Iron nitrate/TEMPO-catalyzed aerobic oxidative synthesis of quinazolinones from alcohols and 2-aminobenzamides with air as the oxidant†
Yongke
Hu
,
Lei
Chen
and
Bindong
Li
*
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, P. R. China. E-mail: libindong@njust.edu.cn; Fax: +86-25-84315514
First published on 4th July 2016
Abstract
A highly efficient, iron nitrate/TEMPO-catalyzed approach for the synthesis of quinazolinones has been achieved via a one-pot, tandem aerobic oxidative cyclization of primary alcohols with 2-aminobenzamides. This practical reaction tolerates a broad scope of substrates and could afford a variety of desirable products in good to excellent yields with air as the terminal green oxidant. Thus, the present synthetic protocol provides an efficient and concise strategy for the synthesis of quinazolinones.
Introduction
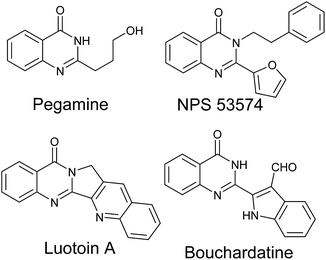
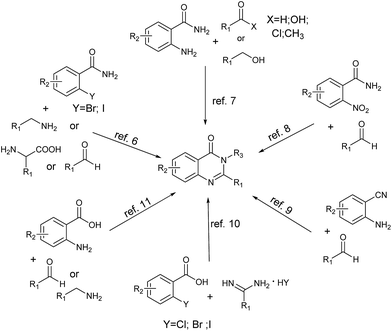
Quinazolinone derivatives as an important class of N-containing heterocyclic compounds exist widely in natural products and synthetic drugs (Scheme 1).1 Specifically, they are assigned as privileged scaffolds in drug discovery and are well-known for their broad range of biological and pharmacological properties such as anticancer,2 antibacterial,3 anticonvulsant4 and hypolipidemic5 activities.Because of their importance, the synthesis of substituted quinazolinones has extensively attracted much attention and numerous synthetic approaches have been developed. In general, the main approaches usually utilize 2-halobenzamides,6 2-aminobenzamides,7 2-nitrobenzamides,8 2-aminobenzonitriles,9 2-halobenzoic acids,10 and 2-aminobenzoic acids11 as suitable substrates to form the quinazolinones (Scheme 2). Among the plentiful methodologies, the most classical and common protocols for the synthesis of quinazolinones are the condensation of 2-aminobenzamides with aldehydes followed by oxidation of the aminal intermediate in the present of stoichiometric or large excess amounts of nonrenewable oxidants, such as KMnO4,12 MnO2,13 DDQ,14 CuCl2 (ref. 15) and PhI(OAc)2.16 However, the drawback of these methods is that chemical unstable aldehydes are required. As is well known, aldehydes can be easily prepared by the oxidation of alcohols and numerous methods have been developed, e.g., Swern oxidation,17 Dess–Martin oxidation18 and TEMPO-catalyzed oxidation.19 Besides, alcohols are inexpensive, benign and readily available chemical feedstock. It would be advantages to synthesize quinazolinones directly from alcohols rather than aldehydes. To the best of our knowledge, several catalytic methods have been reported for the synthesis of quinazolinones derivatives using alcohols as the stating substrates. In 2011, Zhou described a [Cp*IrCl2]2-catalyzed one-pot oxidative cyclization of primary alcohols with 2-aminobenzamides to quinazolinones under hydrogen transfer conditions.20 Although this strategy was green, atomo-efficient and the only by-products are environmentally benign H2 and water, the turnover number of the catalytic system was still low. In 2013, Wei and co-workers reported a one-pot two-step catalytic system for synthesis of quinazolinones from primary alcohols and 2-aminobenzamides.21 In their method, iodine was used as catalyst, DMSO as the oxidant and various quinazolinones were prepared in good yields, but this system produced stoichiometric amount of poisonous dimethyl sulfide. Very recently, Wu et al.22 reported a new strategy for the synthesis of quinazolinones based on ZnI2-catalyzed reaction of benzyl alcohols with 2-aminobenzamides, and soon later Li et al.23 developed a similar FeCl3-catalyzed oxidative system, but both of the two strategies required large excess amounts of tert-butyl hydroperoxide (TBHP) as oxidant. Recently, molecular oxygen, as a green oxidant, has received significant attention because O2 is inexpensive, abundant and water is produced as the only benign by-product. And aerobic oxidation of organic compounds catalyzed by aminoxyl radicals such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) using O2 or air as the terminal oxidant has also been extensively studied. To date, few catalytic systems have been successfully developed for the synthesis of N-heterocyclic compounds using molecular oxygen as the oxidant in the present of TEMPO.24 It is well known that the Fe/TEMPO catalytic system has been well studied for the aerobic oxidation of alcohols and amines, and exhibits broad utilities in both academe and industry.25 However, its application in the catalytic oxidative synthesis of N-heterocycles is rare.
