Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions†
Stephen D.
Ebbs
*a,
Scott J.
Bradfield
a,
Pawan
Kumar
a,
Jason C.
White
b,
Craig
Musante
b and
Xingmao
Ma
c
aDepartment of Plant Biology and Center for Ecology, Southern Illinois University, 1125 Lincoln Drive, Carbondale, IL 62901-6509, USA. E-mail: ebbs@siu.edu; Fax: +1 618 453 3441; Tel: +1 618 453 3226
bDepartment of Analytical Chemistry, The Connecticut Agricultural Experiment Station, 123 Huntington Street, New Haven, CT 06504, USA
cZachry Department of Civil Engineering, Texas A&M University, 3136 TAMU, College Station, TX 77843-3136, USA
First published on 16th October 2015
Abstract
The release of engineered nanoparticles (ENPs) into the environment has raised concerns about the potential risks to food safety and human health. There is a particular need to determine the extent of ENP uptake into plant foods. Belowground vegetables growing in direct contact with the growth substrate are likely to accumulate the highest concentration of ENPs. Carrot (Daucus carota) was grown in sand amended with ZnO, CuO, or CeO2 NPs or the same concentrations of Zn2+, Cu2+, or Ce4+. Treatment with ZnO or Zn2+ produced a concentration-dependent decrease in root and total biomass. Ionic Cu2+ and Ce4+ caused a greater reduction in shoot biomass as compared to the corresponding ENP treatments. Accumulation of Zn, Cu, or Ce in the taproot was restricted to the taproot periderm. Metal concentrations in the taproot periderm were higher for the ionic treatments than for the ENP treatments. Radial penetration of the metals into the taproot and subsequent translocation to shoots were also generally greater for plants receiving the ionic treatment than those receiving the ENP treatment. The distribution of the metals from the ENP treatments across the periderm, taproot, and shoots differed from that observed for the ionic treatments. Overall, the ENPs were no more toxic than the ionic treatments and showed reduced accumulation in the edible tissues of carrot. The results demonstrate that the understanding of ionic metal transport in plants may not accurately predict ENP transport and that an additional comparative study is needed for this and other crop plants.
Nano impactThere is a pressing need for information on the extent to which nanomaterials are accumulated in plant foods, particularly root vegetables that grow in direct contact with the growth substrate. The study here focused on carrot, a plant that has not been studied extensively in this context and has a storage root that displays secondary growth. A specific aspect of carrot taproot anatomy, namely, the outer periderm layer of the taproot surface, was the predominant area where metals from the nanomaterials (ZnO, CuO, or CeO2) accumulated. This periderm layer screened most of the metals from these nanomaterials, preventing a significant accumulation in the interior taproot flesh. The study also incorporated parallel treatments with the ionic metals. The comparison of the data for the nanomaterials to the ionic counterparts showed that at equivalent concentrations, the ionic form more readily penetrated into the edible taproot than the nanomaterials. The results offer specific insight into the potential accumulation of metal oxide nanomaterials in root vegetables with a similar root anatomy and how that accumulation might translate into human exposures through dietary consumption. |
1. Introduction
The nanotechnology industry is a rapidly expanding commercial sector. The market share of commercial products incorporating nanotechnology reached $174 billion in 2007 and is expected to grow to $2.5 trillion by 2015.1 The unique properties that emerge when materials are fabricated at the nanoscale (i.e., at least one dimension <100 nm) have given rise to thousands of applications and proposed inclusion in hundreds of consumer, medical, and industrial products.1 The anticipated increase in the use of nanomaterials, in particular some metallic oxide nanoparticles, has also produced concern that there may be detrimental effects of these materials upon intentional or accidental release into the environment. Assessing the toxicity of nanoparticles to animals, plants, fungi, and microorganisms has been a principle focus to date.2–6 Another concern is possible particle bioaccumulation in terrestrial food webs and the human food supply.7,8Crop plants could potentially come in contact with engineered nanoparticles (ENPs) through application of biosolids to agricultural fields9,10 or the application of nano-enabled agricultural products to plants or to the soil.11–13 Additional routes by which crop plants may be exposed to ENPs include accidental discharges, contact with nanomaterials intended for soil or water remediation, the application of irrigation water containing ENPs, and potential aerial deposition. The presence of ENPs in plant foods represents a likely pathway by which the general public might be exposed to these materials. A variety of studies have focused on the accumulation of Ag and other metal oxide (e.g., CuO, CeO2, ZnO) ENPs in the edible tissues of various crops.2–4,14 Most of these studies have focused on leafy or stem vegetables (e.g., lettuce and spinach), fruits (e.g., tomato, zucchini, legumes), and grains (e.g., barley, rice, maize). Where data on accumulation in these and other plants are presented, the results frequently indicate that plant roots accumulate considerably higher concentrations of ENPs as compared to the aboveground tissues. This tendency of roots to retain ENPs has been attributed to the small pore size (2–20 nm) of the root cell wall network that is generally smaller than most ENPs4,15 and the capacity of the root to act as a selective “sieve” to trap ENPs.16 The propensity of roots to accumulate ENPs suggests that belowground root, tuberous, and bulb vegetables, due to their direct contact with ENPs in the growth substrate, may be more likely to accumulate ENPs.
There is limited information available on the accumulation of ENPs in belowground vegetables; consequently, the study described here had two primary objectives. The first objective was to assess the accumulation of Ce, Cu, or Zn in carrot (Daucus carota) when irrigated with solutions of CeO2, CuO, or ZnO nanoparticles or the corresponding ionic metal form of each. Carrot has been included in some prior studies with nanoparticles,17,18 but the response of this species to these metal oxide nanoparticles and the resulting accumulation have yet to be evaluated. Carrot is also a nutritionally important root vegetable crop with >28 000 ha in cultivation in the US with a market value of >$650 million for fresh carrots alone.19 Carrot can be cultivated in soils receiving biosolid amendments in the US within the guidelines established by the US Environmental Protection Agency.20 The second objective was to derive basic information on the spatial distribution of the accumulated element in the carrot tissue. One approach was to determine the extent to which the metals from the added nanoparticles penetrated the outer “peel” of the carrot into the inner edible taproot flesh. The data derived would help illustrate the degree to which this cell layer of the carrot taproot serves as a filter to limit accumulation. Alternatively, the distribution of the metals across the three tissues (peel, taproot flesh, and shoot) was examined as a function of treatment concentration and chemical form (ENP or ionic) to provide information on the internal partitioning of the accumulated metals.
