Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
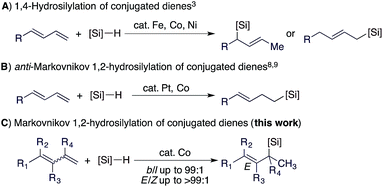
Cobalt-catalyzed regioselective stereoconvergent Markovnikov 1,2-hydrosilylation of conjugated dienes†
Hui Leng
Sang
 ,
Songjie
Yu
,
Songjie
Yu
 and
Shaozhong
Ge
and
Shaozhong
Ge
 *
*
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543, Singapore. E-mail: chmgsh@nus.edu.sg
First published on 27th November 2017
Abstract
We report the first stereoconvergent Markovnikov 1,2-hydrosilylation of conjugated dienes using catalysts generated from bench-stable Co(acac)2 and phosphine ligands. A wide range of E/Z-dienes underwent this Markovnikov 1,2-hydrosilylation in a stereoconvergent manner, affording (E)-allylsilanes in high isolated yields with high stereoselectivities (E/Z = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and high regioselectivities (b/l up to > 99
1) and high regioselectivities (b/l up to > 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Mechanistic studies revealed that this stereoconvergence stems from a σ–π–σ isomerization of an allylcobalt species generated by the 1,4-hydrometalation of Z-dienes. In addition, a cobalt catalyst that can only catalyze the hydrosilylation of the E-isomer of an (E/Z)-diene was identified, which allows the separation of the (Z)-isomer from an isomeric mixture of (E/Z)-dienes. Furthermore, asymmetric hydrosilylation of (E)-1-aryl-1,3-dienes was studied with Co(acac)2/(R)-difluorphos and good enantioselectivities (er up to 90
1). Mechanistic studies revealed that this stereoconvergence stems from a σ–π–σ isomerization of an allylcobalt species generated by the 1,4-hydrometalation of Z-dienes. In addition, a cobalt catalyst that can only catalyze the hydrosilylation of the E-isomer of an (E/Z)-diene was identified, which allows the separation of the (Z)-isomer from an isomeric mixture of (E/Z)-dienes. Furthermore, asymmetric hydrosilylation of (E)-1-aryl-1,3-dienes was studied with Co(acac)2/(R)-difluorphos and good enantioselectivities (er up to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were obtained.
10) were obtained.
Introduction
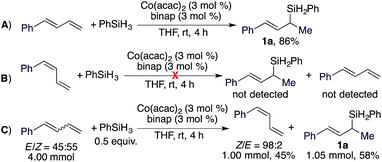
Allylsilanes are synthetically valuable building blocks due to their non-toxicity, high stability and versatile applications in organic synthesis and material science.1 Among the methods for allylsilane synthesis,2 transition metal-catalyzed hydrosilylation of conjugated dienes is the most straightforward approach to prepare these synthetically valuable allylsilanes.3 Recently, the hydrosilylation of alkenes4 and alkynes5 has been extensively studied with cobalt catalysts,6 particularly due to their higher abundance and lower toxicity compared to platinum catalysts for hydrosilylation reactions.7 More importantly, recent studies indicate that cobalt catalysts can offer a precise control of regio- and stereoselectivities.5 However, highly selective cobalt catalysts for the hydrosilylation of conjugated dienes still remain rare and are under development.3c,8Conjugated dienes can undergo 1,4- and 1,2-hydrosilylation and the selectivity is dependent on the catalyst employed. The majority of the catalysts based on Fe, Co, and Ni show high selectivity towards 1,4-hydrosilylation (Scheme 1A).3 For example, Hilt reported a highly selective cobalt catalyst for the 1,4-hydrosilylation of isoprene in the presence of P(n-Bu)3,3c and Ritter reported a well-defined Fe(0) complex ligated by 2-iminopyridines for the 1,4-hydrosilylation of 1,3-dienes.3d In contrast, the 1,2-hydrosilylation of conjugated dienes has been barely studied, and two catalysts based on Pt and Co have been used to catalyze this 1,2-hydrosilylation.8,9 In addition, these two catalysts show high selectivity towards anti-Markovnikov hydrosilylation (Scheme 1B). However, transition metal catalysts for Markovnikov 1,2-hydrosilylation of conjugated dienes, especially for stereoconvergent Markovnikov 1,2-hydrosilylation of E/Z-dienes, still remain unknown. Driven by our research interest in developing base metal catalysts for transformations of unsaturated organic molecules,4f,5f,10 herein we report the highly selective cobalt-catalyzed stereoconvergent Markovnikov 1,2-hydrosilylation of a wide range of functionalized conjugated (E/Z)-dienes (Scheme 1C). In addition, we also identified a cobalt catalyst that selectively catalyzes the hydrosilylation of the (E)-isomer of an (E/Z)-diene with the (Z)-isomer unreacted. This discovery would represent a convenient protocol to purify (Z)-dienes from (E/Z)-isomeric dienes, which are generally more accessible than stereodefined (Z)- or (E)-dienes.