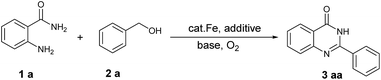
Herein, we report a new approach to quinazolinones from 2-aminobenzamides and primary alcohols using cheap and nontoxic Fe(NO3)3 as the catalyst, TEMPO as the co-catalyst and air as the terminal oxidant. This catalytic system provides an efficient and convenient method for the synthesis of diverse quinazolinones from easily accessible starting materials.
Results and discussion
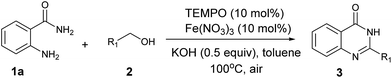
Initially, 2-aminobenzamide (1a) and benzyl alcohol (2a) were employed as model substrates to optimize the reaction conditions. The optimized results including catalyst, solvent, base and reaction temperature were summarized in Table 1. As listed in Table 1, iron salts such as FeCl3, FeBr3, Fe2(SO4)3, Fe(acac)3 and Fe(NO3)3 were screened in the present of KOH at 100 °C under air balloon (Table 1, entries 1–5). To our delight, Fe (NO3)3 showed higher catalytic activity than other iron salts and the desired product 3a was isolated in 60% yield (Table 1, entry 5). When TEMPO (5 mol%) was added in the reaction, it could greatly enhance the product yield to 75% (Table 1, entry 6). Further, we found that 10 mol% of Fe(NO3)3 and 10 mol% of TEMPO were sufficient for this transformation (Table 1, entries 6–8). Next, we screened different bases, the results revealed that KOH and CsOH were the most effective for the reaction and gave good yield of 3a (Table 1, entries, 8, 11–14). The amount of base was also tested (Table 1, entries 8–10). Subsequently, the effect of solvents was investigated to further optimize the reaction. It was also found that toluene showed the best efficiency compared with other solvents such as DMSO, DMF, dioxane, CH3CN, DMC and H2O (Table 1, entries 15–20). In addition, the reaction activities were found inhibited with higher or lower the reaction temperature than 100 °C (Table 1, entries 21 and 22). When the reaction was carried out under nitrogen, the desired product was not observed, which indicated that the importance of molecular oxygen as the terminal oxidant (Table 1, entry 23). Therefore, we chose 10 mol% Fe(NO3)3, 10 mol% TEMPO, 0.5 equiv. KOH in toluene at 100 °C under air as standard reaction conditions (Table 1, entry 8).| Entry | Fe catalystb (mol%) | TEMPO (mol%) | Base (equiv.) | Solvent | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction condition: 1a (1 mmol), 2a (1 mmol), Fe catalyst, TEMPO, base and solvent (2 mL) in a 10 mL Schlenk tube stirred for 12 h at 100 °C under air balloon and then monitored by TLC. b Fe(NO3)3·9H2O = Fe(NO3)3 unless otherwise noted. c Isolated yield. d Reaction at 90 °C. e Reaction at 120 °C. f Reaction carried out under nitrogen. | |||||
| 1 | FeCl3 (5) | — | KOH (0.5) | Toluene | 48 |
| 2 | FeBr3 (5) | — | KOH (0.5) | Toluene | 51 |