2. Materials and methods
2.1 Nanoparticles and plant materials
The three nanoparticles used, ZnO (30–40 nm), CuO (25–55 nm), and CeO2 (30–50 nm), were selected as representatives of the ten most commonly used materials as identified by Keller et al.21 The nanoparticles were obtained from US Research Nanomaterials, Inc. (Houston, TX). The CuO (99.95% purity) was in powder form, while the ZnO (99.5% purity) and CeO2 (99.9% purity) were obtained as aqueous dispersions in deionized water at initial concentrations of 20% wt. The metal salts CuSO4·5H2O and ZnSO4·7H2O were purchased from Fisher Scientific (New Jersey, USA), whereas Ce(SO4)2·4H2O was obtained from Acros Organic (New Jersey, USA). Seeds of carrot (Daucus carota cv Danvers Half Long) were obtained from Burpee Seeds and Plants (W. Atlee Burpee & Co., Warminster, PA).2.2 Preparation and characterization of nanoparticle solutions
Nanoparticle treatment solutions were prepared to provide final concentrations of elemental Zn, Cu, or Ce at 1, 10, 100, and 1000 mg L−1. Preparing the solutions based on the concentration of the metal was to provide an alignment with the corresponding metal ion treatments (see below). The ZnO and CeO2 solutions were prepared by diluting the commercial suspension with 18 mΩ deionized water to achieve the desired concentrations. To prepare the CuO treatment solutions, the required mass of the nanopowder was mixed with deionized water, and each solution was sonicated (130 W, 20 kHz) for 15 min (model VCX 130, Sonics & Materials Inc., Newtown, CT) to facilitate dispersion. The hydrodynamic size was determined using a Zetasizer Nano ZS90 (Malvern Instruments, UK). All the measurements were taken in triplicate. There was significant aggregation of ZnO in solution across the four concentrations, with hydrodynamic sizes ranging from <1200 to >2100 nm (Table S1†). There was some evidence of CuO aggregation in solution, with the greatest aggregation observed at the highest solution concentration. The hydrodynamic size for the lower three CuO concentrations was similar (i.e., ~330–410 nm) but increased to >1200 nm at 1000 mg Cu L−1. There was less aggregation of CeO2 particles, and the hydrodynamic sizes were consistent across the four initial concentrations (i.e., ~250–290 nm). The corresponding solutions of ionic Zn, Cu, and Ce were prepared by dissolving the required mass of the salts in deionized water. All solutions were prepared fresh on the day that the treatments were to be imposed.2.3 Plant growth and exposure
Plastic pots (0.95 L total volume) were filled with 1.3 kg of dry coarse sand and wetted to the equivalent of 80% field capacity with deionized water. Three carrot seeds were planted approximately 1.5 cm below the sand surface. The pots were transferred to a phytotron growth chamber (16 h photoperiod, light intensity 200–350 μmol m−2 s−1, 18–22 °C, and ambient relative humidity) to facilitate germination. Pots were maintained during germination at 80% field capacity by watering with deionized water every other day. The amount of water needed to maintain 80% field capacity was determined by weighing the pots to estimate the water losses due to evaporation. After emergence, the plants were thinned to one seedling per pot. Once per week after emergence, each pot received 0.05 L of nutrient solution with the following composition: 0.6 mM KNO3, 0.4 mM Ca(NO3)2, 0.05 mM NH4H2PO4, 0.1 mM MgSO4, 50 μM KCl, 12.5 μM H3BO3, 1 μM MnSO4, 1 μM ZnSO4, 0.5 μM CuSO4, 0.1 μM NiSO4, and 0.1 μM H2MoO4. The solution was buffered with 1 mM n-morpholinoethanesulfonic acid (MES), titrated to pH 6.0 with KOH. Iron was provided as 10 μM Fe-EDDHA. Pots were weighed intermittently during the course of each week to assess water loss due to evapotranspiration. Deionized water was added when needed during the week to maintain the soil moisture near 80% of field capacity. Plants were grown for 16 weeks to establish biomass and initiate formation of the taproot before treatments began.For each of the three metals, the experimental design consisted of either the nanoparticle or the corresponding ionic solution at one of four concentrations (1, 10, 100, or 1000 mg L−1) and a control treatment (i.e., no metal). Each treatment was replicated five times, giving 45 pots (one plant per pot) per metal. The pots were randomly assigned to a treatment and arrayed in a completely random pattern. The treatments were imposed by watering the pots once per week for 13 weeks with 0.05 L of nanoparticle or ionic solution or with deionized water. By the end of the 13 week treatment period, the calculated final concentration of these metals in the pots was 0.5, 5, 50, or 500 mg kg DW−1, respectively. This broad range of concentrations was chosen to be conservative due to the lack of information on the concentrations of ENPs in the environment. The pots were also watered once per week with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution of the nutrient solution described above at a different time from the treatment irrigation. The nutrient solution used to irrigate the pots prior to and through the entire treatment period introduced <0.1 and <0.05 mg of total Zn or Cu, respectively, and therefore had a minimal effect on the total concentration of either elements in the pots.
2 dilution of the nutrient solution described above at a different time from the treatment irrigation. The nutrient solution used to irrigate the pots prior to and through the entire treatment period introduced <0.1 and <0.05 mg of total Zn or Cu, respectively, and therefore had a minimal effect on the total concentration of either elements in the pots.
At harvest, plants were removed from the pots, separated into green, aboveground petioles and stems (hereafter referred to as shoots) and belowground taproot and then rinsed with deionized water. The carrot taproot was gently abraded with a vegetable brush to insure removal of any adhering sand particles. The taproot was peeled with a standard vegetable peeler, removing the outer 1–2 mm of the carrot taproot periderm (for simplicity, this tissue layer will be referred to hereafter as the “peel”). The peeled taproot, composed primarily of the secondary phloem and xylem, was considered as the edible “flesh” of the carrot. The fresh weight of the tissues was determined and all tissues were then dried to constant mass at 60 °C. The dried peel and flesh mass for each replicate were combined to represent total root dry weight. The dried tissues were ground to a particle size of <5 mm and digested using EPA method 3050b22 using a combination of trace metal grade nitric acid and 30% hydrogen peroxide. The digested samples were analysed for Zn, Cu, or Ce using inductively coupled plasma mass spectroscopy (ICP-MS, Agilent 7500ce, Santa Clara, CA).
The bioconcentration factor (BCF) in the tissues in response to treatment was calculated by dividing the metal concentration in that tissue by the final metal concentration in the sand growth medium. Using the tissue concentration and the total dry mass data, the total mass of Zn, Cu, or Ce in each tissue was calculated. The transfer factor (TF) was calculated as the mass of a metal in the shoot divided by the total mass of that metal in the taproot (i.e., total metal in the peel and flesh). The mass of an element in those three tissues was also summed to obtain the total mass of that element per plant. The percent of Zn, Cu, or Ce element in each tissue was calculated by dividing the mass of the element in that tissue by the total mass for that plant. The percent of total added Zn, Cu, or Ce removed by a plant was determined by dividing the sum of the total Zn, Cu, or Ce in an entire plant by the total mass of each element added to the substrate.
2.4 Data analysis
Each biomass parameter, i.e., the root![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) shoot ratio, shoot metal concentration, shoot BCF, shoot TF, and the percent of added metal removed per plant for a given element, was analyzed using a two-way ANOVA with treatment concentration and chemical form (nanoparticle or ionic) as the main effects. For root dry weight concentration and root BCF, a three-way ANOVA was used with treatment concentration, chemical form, and root tissue (peel or flesh) as the main effects for a given element. As the plants used for the Zn treatments, the Cu treatments, and the Ce treatments were grown sequentially and not simultaneously during the course of this research, the data were not compared between elements but only within each element.
shoot ratio, shoot metal concentration, shoot BCF, shoot TF, and the percent of added metal removed per plant for a given element, was analyzed using a two-way ANOVA with treatment concentration and chemical form (nanoparticle or ionic) as the main effects. For root dry weight concentration and root BCF, a three-way ANOVA was used with treatment concentration, chemical form, and root tissue (peel or flesh) as the main effects for a given element. As the plants used for the Zn treatments, the Cu treatments, and the Ce treatments were grown sequentially and not simultaneously during the course of this research, the data were not compared between elements but only within each element.