Results and discussion
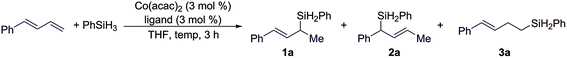
We chose the reaction of (E)-1-phenyl-1,3-butadiene with PhSiH3 to evaluate the reaction conditions for this Co-catalyzed hydrosilylation of conjugated dienes. We tested this reaction with cobalt catalysts generated from Co(acac)2 and various nitrogen- and phosphine-based ligands. The selected examples are summarized in Table 1. In general, these reactions were conducted with 3 mol% of Co(acac)2 and 3 mol% ligand at 50 °C for 3 h.| Entry | Ligand | Temperature | Conversionb (%) | Yield of 1ab (%) | 1a/2a/3ab |
|---|---|---|---|---|---|
a Conditions: (E)-diene (0.400 mmol), PhSiH3 (0.500 mmol), Co(acac)2 (12.0 μmol), ligand (12.0 μmol), and THF (1 mL) for 3 h.
b The conversion of diene, yield of 1a, and the ratio of products 1a, 2a, and 3a were determined by GC analysis with tridecane as the internal standard.
c Catalyst (1 mol%).
d A mixture of (E/Z)-1-phenyl-1,3-butadiene (E/Z = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55) was used. 55) was used.
|
|||||
| 1 | PhPDI | 50 °C | >99 | 27 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
| 2 | TFAPDI | 50 °C | >99 | 33 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
| 3 | PyBox | 50 °C | >99 | 49 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| 4 | dppm | 50 °C | 28 | 23 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 5 | dppe | 50 °C | 78 | 77 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 6 | dppbz | 50 °C | 9 | 5 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 7 | binap | 50 °C | 93 | 76 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 8 | xantphos | 50 °C | >99 | 81 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 9c | xantphos | rt | >99 | 87 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — —![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 10c,d | xantphos | rt | >99 | 83 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
|
|||||
Cobalt catalysts generated from the combination of Co(acac)2 and nitrogen-based ligands, such as PhPDI, TFADPI or PyBox, did catalyze the 1,2-hydrosilylation of (E)-1-phenyl-1,3-butadiene to full conversions, but these reactions produced a mixture of products 1a and 3a with low selectivities (entries 1–3 in Table 1).8 The reactions conducted with a combination of Co(acac)2 and bisphosphine ligands, such as dppm, dppe, or dppbz, proceeded to low to modest conversions, but with complete selectivities (>99%) for Markovnikov 1,2-hydrosilylation (entries 4–6 in Table 1). In particular, the reactions catalyzed by the combination of Co(acac)2 and binap or xantphos proceeded to high or full conversions with excellent selectivities to branched allylsilane 1a (entries 7 and 8 in Table 1). As dienes are thermally less stable and can undergo polymerization, we tested this hydrosilylation at lower temperatures. The reaction conducted with 1 mol% of Co(acac)2 and 1 mol% of xantphos at room temperature proceeded to full conversion and afforded the desired allylsilane 1a in an increased yield with an excellent Markovnikov selectivity (entry 9 in Table 1).
As (E/Z)-isomeric mixtures of dienes are synthetically more accessible than stereodefined (E)- or (Z)-dienes, we tested this hydrosilylation with a mixture of (E/Z)-isomeric 1-phenyl-1,3-butadiene to check the stereoconvergency for this reaction. To our delight, both (Z)- and (E)-1-phenyl-1,3-dienes underwent this cobalt-catalyzed Markovnikov 1,2-hydrosilylation and produced branched allylsilane 1a in high yield with high selectivity (entry 10 in Table 1). Different from the hydrosilylation of (E)-1-phenyl-1,3-diene (entry 9 in Table 1), the reaction with a mixture of (E/Z)-1-phenyl-1,3-butadienes also generated a detectable amount (4%) of branched allylsilane 2a, a product resulting from the 1,4-hydrosilylation of a diene.