| 3 | Fe2(SO4)3 (5) | — | KOH (0.5) | Toluene | 43 |
| 4 | Fe(acac)3 (5) | — | KOH (0.5) | Toluene | 38 |
| 5 | Fe(NO3)3 (5) | — | KOH (0.5) | Toluene | 60 |
| 6 | Fe(NO3)3 (5) | 5 | KOH (0.5) | Toluene | 75 |
| 7 | Fe(NO3)3 (5) | 10 | KOH (0.5) | Toluene | 81 |
| 8 | Fe(NO 3 ) 3 (10) | 10 | KOH (0.5) | Toluene | 95 |
| 9 | Fe(NO3)3 (10) | 10 | KOH (1.0) | Toluene | 94 |
| 10 | Fe(NO3)3 (10) | 10 | — | Toluene | 65 |
| 11 | Fe(NO3)3 (10) | 10 | K2CO3 (0.5) | Toluene | 75 |
| 12 | Fe(NO3)3 (10) | 10 | K3PO4 (0.5) | Toluene | 61 |
| 13 | Fe(NO3)3 (10) | 10 | t-BuOK (0.5) | Toluene | 85 |
| 14 | Fe(NO3)3 (10) | 10 | CsOH (0.5) | Toluene | 93 |
| 15 | Fe(NO3)3 (10) | 10 | KOH (0.5) | DMSO | 87 |
| 16 | Fe(NO3)3 (10) | 10 | KOH (0.5) | DMF | 59 |
| 17 | Fe(NO3)3 (10) | 10 | KOH (0.5) | Dioxane | 43 |
| 18 | Fe(NO3)3 (10) | 10 | KOH (0.5) | CH3CN | 75 |
| 19 | Fe(NO3)3 (10) | 10 | KOH (0.5) | DMC | Trace |
| 20 | Fe(NO3)3 (10) | 10 | KOH (0.5) | H2O | Trace |
| 21d | Fe(NO3)3 (10) | 10 | KOH (0.5) | Toluene | 87 |
| 22e | Fe(NO3)3 (10) | 10 | KOH (0.5) | Toluene | 90 |
| 23f | Fe(NO3)3 (10) | 10 | KOH (0.5) | Toluene | Trace |
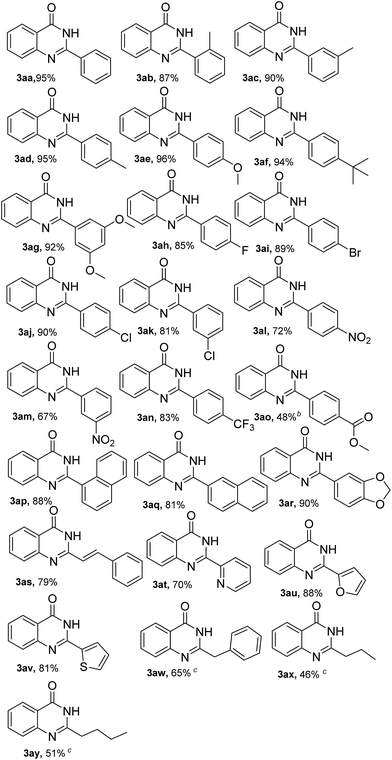
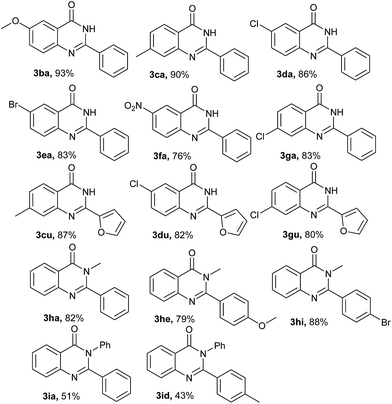
With the optimized reaction conditions in hand, the scope and generality of this Fe/TEMPO-catalyzed tandem reaction for synthesis of 2-substituted quinazolinones were explored and the results were summarized in Table 2. Firstly, we investigated the reaction between 2-aminobenzamide (1a) and various primary alcohols (2) under standard condition. To our delights, electron-donating and electron-withdrawing groups were well tolerated in the reactions. Generally, as is shown in Table 2, primary benzylic alcohols bearing electron-donating groups such as methyl, methoxyl, tert-butyl gave the target products in excellent isolated yields of 87–96% (Table 2, 3ab–3ag). However, the electron-withdrawing groups such as –F, –Br, –Cl, –NO2 and –CF3 substituted benzylic alcohols gave comparably low yields of products (Table 2, 3ah–3an). The methyl 4-(hydroxymethyl)benzoate could also be converted to the desired products in the absence of KOH (Table 2, 3ao). The reaction of 1-naphthylmethanol and 2-naphthylmethanol with 1a produced the corresponding products 3ap and 3aq in 88% and 81% yields, respectively. Notably, both piperonyl alcohol and cinnamyl alcohol were well-tolerated in the present catalytic system (Table 2, 3ar and 3as). In addition, heterocyclic alcohols such as 2-pyridinemethanol, 2-furanemethanol and 2-thiophenemethanol were tested successively and the desired products were obtained in 70–88% yields as well (Table 2, 3at–3av). In the case of aliphatic alcohols such as phenethyl alcohol, n-butyl alcohol and n-pentyl alcohol could also be transformed to the desired products in 46–65% yields (Table 2, 3aw–3ay).