3. Results
3.1 Influence of nanoparticle and ionic treatments on plant growth and development
During the course of the experiment, there were no overt signs of toxicity or stress (e.g., chlorosis, necrosis) in any plants. There was no evident malformation or splitting of the taproots. Within each metal treatment, the effect of the nanoparticle and ionic treatments had statistically significant (Table S2†) yet modest effects on tissue biomass and primarily at the highest concentration of the ionic metal treatment. For Zn, there was no significant effect of treatment on shoot biomass (Fig. 1A) or root![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) shoot ratio (Fig. 2A). There was, for root mass, a significant concentration effect across both chemical forms of Zn but not an effect of Zn chemical form (nanoparticle versus ionic). The effect of Zn concentration can be seen most notably in the reduction in root biomass at the highest ionic Zn treatment concentration. Total plant biomass showed the same pattern as root biomass in response to Zn treatment (Table S2†). Both concentration and chemical form are significant factors for shoot biomass (Fig. 1B and Table S2†). There was no effect of Cu treatment on root biomass or root
shoot ratio (Fig. 2A). There was, for root mass, a significant concentration effect across both chemical forms of Zn but not an effect of Zn chemical form (nanoparticle versus ionic). The effect of Zn concentration can be seen most notably in the reduction in root biomass at the highest ionic Zn treatment concentration. Total plant biomass showed the same pattern as root biomass in response to Zn treatment (Table S2†). Both concentration and chemical form are significant factors for shoot biomass (Fig. 1B and Table S2†). There was no effect of Cu treatment on root biomass or root![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) shoot ratio (Fig. 2B). A significant interaction between concentration and chemical form was obtained for Cu for total plant biomass, driven primarily by the reduction in root biomass seen for the two highest concentrations of the ionic Cu treatment. There was a significant interaction (Table S2†) between the main effects for shoot biomass (Fig. 1C) and for root
shoot ratio (Fig. 2B). A significant interaction between concentration and chemical form was obtained for Cu for total plant biomass, driven primarily by the reduction in root biomass seen for the two highest concentrations of the ionic Cu treatment. There was a significant interaction (Table S2†) between the main effects for shoot biomass (Fig. 1C) and for root![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) shoot (Fig. 2C) ratio in response to the Ce treatments. There was no significant effect of Ce treatment on root biomass and for total plant biomass; the only significant effect was in response to the treatment concentration. Overall, the change in biomass resulting from the nanoparticle treatments was generally not different from the effects of the ionic metal treatments. A difference in biomass was not observed until the highest treatment concentration for each element, and it was the ionic treatment for Zn2+ or Cu2+ that produced the greatest decrease in biomass.
shoot (Fig. 2C) ratio in response to the Ce treatments. There was no significant effect of Ce treatment on root biomass and for total plant biomass; the only significant effect was in response to the treatment concentration. Overall, the change in biomass resulting from the nanoparticle treatments was generally not different from the effects of the ionic metal treatments. A difference in biomass was not observed until the highest treatment concentration for each element, and it was the ionic treatment for Zn2+ or Cu2+ that produced the greatest decrease in biomass.
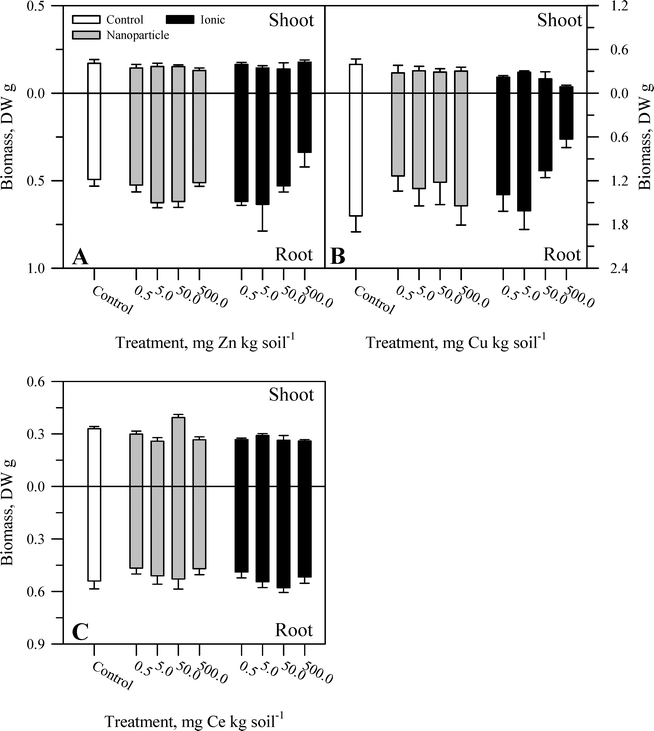 | ||
| Fig. 1 Shoot and root biomass for carrot grown in sand culture for 13 weeks and exposed to 0.5, 5, 50, or 500 mg kg−1 of Zn (A), Cu (B), or Ce (C) as either a metal oxide nanoparticle (ZnO, CuO, or CeO2) or an ionic metal (Zn2+, Cu2+, or Ce4+ as sulfate salts). The data represent the mean and standard error (n = 4–5). The results of the statistical analysis of the data are shown in Table S2.† | ||