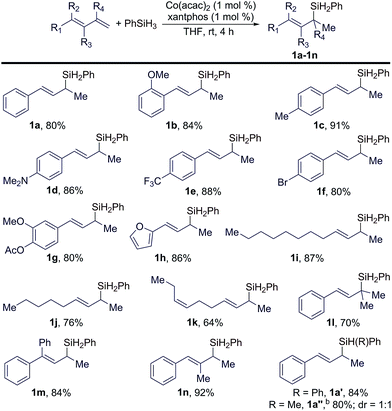
Under the identified conditions (entry 9 in Table 1), we studied the scope of conjugated trans-dienes for this reaction. These results are summarized in Table 2. In general, a wide range of conjugated trans-dienes reacted smoothly with PhSiH3 in the presence of 1 mol% of Co(acac)2 and xantphos at room temperature, affording the corresponding (E)-allylsilanes (1a–1n in Table 2) in high yields (64–92%) with excellent regioselectivities (b/l = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The scope of these trans-dienes encompassed aryl-substituted (1a–1h in Table 2), alkyl-substituted (1i–1k in Table 2), and multiple-substituted dienes (1a–1h in Table 2). The GC-MS analysis of the crude mixtures of these reactions revealed that organosilane products from either 1,4-hydrosilylation or anti-Markovnikov 1,2-hydrosilylation of these trans-dienes were not formed during the Co-catalyzed Markovnikov 1,2-hydrosilylation. In addition, we also tested this hydrosilylation with secondary hydrosilanes (Ph2SiH2 and PhMeSiH2), and these reactions proceeded smoothly to afford tertiary cinnamylsilanes (1a′ and 1a′′ in Table 2) in high isolated yields. However, this Co-catalyzed hydrosilylation did not occur with dialkylsilane (Et2SiH2) or tertiary hydrosilanes, such as (EtO)3SiH and (EtO)2MeSiH.
1). The scope of these trans-dienes encompassed aryl-substituted (1a–1h in Table 2), alkyl-substituted (1i–1k in Table 2), and multiple-substituted dienes (1a–1h in Table 2). The GC-MS analysis of the crude mixtures of these reactions revealed that organosilane products from either 1,4-hydrosilylation or anti-Markovnikov 1,2-hydrosilylation of these trans-dienes were not formed during the Co-catalyzed Markovnikov 1,2-hydrosilylation. In addition, we also tested this hydrosilylation with secondary hydrosilanes (Ph2SiH2 and PhMeSiH2), and these reactions proceeded smoothly to afford tertiary cinnamylsilanes (1a′ and 1a′′ in Table 2) in high isolated yields. However, this Co-catalyzed hydrosilylation did not occur with dialkylsilane (Et2SiH2) or tertiary hydrosilanes, such as (EtO)3SiH and (EtO)2MeSiH.
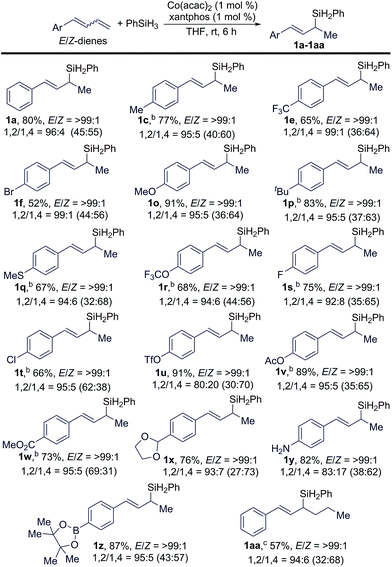
Subsequently, we studied the scope of conjugated dienes containing a mixture of (E/Z)-isomeric 1,3-dienes for this hydrosilylation reaction, and the results are summarized in Table 3. Generally, a wide range of (E/Z)-dienes, with E/Z ratios given in the brackets in Table 3, underwent this Markovnikov 1,2-hydrosilylation in a stereoconvergent manner with full conversions, affording the corresponding (E)-allylsilanes (1a–1z in Table 3) in high isolated yields with high regio- and stereoselectivities (b/l = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; E/Z = >99
1; E/Z = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The GC-MS analysis of the crude reaction mixtures indicated that these reactions also produced small amounts of 1,4-hydrosilylation products, and the ratios of the products from 1,2- and 1,4-hydrosilylation are listed in Table 3 and abbreviated as 1,2/1,4 ratios.