To further expand the substrate scope, we investigated the reactions of substituted 2-aminobenzamides with primary alcohols and the results were summarized in Table 3. It was observed that 2-aminobenzamides bearing electron-withdrawing or electron-donating functional groups could be smoothly converted to the desired products in good yields of 76–93% (Table 3, 3ba–3ga). And the reaction of 2-furanemethanol with different substituted 2-aminobenzamides proceeded smoothly to generate the corresponding products 3cu, 3du and 3gu in the yield of 87%, 82% and 80%, respectively. Furthermore, the reaction of sterically demanding 2-amino-N-methylbenzamide and 2-amino-N-phenylbenzamide were found to be effective and the corresponding products were produced in good yields (Table 3, 3ha, 3he, 3hi, 3ia and 3id).
| a Reaction condition: 1 (1 mmol), 2 (1 mmol), Fe(NO3)3·9H2O (10 mol%), TEMPO (10 mol%), KOH (0.5 equiv.), toluene (2 mL), in a 10 mL Schlenk tube stirred for 12 h at 100 °C under air balloon and then monitored by TLC. Isolated yield. |
|---|
|
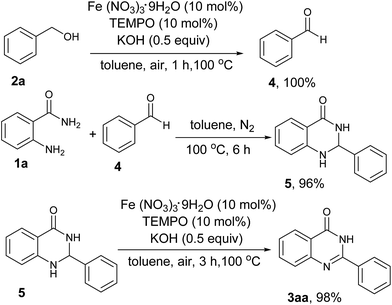
To elucidate the reaction mechanism, the following control experiments were carried out. As shown in Scheme 3, firstly, benzaldehyde (4) was generated in suit from benzyl alcohol (2a) under standard condition. Next, we conducted the reaction of 2-aminobenzamide (1a) and benzaldehyde (4) in the absence of Fe(NO3)3, TEMPO and KOH under nitrogen atmosphere in toluene, which resulted in 96% yield of 2-phenyl-2,3-dihydroquinazolin-4(1H)-one (5). Then, the aminal intermediate (5) was oxidized to the desired product in 98% yield under standard condition.
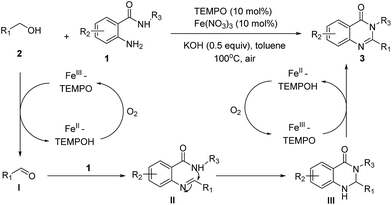
Based on the results above, a plausible mechanism of the oxidative condensation of 2-aminobenzamides with primary alcohols is presented in Scheme 4. The initial step involves the aerobic oxidation of alcohols to the corresponding aldehydes (I) catalyzed by Fe/TEMPO which has been already reported by Ma et al.25b Next, the aldehydes react with 2-aminobenzamides to form the imine intermediates (II), which after cyclization generates 2,3-dihydroquinazolinones (III). At last, the aminal intermediates (III) undergo an aerobic oxidative dehydrogenation to give the corresponding quinazolinone products.