 | ||
Fig. 2 Root![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) shoot biomass ratio for carrot grown in sand culture for 13 weeks and exposed to 0.5, 5, 50, or 500 mg kg−1 of Zn (A), Cu (B), or Ce (C) as either a metal oxide nanoparticle (ZnO, CuO, or CeO2) or an ionic metal (Zn2+, Cu2+, or Ce4+ as sulfate salts). The data represent the mean and standard error (n = 4–5). The results of the statistical analysis of the data are shown in Table S2.† shoot biomass ratio for carrot grown in sand culture for 13 weeks and exposed to 0.5, 5, 50, or 500 mg kg−1 of Zn (A), Cu (B), or Ce (C) as either a metal oxide nanoparticle (ZnO, CuO, or CeO2) or an ionic metal (Zn2+, Cu2+, or Ce4+ as sulfate salts). The data represent the mean and standard error (n = 4–5). The results of the statistical analysis of the data are shown in Table S2.† | ||
3.2 Influence of nanoparticle and ionic treatments on metal accumulation and partitioning
Within each of the three elemental treatments, there were highly significant interactions between the main effects with respect to the metal concentration in the taproot tissues or the shoots (Table S3†). For the taproot tissues, there were three patterns that were generally consistent across the three metals. The first and most consistent pattern was that the peel tissues from the taproot had significantly higher concentrations of the metal than the underlying flesh tissues (Fig. 3–5 and Table S3†). The peel concentrations of Zn, Cu, or Ce generally increased in a concentration-dependent manner. One exception observed was for the two lowest concentrations of the ZnO treatment where the peel concentrations did not differ significantly from the control plants. The concentration of Zn, Cu, or Ce in peels varied from as little as two-fold greater than the flesh concentrations for the lowest treatment concentrations to an order of magnitude higher than the flesh at the highest treatment concentration.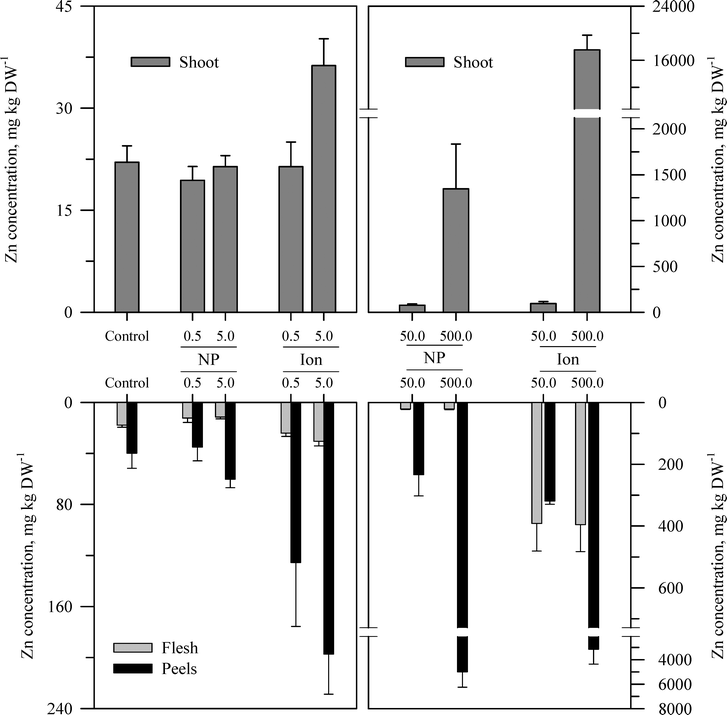 | ||
| Fig. 3 Concentration of Zn in the shoots, peels (outer periderm), and taproot flesh of carrot grown in sand culture for 13 weeks and exposed to 0.5, 5, 50, or 500 mg Zn kg−1 as either ZnO or Zn2+ (sulfate salt). The data represent the mean and standard error (n = 4–5). The results of the statistical analysis of the data are shown in Table S3.† | ||
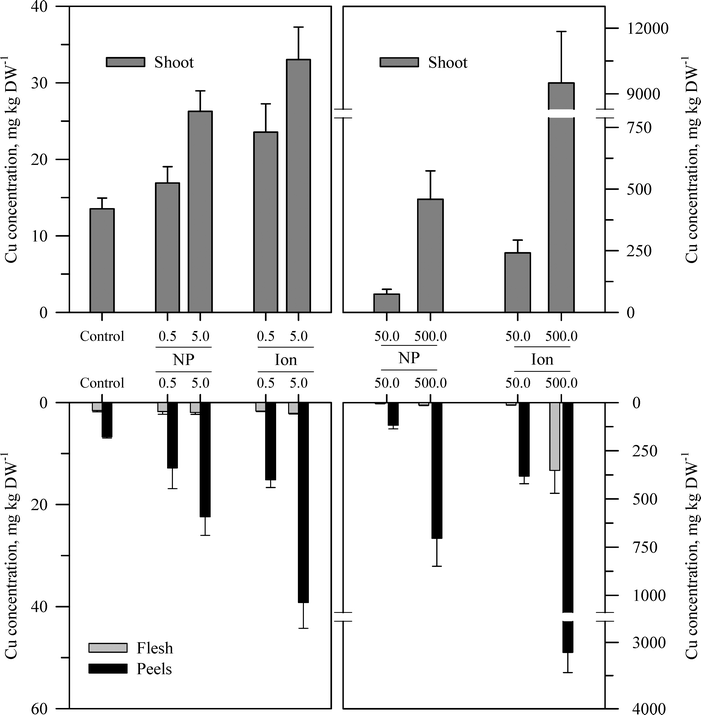 | ||
| Fig. 4 Concentration of Cu in the shoots, peels (outer periderm), and taproot flesh of carrot grown in sand culture for 13 weeks and exposed to 0.5, 5, 50, or 500 mg Cu kg−1 as either CuO or Cu2+ (sulfate salt). The data represent the mean and standard error (n = 4–5). The results of the statistical analysis of the data are shown in Table S3.† | ||
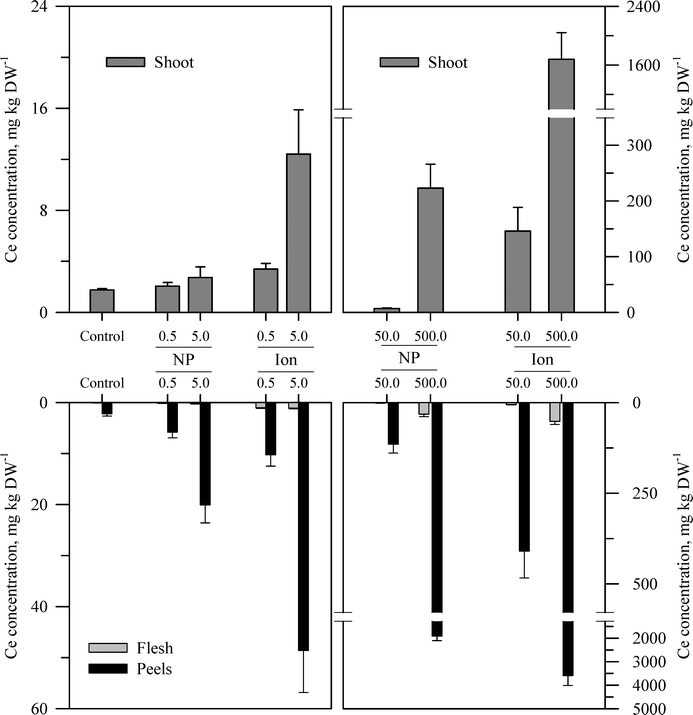 | ||
| Fig. 5 Concentration of Ce in the shoots, peels (outer periderm), and taproot flesh of carrot grown in sand culture for 13 weeks and exposed to 0.5, 5, 50, or 500 mg Ce kg−1 as either CeO2 or Ce4+ (sulfate salt). The data represent the mean and standard error (n = 4–5). The results of the statistical analysis of the data are shown in Table S3.† | ||
The second recurring pattern was that for a given concentration and element, the ionic treatment resulted in a significantly higher concentration in both the peels and the flesh as compared to the nanoparticle treatment (Fig. 3–5 and Table S3†). There were only a few exceptions to this trend, namely, the peel Zn concentration for the highest concentration applied and also for the peel and flesh Cu concentrations for the lowest concentration applied. The third pattern was also associated with a difference between the nanoparticle and the ionic treatment. For the three highest Zn and Cu ionic treatments, the concentration of that metal in the flesh increased significantly relative to the untreated control and the lowest ionic treatment concentration. The flesh Ce concentration increased significantly compared to the control for all the ionic Ce treatments. In sharp contrast, the concentration of Zn, Cu, or Ce in the edible flesh from the nanoparticle treatment was significantly greater only for the highest treatment, which was still less than that for the corresponding ionic treatment.
