe, C. Mottillo, R. S. Stein, D.-K. Bučar and T. Friščić, Chem. Sci., 2012, 3, 2495 RSC; (b) X. Feng, C. Jia, J. Wang, X. Cao, P. Tang and W. Yuan, Green Chem., 2015, 17, 3740 RSC; (c) D. Braga, S. L. Giaffreda, F. Grepioni, M. R. Chierotti, R. Gobetto, G. Palladino and M. Polito, CrystEngComm, 2007, 9, 879 RSC; (d) F. Qi, R. S. Stein and T. Friščić, Green Chem., 2014, 16, 121 RSC.
- J. S. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700 RSC.
- D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewiński, Chem. Commun., 2015, 51, 4032 RSC.
- G. Kickelbick, P. Wiede and U. Schubert, Inorg. Chim. Acta, 1999, 287, 1 CrossRef.
- G. Kickelbick and U. Schubert, Chem. Ber., 1997, 130, 473 CrossRef CAS.
- BET surface areas for UiO-66 range from 700–1600 m2 g−1. Values above theoretically expected one are explained by structural defects, e.g. missing linkers.[16].
- (a) M. J. Katz, S.-Y. Moon, J. E. Mondloch, M. H. Beyzavi, C. J. Stephenson, J. T. Hupp and O. K. Farha, Chem. Sci., 2015, 6, 2286 RSC; (b) P. Li, R. C. Klet, S.-Y. Moon, T. C. Wang, P. Deria, A. W. Peters, B. M. Klahr, H.-J. Park, S. S. Al-Juaid, J. T. Hupp and O. K. Farha, Chem. Commun., 2015, 51, 10925 RSC.
- (a) O. G. Nik, X. Y. Chen and S. Kaliaguine, J. Membr. Sci., 2012, 48, 413 Search PubMed; (b) P. M. Schoenecker, G. A. Belancik, B. E. Grabicka and K. S. Walton, AIChE J., 2012, 59, 1255 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Selected PXRD, FTIR-ATR, BET, TGA, SEM and DLS data. See DOI: 10.1039/c5cc08972g |
| ‡ On academic leave from Ruđer Bošković Institute, Zagreb, Croatia. |
| This journal is © The Royal Society of Chemistry 2016 |