Conclusions
In summary, we have disclosed a one-pot efficient Fe(NO3)3/TEMPO-catalyzed protocol for aerobic oxidative synthesis of quinazolinones from easily accessible primary alcohols and 2-aminobenzamides with molecular oxygen as the terminal oxidant. This synthetically valuable procedure exhibits good functional group tolerance and a variety of quinazolinones are obtained in moderate to excellent yields. In addition, a plausible reaction mechanism has been proposed and further more synthetic applications of this catalytic system are under way in our laboratory.Experimental section
Experimental details
All reagents were purchased from commercial suppliers without further purification. Column chromatography was carried out over 200–300 mesh silica gel. The 1H and 13C NMR spectra were recorded on a Bruker Avance III 500 MHz spectrometer with deuterated dimethyl sulfoxide (DMSO-d6) or CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard at room temperature. Chemical shifts are given in δ relative to TMS, and the coupling constants J are given in hertz. High-resolution mass spectra (HRMS) were obtained on an Agilent mass spectrometer using ESI-TOF (electrospray ionization-time of flight). IR spectra were recorded on a NicoletiS10 FT-IR spectrometer as KBr pellets. Melting points were determined using an electrothermal capillary melting point apparatus.Typical procedure for the synthesis of quinazolinones
To a mixture of 2-aminobenzamide 1a (1 mmol), benzyl alcohol 2a (1 mmol), Fe(NO3)3·9H2O (10 mol%), TEMPO (10 mol%), KOH (0.5 equiv.) and toluene (2 mL) were added in a 10 mL Schlenk tube. Then the mixture was stirred at 100 °C for 12 h under air balloon. The progress of the reaction was monitored by TLC. After completion of the reaction, the solution was diluted with ethyl acetate, washed with water, and then the organic layer was separated and dried over anhydrous MgSO4. The solvent was concentrated under reduced pressure and the crude product was purified by column chromatography on silica gel using PE/EtOAc as eluent to afford the pure product 3a.Procedure for the preparation of compound 5
A mixture of 1a (1 mmol) and benzaldehyde (1 mmol) in toluene (2 mL) was stirred at 100 °C for 6 h under a N2 atmosphere. After completion of the reaction, the solution was diluted with ethyl acetate, washed with water, and then the organic layer was separated and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure and the crude product was purified by chromatography on silica gel, eluting with PE/EtOAc, to afford 5 as white solid in 90% isolated yield.Notes and references
- (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787–9826 CrossRef CAS; (b) I. Khan, A. Ibrar, N. Abbas and A. Saeed, Eur. J. Med. Chem., 2014, 76, 193–244 CrossRef CAS; (c) Z.-Z. Ma, Y. Hano, T. Nomura and Y.-J. Chen, Heterocycles, 1997, 46, 541–546 CrossRef CAS; (d) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef CAS.
- S.-L. Cao, Y.-P. Feng, Y.-Y. Jiang, S.-Y. Liu, G.-Y. Ding and R.-T. Li, Bioorg. Med. Chem. Lett., 2005, 15, 1915–1917 CrossRef CAS PubMed.
- P. P. Kung, M. D. Casper, K. L. Cook, L. Wilson-Lingardo, L. M. Risen, T. A. Vickers, R. Ranken, L. B. Blyn, J. R. Wyatt, P. D. Cook and D. J. Ecker, J. Med. Chem., 1999, 42, 4705–4713 CrossRef CAS.
- M. M. Aly, Y. A. Mohamed, K. A. El-Bayouki, W. M. Basyouni and S. Y. Abbas, Eur. J. Med. Chem., 2010, 45, 3365–3373 CrossRef CAS.
- Y. Kurogi, Y. Inoue, K. Tsutsumi, S. Nakamura, K. Nagao, H. Yoshitsugu and Y. Tsuda, J. Med. Chem., 1996, 39, 1433–1437 CrossRef CAS.
- (a) W. Xu and H. Fu, J. Org. Chem., 2011, 76, 3846–3852 CrossRef CAS; (b) W. Xu, Y. Jin, H. Liu, Y. Jiang and H. Fu, Org. Lett., 2011, 13, 1274–1277 CrossRef CAS; (c) S. Guo, Y. Li, L. Tao, W. Zhang and X. Fan, RSC Adv., 2014, 4, 59289–59296 RSC; (d) T. Kotipalli, V. Kavala, D. Janreddy, V. Bandi, C.-W. Kuo and C.-F. Yao, Eur. J. Org. Chem., 2016, 2016, 1182–1193 CrossRef CAS.