There were also highly significant differences and several significant interactions between the main effects for the calculated BCF values for the root peel and flesh tissues (Tables 1 and S3†). There were also three notable trends in the BCF results. Two of these trends were the same as for the concentration data in that the BCF values were greater for peels as compared to the taproot flesh and were significantly higher for carrot grown in the presence of ionic form than that grown in the presence of the nanoparticle form. The third trend was an inverse relationship, where in most cases the BCF value for peels and taproot flesh decreased as treatment concentration increased. The BCF values were the largest at the lowest concentration and then decreased sharply from the 0.5 to 5 mg kg sand−1 treatments.
| Element | Treatment (mg kg DW−1) | Bioconcentration factors for storage root tissues | |||
|---|---|---|---|---|---|
| Nanoparticle | Ionic | ||||
| Peel | Flesh | Peel | Flesh | ||
| Zinc | 0.5 | 70.2 ± 21.5 | 24.7 ± 6.8 | 251.0 ± 100.2 | 48.2 ± 2.6 |
| 5 | 12.9 ± 1.5 | 2.3 ± 0.3 | 39.5 ± 7.1 | 6.1 ± 0.7 | |
| 50 | 4.7 ± 1.4 | 0.4 ± 0.03 | 6.5 ± 0.3 | 9.4 ± 1.7 | |
| 500 | 10.0 ± 2.5 | 0.04 ± 0.01 | 4.3 ± 1.8 | 0.9 ± 0.2 | |
| Copper | 0.5 | 25.6 ± 10.0 | 3.6 ± 1.3 | 30.3 ± 3.0 | 3.3 ± 0.3 |
| 5 | 4.5 ± 0.8 | 0.4 ± 0.08 | 7.9 ± 1.0 | 0.4 ± 0.02 | |
| 50 | 2.4 ± 0.4 | 0.1 ± 0.01 | 7.7 ± 0.8 | 0.2 ± 0.04 | |
| 500 | 1.4 ± 0.3 | 0.03 ± <0.01 | 6.3 ± 0.6 | 0.7 ± 0.2 | |
| Cerium | 0.5 | 11.6 ± 2.5 | 0.3 ± 0.1 | 20.5 ± 5.0 | 2.1 ± 0.3 |
| 5 | 4.0 ± 0.7 | 0.05 ± 0.01 | 9.7 ± 1.8 | 0.2 ± 0.03 | |
| 50 | 2.3 ± 0.5 | 0.03 ± <0.01 | 8.2 ± 1.5 | 0.1 ± 0.02 | |
| 500 | 3.8 ± 0.4 | 0.06 ± 0.01 | 7.2 ± 0.8 | 0.1 ± 0.03 | |
The concentration of each metal in the carrot shoots displayed the same pattern in response to the nanoparticle and ionic treatments as did the taproot tissues (Fig. 3–5 and Table S3†). The BCF for each element in shoot tissues (Table 2) followed much the same patterns as for the root tissues with the same three general trends. With respect to the change in the BCF value as a function of treatment concentration, there were evident exceptions at the higher concentrations of Zn and Cu for plants receiving the ionic treatment where the BCF values showed a marked increase from the 50 to 500 mg kg−1 treatment. There were significant effects of form and concentration for all three elements, but the interaction between these main effects was significant only for Cu (Table S3†). The transfer factors (TF), which express the ratio of concentration in the shoot to that in the taproot, were between 0.01 and 0.5 for all Zn treatments with the exception of the highest ionic treatment concentration (Tables 2 and S3†). The TF values for the ionic and nanoparticle Cu treatments were not significantly different from one another, while for the Ce treatments, there was a significant interaction between form and concentration but not for either factor alone (Table S3†).
| Element | Treatment (mg kg DW−1) | Bioconcentration factor for shoot tissues | Transfer factor for shoot tissues | ||
|---|---|---|---|---|---|
| Nanoparticle | Ionic | Nanoparticle | Ionic | ||
| Zinc | 0.5 | 38.8 ± 4.0 | 74.2 ± 25.2 | 0.4 ± 0.04 | 0.3 ± 0.05 |
| 5 | 4.3 ± 0.3 | 7.3 ± 0.8 | 0.3 ± 0.07 | 0.2 ± 0.02 | |
| 50 | 1.6 ± 0.3 | 1.9 ± 0.4 | 0.3 ± 0.1 | 0.08 ± 0.03 | |
| 500 | 2.7 ± 1.0 | 37.1 ± 5.0 | 0.5 ± 0.3 | 10.3 ± 3.3 | |
| Copper | 0.5 | 33.8 ± 4.3 | 47.1 ± 7.4 | 1.3 ± 0.4 | 1.3 ± 0.3 |
| 5 | 5.3 ± 0.5 | 6.6 ± 0.9 | 1.2 ± 0.3 | 0.7 ± 0.2 | |
| 50 | 1.5 ± 0.4 | 4.8 ± 1.0 | 0.9 ± 0.4 | 0.5 ± 0.1 | |
| 500 | 0.9 ± 0.2 | 19.0 ± 4.7 | 0.8 ± 0.2 | 1.1 ± 0.3 | |
| Cerium | 0.5 | 4.1 ± 0.6 | 6.8 ± 0.9 | 2.3 ± 0.9 | 0.9 ± 0.3 |
| 5 | 0.5 ± 0.2 | 2.5 ± 0.7 | 0.4 ± 0.1 | 1.1 ± 0.7 | |
| 50 | 0.1 ± 0.02 | 2.9 ± 0.9 | 0.2 ± 0.1 | 0.7 ± 0.3 | |
| 500 | 0.4 ± 0.1 | 3.4 ± 0.7 | 0.3 ± 0.05 | 0.9 ± 0.3 | |
Another perspective from which to consider the results, and perhaps the best to visualize the distribution of each metal, is to express the data as the percent of total metal within each plant tissue (Fig. 6). This approach illustrates the partitioning of the metals from each treatment across the taproot peel, taproot flesh, and shoot tissues. For the untreated control plants, the peel and shoots accounted for the majority of the metal in each plant, 71.7% of the total Zn in the carrot, 75.2% of the total Cu, and 96.4% of the total Ce. More Zn was associated with peels than shoots, but the converse was observed for Cu and Ce. The partitioning of the metals between these three tissues differed between metals and in some cases between the nanoparticle and ionic treatments. The percentage of total Zn associated with the root tissues increased with nanoparticle concentrations, but the percentage of Zn associated with the edible flesh decreased. The pattern for ionic Zn was different, with both the total root Zn and the percentage of Zn associated with the edible tissues increasing with concentration, except for the highest ionic Zn treatment where the majority of the Zn was associated with the shoot tissues. In contrast to the difference between the two chemical forms of Zn, the partitioning of Cu within the plant tissues was somewhat similar between the nanoparticle and ionic treatments at each concentration, with the flesh Cu concentration generally decreasing with increasing treatment concentration. The results for Ce were comparable to those for Zn except that the concentration of Ce in the flesh was similar across the nanoparticle treatments and decreased compared to the ionic Ce treatments.
While the concentration of each element in the various tissues tended to increase with the treatment concentrations, the proportion of the total Zn, Cu, or Ce added to the substrate that accumulated in the carrot plants showed a significant decrease along the same gradient (Tables 3 and S3†). Aside from the lowest concentration of each treatment, the plants removed ≤5% of the added metal, and for Ce, the values were <2%. For Zn and Ce, significantly more of the ionic metal added to the substrate was removed into the plant tissues than the corresponding nanoparticle treatment, which is expected given the higher carrot tissue concentrations for each element observed for the plants receiving the ionic treatments. There was no significant difference for Cu in terms of chemical form.
| Element | Treatment (mg kg DW−1) | Percent of total added metal removed | |
|---|---|---|---|
| Nanoparticle | Ionic | ||
| Zinc | 0.5 | 16.7 ± 2.7 | 43.1 ± 9.8 |
| 5 | 2.6 ± 0.5 | 4.1 ± 1.0 | |
| 50 | 1.0 ± 0.2 | 2.5 ± 0.3 | |
| 500 | 1.3 ± 0.3 | 5.5 ± 0.7 | |
| Copper | 0.5 | 13.1 ± 4.2 | 20.6 ± 5.0 |
| 5 | 2.5 ± 0.5 | 3.0 ± 0.3 | |
| 50 | 0.8 ± 0.1 | 3.0 ± 0.4 | |
| 500 | 0.5 ± 0.1 | 2.3 ± 0.6 | |
| Cerium | 0.5 | 1.7 ± 0.3 | 3.5 ± 0.6 |
| 5 | 0.6 ± 0.2 | 1.5 ± 0.4 | |
| 50 | 0.3 ± 0.1 | 1.7 ± 0.3 | |
| 500 | 0.4 ± 0.1 | 1.4 ± 0.2 | |
4. Discussion
Even though there have been many studies that examined the uptake and accumulation of ENPs by plant, only a handful of studies examined accumulation in comparison to the accumulation of the ionic forms of each metal, especially for underground vegetables.23,24 Carrot was chosen both because of its popularity as a vegetable and its unique anatomical structure. Instead of a typical dicotyledonous root structure of central vascular bundle, endodermis, cortex, and epidermis (i.e., anatomical organization from center radially outward), the carrot taproot displays secondary growth analogous to that of woody plants. That is, as the carrot root enlarges radially, that growth is achieved by replacing the cortex and epidermis with concentric rings of secondary vascular tissues laid down by mitotically active cambium cell layers.25,26 The inner secondary vascular tissue is the secondary xylem, and the outer is the secondary phloem. Immediately to the outside of the secondary phloem is the periderm. The periderm is a layer of dead cells that form the protective outer surface of the taproot. The “peel” collected here would have been composed mostly of periderm.The patterns observed in the data for metal accumulation were likely dictated by the anatomy of the carrot taproot. The periderm, for example, displayed a clear capacity to retain a large fraction of metals from either the ENP or the ionic treatments. Although correlating the histological distribution to ENP to regions within the periderm is certainly of interest, this was not a goal in the current investigation. Previous studies have shown that the cell wall of the root epidermal layer has the capacity to trap ENPs. The typical pore size of the plant cell wall is 2–20 nm,4,15 which is smaller than most metal oxide ENPs and smaller than the hydrodynamic size measured for the ENP suspensions used here (Table S1†). Some studies have reported that ENPs can be found sorbed only to the epidermal cell wall surface.23,27 Others have reported that ENPs or the metal ions that dissociate from the ENPs penetrate into the root but may then precipitate or aggregate in the root cell wall network, restricting further transport and accumulation.28 The cork cell layer of the carrot taproot periderm is thicker than a typical root epidermis. Moreover, as dead cells, the cork layer could not only sorb metals in the cell wall network but perhaps also retain metals within the cells themselves. One recent study examined the distribution of potassium across the radius of 90 day old carrot taproots and found the periderm cells to have the highest concentration of that element.25 The periderm of the potato tuber has also been shown to retain Cd from the external media and restrict the penetration of that element into the tuber interior.29 Such results suggest that the periderm has a large capacity to retain ions sorbed from the external media, as indicated by the BCF values obtained for the peel tissues (Table 1). The affinity of the periderm for ions such as Zn, Cu, and Ce (whether as ENPs or ions) must be quite high as the BCF values for those tissues were highest at the lowest treatment concentrations. One could speculate that the periderm cells associated with the peel sorbed a large fraction of the metals and that at low concentrations this accounted for a large fraction of the total metal in solution. In other words, the sorption capacity of the periderm cells was large enough to bind a large fraction of the metal in solution, hence the large BCF values. Sorption capacity was likely finite; however, as the treatment concentrations increased, the cells could have become saturated with the metals giving rise to the decreasing BCF values with increasing treatment concentration. Comparable inverse trends between tissue BCF values and the external metal concentration have been observed in other studies with plants and metal absorption.30–33 The same rationale would likely explain the parallel trends in the taproot flesh but the correspondingly lower BCF values given the lower tissue concentrations for this taproot tissue.
The capacity of the periderm peel to screen ENPs is evident when contrasted with the results observed for the ionic treatments. The ions more readily migrated through the periderm layer into the carrot taproot flesh and reached the secondary xylem for translocation to the shoots as indicated by the significantly larger shoot concentrations and shoot BCF values for the ionic treatments. The radial transport and translocation of Zn2+ and Cu2+ are not unexpected as these two elements are essential micronutrients for plants, needed in both the belowground and aboveground tissues. Cerium from rare earth element fertilizers and other sources has been detected in plant shoots, indicating that this element can be translocated to plant shoots in the ionic form.8,34,35 The significant aggregation of the ENPs in the initial suspensions (Table S1†) suggested that these ENPs were not stable in liquid suspension and quite likely contributed to their greater association with the periderm and the lower translocation of the associated elements to shoots.
The accumulation of Zn, Cu, or Ce from all the treatments was generally greater in the shoots than in the flesh of the carrot taproot, which may also be attributable to the anatomy of the carrot taproot. The majority of the carrot taproot is vascular tissue (secondary phloem and secondary xylem) rather than the cortex tissues found in most herbaceous plant roots. While these secondary vascular tissues do have a storage capacity, that storage is devoted principally to the accumulation of sugars and starch, along with osmotically active solutes to maintain the proper water status of those cells.25 If the distribution of potassium in the carrot taproot is used as a general guide for the pattern of ion distribution, the results have shown that the concentrations are highest in the periderm and pith of the mature taproot and significantly lower in the secondary phloem and xylem tissues.25 Potassium was found predominantly in the apoplasm, which likely allowed this element to migrate through the wall space radially to the carrot taproot core (i.e., secondary xylem and pithlike center), giving rise to the reported pattern. The same might not necessarily be observed for every nutrient or trace element, but such information is lacking for this plant species. The results for Cu and Ce from this study, and for the ZnO treatment, suggest a similar radial migration pattern for Zn, Cu, and Ce, with the elements readily reaching the secondary xylem for translocation to shoots and retained to only a modest extent in the secondary phloem that comprises the bulk of the taproot diameter. The results for the ionic Zn treatments demonstrate a more extensive retention in the taproot flesh. The reason for this specific pattern of Zn accumulation is not clear at present but may be related to the aforementioned control of osmotically active solutes and ions in the taproot. The flush of Zn2+ that was translocated to the carrot shoots at the highest Zn2+ concentration was unexpected but may be related to the decrease in biomass of the taproots in this treatment and possibly the onset of phytotoxic effects. Such conclusions are speculative and would require further study to clarify.