- (a) T. M. Potewar, R. N. Nadaf, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Synth. Commun., 2005, 35, 231–241 CrossRef CAS; (b) X.-S. Wang, K. Yang, M.-M. Zhang and C.-S. Yao, Synth. Commun., 2010, 40, 2633–2646 CrossRef CAS; (c) N. Y. Kim and C. H. Cheon, Tetrahedron Lett., 2014, 55, 2340–2344 CrossRef CAS; (d) X. Tian, L. Song, E. Li, Q. Wang, W. Yu and J. Chang, RSC Adv., 2015, 5, 62194–62201 RSC; (e) X. Chen, T. Chen, F. Ji, Y. Zhou and S.-F. Yin, Catal. Sci. Technol., 2015, 5, 2197–2202 RSC; (f) S. Mohammed, R. A. Vishwakarma and S. B. Bharate, J. Org. Chem., 2015, 80, 6915–6921 CrossRef CAS; (g) F. Li, L. Lu and J. Ma, Org. Chem. Front., 2015, 2, 1589–1597 RSC; (h) Z. Zhang, M. Wang, C. Zhang, Z. Zhang, J. Lu and F. Wang, Chem. Commun., 2015, 51, 9205–9207 RSC.
- A. H. Romero, J. Salazar and S. E. Lopez, Synthesis, 2013, 45, 2043–2050 CrossRef CAS.
- (a) H. Q. Li, L. He, H. Neumann, M. Beller and X.-F. Wu, Green Chem., 2014, 16, 1336–1343 RSC; (b) W. Zhao, P. Liu and F. Li, ChemCatChem, 2016, 8, 1523–1530 CrossRef CAS.
- (a) X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348–351 CrossRef CAS; (b) X. Zhang, D. Ye, H. Sun, D. Guo, J. Wang, H. Huang, X. Zhang, H. Jiang and H. Liu, Green Chem., 2009, 11, 1881–1888 RSC.
- (a) D. J. Connolly and P. J. Guiry, Synlett, 2001, 2001, 1707–1710 CrossRef; (b) J.-F. Liu, J. Y. Lee, A. M. Dalton, G. Bi, L. B. Yu, C. M. Baldino, E. McElory and M. Brown, Tetrahedron Lett., 2005, 46, 1241–1244 CrossRef CAS; (c) K. Ighilahriz, B. Boutemeur, F. Chami, C. Rabia, M. Hamdi and S. M. Hamdi, Molecules, 2008, 13, 779–789 CrossRef CAS.
- T. Hisano, M. Ichikawa, A. Nakagawa and M. Tsuji, Chem. Pharm. Bull., 1975, 23, 1910–1916 CrossRef CAS.
- C. Balakumar, P. Lamba, D. P. Kishore, B. L. Narayana, K. V. Rao, K. Rajwinder, A. R. Rao, B. Shireesha and B. Narsaiah, Eur. J. Med. Chem., 2010, 45, 4904–4913 CrossRef CAS.
- Y. Mitobe, S. Ito, T. Mizutani, T. Nagase, N. Sato and S. Tokita, Bioorg. Med. Chem. Lett., 2009, 19, 4075–4078 CrossRef CAS.
- R. J. Abdel-Jalil, W. Voelter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475–3476 CrossRef CAS.
- R. Cheng, T. Guo, D. Zhang-Negrerie, Y. Du and K. Zhao, Synthesis, 2013, 45, 2998–3006 CrossRef CAS.
- (a) A. J. Mancuso, S.-L. Huang and D. Swern, J. Org. Chem., 1978, 43, 2480–2482 CrossRef CAS; (b) M. Marx and T. T. Tidwell, J. Org. Chem., 1984, 49, 788–793 CrossRef CAS.
- (a) D. B. Dess and J. C. Martin, J. Am. Chem. Soc., 1991, 113, 7277–7287 CrossRef CAS; (b) D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 48, 4155–4156 CrossRef CAS.