The study performed here did not attempt for the ENP treatments to determine whether the Zn, Cu, or Ce accumulated in the taproot tissues or translocated to the shoots was parent ENP or dissociated ions from the ENP. There have been reports that intact ENPs are translocated to aboveground plant tissues,36 but most studies report xylem translocation of the metal from the ENP but not the intact metal oxide ENPs themselves.23,37–39 Intact ENPs could readily reach the secondary xylem for translocation if there was splitting of the carrot taproot to expose the core, but no splitting was observed in this study. In the absence of splitting, intact ENPs would either have to migrate radially across the taproot diameter across the secondary phloem and vascular cambium or would have to reach the secondary xylem via the more direct connections created by xylem rays (Fig. S1†). Xylem rays are reportedly present however only in the early stages of taproot development and are lost later in development as secondary growth continues and the taproot matures,26 but this conclusion was based on one anatomical study. Small xylem rays have been observed in field grown carrot after harvest.40 No splitting of the taproots was evident in this study, but an anatomical examination for the presence or absence of xylem rays was not performed. Both ZnO and CuO can undergo dissolution, releasing Zn2+ or Cu2+, respectively.24,41 Dissociation of ZnO or CuO in the sand culture and/or in the periderm may have released ions which were transported radially and then translocated. The dissolution of ZnO or CuO was either limited in extent or kinetically slow; otherwise, the accumulation of Zn and Cu from the ZnO and CuO treatments would have been more similar to the comparable ionic treatments. CeO2 ENPs are more stable and reportedly do not undergo significant dissolution,35 which likely explains the greater retention of Ce from the ENP treatment as compared to the ionic treatment. Nonetheless, the results here demonstrate that Ce from the added CeO2 ENPs migrated radially through the flesh and was translocated to the shoots. Similar results for Ce translocation have been reported for other food crops,8,28,38,42 including radish,43 which shows secondary growth comparable to that of carrot. There is little evidence that intact CeO2 ENPs are translocated to plant shoots; most studies have detected cerium in plant shoots but not necessarily intact CeO2 ENPs.35,37,38 One recent study demonstrated that Ce uptake from CeO2 ENPs may involve dissolution of Ce to the ionic form, uptake of the ion, and reassembly of the Ce into the ENP form within the plant.44 The tissue distribution data for each element (Fig. 6) demonstrated that the metal from the nanoparticles was distributed quite differently from the ionic forms. This underscores the need to understand the specific aspects of the interaction of plants with each ENP.
5. Conclusions
In conclusion, carrot showed significantly less accumulation of Zn, Cu, or Ce from ENPs than from the ionic forms of each element. These results are in agreement with other studies that have compared the uptake of metal oxide ENPs to their ionic counterparts.23,24 The accumulation of the elements principally in the taproot peel (which we presume corresponds primarily to the periderm layer of the carrot taproot structure) and the shoots, along with lower accumulation in the edible flesh (i.e., secondary tissues and pith), is probably a function of the specific anatomy of the carrot taproot and is similar to the distribution of cerium accumulation reported for radish,43 another vegetable with a comparable root anatomy and secondary growth. The contribution of xylem rays to radial movement and translocation is one particular aspect of the root anatomy that may be important. In the absence of specific data on the dissolution of the ENPs in the sand culture system or the chemical form of Zn, Cu, or Ce in the carrot tissues, no specific conclusion can be drawn here as to whether intact ENPs are transported radially within the taproot or translocated to shoots. An additional study will be required to address these questions, including a focus on how ENP aggregation alters the hydrodynamic size and properties of the nanomaterials. Although there were obvious differences in most data between the ENP and ionic treatments, when viewed from the perspective of the overall mass of added metal removed from the sand culture systems, the results were much more similar for the two chemical forms of each element except at the lowest treatment concentration. On the other hand, given the results in Fig. 6 showing the distribution of the metal across the peel, taproot flesh, and shoots as a percentage of total metal in the plant, there are more obvious differences between the two chemical forms (ENP versus ionic) and between the three metals in terms of their fate within the plant tissues. This distribution is directly germane to human health as it relates specifically to the presence of those metals in the edible tissues as well as to decisions associated with the basic preparation of that plant food for consumption (i.e., how important is peeling the taproot). In order to corroborate such an assumption, more detailed anatomical and histological studies will be needed to relate specific aspects of the carrot taproot structure to the capacity to retain ENPs. Further comparative studies between the ENPs and their ionic equivalents will be necessary to understand the difference in behavior of the two chemical forms of these elements. The information obtained from these continued studies will be necessary to understand the fate and transport of these ENPs and to assess the potential food safety risks that may be associated with the consumption of carrots or other edible plants grown in the presence of these metal oxide nanomaterials.Acknowledgements
Funding for this research was provided by a grant from USDA-NIFA (#2012-67005-19585).References
- A. Nel, T. Xia, L. Madler and N. Li, Toxic potential of materials at the nanolevel, Sci. Total Environ., 2006, 311, 622–627 CAS.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Interaction of nanoparticles with edible plants and their possible implications in the food chain, J. Agric. Food Chem., 2011, 59(8), 3485–3498 CrossRef CAS PubMed.
- P. Miralles, T. L. Church and A. T. Harris, Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants, Environ. Sci. Technol., 2012, 46(17), 9224–9239 CrossRef CAS PubMed.
- X. Ma, J. Geiser-Lee, Y. Deng and A. Kolmakov, Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation, Sci. Total Environ., 2010, 408(16), 3053–3061 CrossRef CAS PubMed.
- B. Nowack and T. D. Bucheli, Occurrence, behavior and effects of nanoparticles in the environment, Environ. Pollut., 2007, 150(1), 5–22 CrossRef CAS PubMed.
- J. H. Niazi and M. B. Gu, Toxicity of metallic nanoparticles in microorganisms—A review, in Atmospheric and Biological Environmental Monitoring, ed. Y. J. Kim, U. Platt, M. B. Gu and H. Iwahashi, Springer, Netherlands, 2009, pp. 193–206 Search PubMed.
- J. L. Gardea-Torresdey, C. M. Rico and J. C. White, Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments, Environ. Sci. Technol., 2014, 48(5), 2526–2540 CrossRef CAS PubMed.
- J. Hawthorne, R. De la Torre Roche, B. Xing, L. A. Newman, X. Ma, S. Majumdar, J. Gardea-Torresdey and J. C. White, Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain, Environ. Sci. Technol., 2014, 48(22), 13102–13109 CrossRef CAS PubMed.
- M. A. Kiser, H. Ryu, H. Y. Jang, K. Hristovski and P. Westerhoff, Biosorption of nanoparticles to heterotrophic wastewater biomass, Water Res., 2010, 44, 4105–4114 CrossRef CAS PubMed.
- S. K. Brar, M. Verma, R. D. Tyagi and R. Y. Surampalli, Engineered nanoparticles in wastewater and wastewater sludge—evidence and impacts, Waste Manage., 2010, 30, 504–520 CrossRef CAS PubMed.
- A. Gogos, K. Knauer and T. D. Bucheli, Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities, J. Agric. Food Chem., 2012, 60, 9781–9792 CrossRef CAS PubMed.
- S. Suppan, Nanomaterials in Soil: Our Future Food Chain?, Institute for Agriculture and Trade Policy, 2013 Search PubMed.
- S. Mura, G. Seddaiu, F. Bacchini and P. P. Roggero, Advances of nanotechnology in agro-environmental studies, Italian Journal of Agronomy, 2013, 8, e18 CrossRef.
- H. T. Ratte, Bioaccumulation and toxicity of silver compounds: A review, Environ. Toxicol. Chem., 1999, 18(1), 89–108 CrossRef CAS.