- (a) M. R. Leanna, T. J. Sowin and H. E. Morton, Tetrahedron Lett., 1992, 33, 5029–5032 CrossRef CAS; (b) P. Gamez, I. W. Arends, J. Reedijk and R. A. Sheldon, Chem. Commun., 2003, 2414–2415 RSC; (c) P. Gamez, I. W. C. E. Arends, R. A. Sheldon and J. Reedijk, Adv. Synth. Catal., 2004, 346, 805–811 CrossRef CAS.
- J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730–7736 CrossRef CAS.
- W. Ge, X. Zhu and Y. Wei, RSC Adv., 2013, 3, 10817–10822 RSC.
- M. Sharif, J. Opalach, P. Langer, M. Beller and X.-F. Wu, RSC Adv., 2014, 4, 8–17 RSC.
- D. Zhao, Y.-R. Zhou, Q. Shen and J.-X. Li, RSC Adv., 2014, 4, 6486–6489 RSC.
- (a) Z. Chen, J. Chen, M. Liu, J. Ding, W. Gao, X. Huang and H. Wu, J. Org. Chem., 2013, 78, 11342–11348 CrossRef CAS; (b) B. Han, X.-L. Yang, C. Wang, Y.-W. Bai, T.-C. Pan, X. Chen and W. Yu, J. Org. Chem., 2012, 77, 1136–1142 CrossRef CAS; (c) Y.-X. Chen, L.-F. Qian, W. Zhang and B. Han, Angew. Chem., Int. Ed., 2008, 47, 9330–9333 CrossRef CAS; (d) J. C. A. Flanagan, L. M. Dornan, M. G. McLaughlin, N. G. McCreanor, M. J. Cook and M. J. Muldoon, Green Chem., 2012, 14, 1281–1283 RSC; (e) J. Yu, J. Xu and M. Lu, Appl. Organomet. Chem., 2013, 27, 606–610 CrossRef CAS.
- (a) N. Wang, R. Liu, J. Chen and X. Liang, Chem. Commun., 2005, 5322–5324 RSC; (b) S. Ma, J. Liu, S. Li, B. Chen, J. Cheng, J. Kuang, Y. Liu, B. Wan, Y. Wang, J. Ye, Q. Yu, W. Yuan and S. Yu, Adv. Synth. Catal., 2011, 353, 1005–1017 CrossRef CAS; (c) E. Zhang, H. Tian, S. Xu, X. Yu and Q. Xu, Org. Lett., 2013, 15, 2704–2707 CrossRef CAS; (d) J. Liu and S. Ma, Tetrahedron, 2013, 69, 10161–10167 CrossRef CAS; (e) J. Liu and S. Ma, Org. Biomol. Chem., 2013, 11, 4186–4193 RSC; (f) S. U. Dighe, D. Chowdhury and S. Batra, Adv. Synth. Catal., 2014, 356, 3892–3896 CrossRef CAS.
- D. Zhao, T. Wang and J.-X. Li, Chem. Commun., 2014, 50, 6471–6474 RSC.
- J. Chen, D. Wu, F. He, M. Liu, H. Wu, J. Ding and W. Su, Tetrahedron Lett., 2008, 49, 3814–3818 CrossRef CAS.
- Q. Li, Y. Huang, T. Chen, Y. Zhou, Q. Xu, S.-F. Yin and L.-B. Han, Org. Lett., 2014, 16, 3672–3675 CrossRef CAS PubMed.
- Y. Feng, Y. Li, G. Cheng, L. Wang and X. Cui, J. Org. Chem., 2015, 80, 7099–7107 CrossRef CAS.
- A. V. Bogolubsky, S. V. Ryabukhin, A. S. Plaskon, S. V. Stetsenko, D. M. Volochnyuk and A. A. Tolmachev, J. Comb. Chem., 2008, 10, 858–862 CrossRef CAS.
- N. Kausar, I. Roy, D. Chattopadhyay and A. R. Das, RSC Adv., 2016, 6, 22320–22330 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12164k |
| This journal is © The Royal Society of Chemistry 2016 |