- N. C. Carpita and D. M. Gibeaut, Structural models of primary cell walls in flowering plants: consistency of molecular-structure with the physical properties of walls during growth, Plant J., 1993, 3, 1–30 CrossRef CAS PubMed.
- E. Navarro, A. Baun, R. Behra, N. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P. Santschi and L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi, Ecotoxicology, 2008, 17(5), 372–386 CrossRef CAS PubMed.
- J. E. Cañas, M. Long, S. Nations, R. Vadan, L. Dai, M. Luo, R. Ambikapathi, E. H. Lee and D. Olszyk, Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species, Environ. Toxicol. Chem., 2008, 27(9), 1922–1931 CrossRef.
- L. Yang and D. J. Watts, Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles, Toxicol. Lett., 2005, 158(2), 122–132 CrossRef CAS PubMed.
- National Agricultural Statistics Service, Carrots, Statistics by subject, In US Department of Agriculture, 2014 Search PubMed.
- USEPA, A Guide for Land Appliers on the Requirements of the Federal Standards for the Use or Disposal of Sewage Sludge, 40 CFR Part 503, EPA/831-B-93-002b; Office of Enforcement and Compliance Assurance, US Environmental Protection Agency, Washington, DC, 1994, p. 62 Search PubMed.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, Global life cycle releases of engineered nanomaterials, J. Nanopart. Res., 2013, 15(6), 1692 CrossRef.
- USEPA, Method 3050b: Acid Digestion of Sediments, Sludges, and Soils, in Test Methods for Evaluating Solid Waste Physical/Chemical Methods, SW-846, Environmental Protection Agency, Office of Solid Waste, 1996, p. 12 Search PubMed.
- D. Lin and B. Xing, Root uptake and phytotoxicity of ZnO nanoparticles, Environ. Sci. Technol., 2008, 42, 5580–5585 CrossRef CAS PubMed.
- N. M. Franklin, N. J. Rogers, S. C. Apte, G. E. Batley, G. E. Gadd and P. S. Casey, Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility, Environ. Sci. Technol., 2007, 41(24), 8484–8490 CrossRef CAS PubMed.
- A. V. Korolev, A. D. Tomos, R. Bowtell and J. F. Farrar, Spatial and temporal distribution of solutes in the developing carrot taproot measured at single cell resolution, J. Exp. Bot., 2000, 51(344), 567–577 CrossRef CAS PubMed.
- L. Havis, Anatomy of the hypocotyl and roots of Daucus carota, J. Agric. Res., 1939, 58(8), 557–564 Search PubMed.
- E. Wild and K. C. Jones, Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants, Environ. Sci. Technol., 2009, 43(14), 5290–5294 CrossRef CAS PubMed.
- M. L. López-Moreno, G. de la Rosa, J. A. Hernández-Viezcas, J. R. Peralta-Videa and J. L. Gardea-Torresdey, X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species, J. Agric. Food Chem., 2010, 58(6), 3689–3693 CrossRef PubMed.
- R. J. Reid, K. R. Dunbar and M. J. McLaughlin, Cadmium loading into potato tubers: the roles of the periderm, xylem and phloem, Plant, Cell Environ., 2003, 26(2), 201–206 CrossRef CAS.
- M. Sakakibara, Y. Ohmori, N. T. H. Ha, S. Sano and K. Sera, Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis acicularis, Clean: Soil, Air, Water, 2011, 39(8), 735–741 CrossRef CAS.
- R. K. Sharma, M. Agrawal and S. B. Agrawal, Physiological, biochemical and growth responses of lady's finger (Abelmoschus esculentus L.) plants as affected by Cd contaminated soil, Bull. Environ. Contam. Toxicol., 2010, 84(6), 765–770 CrossRef CAS PubMed.
- M. Dogan, S. Saygideger and U. Colak, Effect of lead toxicity on aquatic macrophyte Elodea canadensis Michx, Bull. Environ. Contam. Toxicol., 2009, 83(2), 249–254 CrossRef CAS PubMed.
- L. Samsøe-Petersen, E. H. Larsen, P. B. Larsen and P. Bruun, Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils, Environ. Sci. Technol., 2002, 36(14), 3057–3063 CrossRef.
- P. Babula, V. Adam, R. Opatrilova, J. Zehnalek, L. Havel and R. Kizek, Uncommon heavy metals, metalloids and their plant toxicity: a review, Environ. Chem. Lett., 2008, 6(4), 189–213 CrossRef CAS.
- M. L. López-Moreno, G. de la Rosa, J. Á. Hernández-Viezcas, H. Castillo-Michel, C. E. Botez, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants, Environ. Sci. Technol., 2010, 44(19), 7315–7320 CrossRef PubMed.
- Z. Wang, X. Xie, J. Zhao, X. Liu, W. Feng, J. C. White and B. Xing, Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.), Environ. Sci. Technol., 2012, 46(8), 4434–4441 CrossRef CAS PubMed.
- K. Birbaum, R. Brogioli, M. Schellenberg, E. Martinoia, W. J. Stark, D. Günther and L. K. Limbach, No evidence for cerium dioxide nanoparticle translocation in maize plants, Environ. Sci. Technol., 2010, 44(22), 8718–8723 CrossRef CAS PubMed.
- J. Hong, J. R. Peralta-Videa, C. Rico, S. Sahi, M. N. Viveros, J. Bartonjo, L. Zhao and J. L. Gardea-Torresdey, Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants, Environ. Sci. Technol., 2014, 48(8), 4376–4385 CrossRef CAS PubMed.
- J. H. Priester, Y. Ge, R. E. Mielke, A. M. Horst, S. C. Moritz, K. Espinosa, J. Gelb, S. L. Walker, R. M. Nisbet, Y.-J. An, J. P. Schimel, R. G. Palmer, J. A. Hernandez-Viezcas, L. Zhao, J. L. Gardea-Torresdey and P. A. Holden, Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(37), E2451–E2456 CrossRef CAS PubMed.
- N. Halloran, M. U. Kasim and R. Kasim, Pre- and post-harvest hormonal changes of carrots, Acta Hortic., 2005, 682, 299–306 CrossRef CAS.
- C. O. Dimkpa, J. E. McLean, D. E. Latta, E. Manangón, D. W. Britt, W. P. Johnson, M. I. Boyanov and A. J. Anderson, CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat, J. Nanopart. Res., 2012, 14(9), 1–15 CrossRef.
- Q. Wang, S. Ebbs, Y. Chen and X. Ma, Trans-generational impact of cerium oxide nanoparticles on tomato plants, Metallomics, 2013, 5, 753–759 RSC.
- W. Zhang, S. Ebbs, C. Musante, J. White, C. Gao and X. Ma, Uptake and accumulation of bulk and nano-sized cerium oxide particles and ionic cerium by radish (Raphanus sativus L.), J. Agric. Food Chem., 2015, 63(2), 382–390 CrossRef CAS PubMed.
- F. Schwabe, S. Tanner, R. Schulin, A. Rotzetter, W. Stark, A. von Quadt and B. Nowack, Dissolved cerium contributes to uptake of Ce in the presence of differently sized CeO2-nanoparticles by three crop plants, Metallomics, 2015, 7(3), 466–477 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5en00161g |
| This journal is © The Royal Society of Chemistry 2016 |