1). The GC-MS analysis of the crude reaction mixtures indicated that these reactions also produced small amounts of 1,4-hydrosilylation products, and the ratios of the products from 1,2- and 1,4-hydrosilylation are listed in Table 3 and abbreviated as 1,2/1,4 ratios.
| a Conditions: (E/Z)-diene (0.400 mmol), PhSiH3 (0.500 mmol), Co(acac)2 (4.0 μmol), xantphos (4.0 μmol), and THF (1 mL) at rt for 6 h; yield of isolated product; 1,2/1,4 ratios refer to 1,2-/1,4-hydrosilylation, the ratios in brackets are the E/Z ratios of the conjugated butadiene reagents. b Reactions were conducted at 5 °C for 24 h. c 3 mol% catalyst at rt for 48 h. |
|---|
|
The data in Table 3 indicate that the electronic properties of the aryl substituents do not have a significant influence on the stereoconvergence and regioselectivity of these hydrosilylation reactions (e.g.1a, 1e, and 1o in Table 3). This Co-catalyzed transformation can tolerate various reactive groups, such as trifluoromethyl ether (1r), halogens (1f, 1s, and 1t), triflate (1u), ester (1v and 1w), acetal (1x), unprotected primary aniline (1y), and pinacol boronic ester (1z). In addition, ((1E/Z,3E)-hexa-1,3-dien-1-yl)benzene, an internal 1,3-diene, also underwent this stereoconvergent hydrosilylation to afford allylsilane 1aa in high isolated yield with high stereoselectivity (E/Z = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
As both the Co(acac)2 and xantphos used for this hydrosilylation reaction are bench-stable, we tested the hydrosilylation of 1-(buta-1,3-dien-1-yl)-4-methoxybenzene with PhSiH3 on a 10 mmol scale with 1 mol% of Co(acac)2/xantphos weighed on the benchtop without using a dry box. This reaction proceeded to the full conversion of the diene substrate and afforded 1o in 87% isolated yield (eqn (1)).
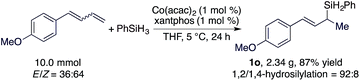 | (1) |
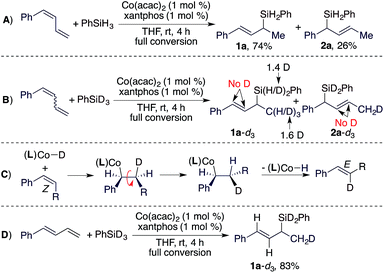
To understand the stereoconvergence of this hydrosilylation of (E/Z)-dienes, we analyzed the reaction of (E/Z)-1-phenyl-1,3-butadiene and found that the (E)-isomer was consumed at a significantly higher rate than the (Z)-isomer.11 In addition, the hydrosilylation of (Z)-1-phenyl-1,3-butadiene was studied, and this reaction afforded the 1,2-hydrosilylation product 1a together with a significant amount of the 1,4-hydrosilylation product 2a (1a:2a = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26, Scheme 2A). The results of this reaction and the reactions of the E-isomer (entry 9 in Table 1) and the mixture of the (E/Z)-isomers (entry 10 in Table 1) suggest that allylsilane 2a was formed by 1,4-hydrosilylation of the (Z)-isomer. To provide insight into the isomerization of the internal Z-alkene in the diene to the E-alkene in product 1a, we subsequently conducted a deuterium-labelling experiment using the (E/Z)-isomers and PhSiD3 (Scheme 2B) and found that deuterium was solely incorporated into the methyl groups of 1a and 2a. This lack of deuterium incorporation onto the internal vinylic carbons suggests that this E/Z-isomerization through migratory insertion of the Z-alkene into a Co–H species followed by β-H elimination,12 as indicated in Scheme 2C, is unlikely. Furthermore, we also tested the reaction of the (E)-isomer with PhSiD3 and the same deuterium incorporation was observed (Scheme 2D).
26, Scheme 2A). The results of this reaction and the reactions of the E-isomer (entry 9 in Table 1) and the mixture of the (E/Z)-isomers (entry 10 in Table 1) suggest that allylsilane 2a was formed by 1,4-hydrosilylation of the (Z)-isomer. To provide insight into the isomerization of the internal Z-alkene in the diene to the E-alkene in product 1a, we subsequently conducted a deuterium-labelling experiment using the (E/Z)-isomers and PhSiD3 (Scheme 2B) and found that deuterium was solely incorporated into the methyl groups of 1a and 2a. This lack of deuterium incorporation onto the internal vinylic carbons suggests that this E/Z-isomerization through migratory insertion of the Z-alkene into a Co–H species followed by β-H elimination,12 as indicated in Scheme 2C, is unlikely. Furthermore, we also tested the reaction of the (E)-isomer with PhSiD3 and the same deuterium incorporation was observed (Scheme 2D).
Based on the results of the experiments in Scheme 2 and the precedent of Co-catalyzed hydrosilylation of alkenes,4f,6 we proposed a hydrometalation pathway with a Co(I)–H intermediate for this Co-catalyzed Markovnikov hydrosilylation of conjugated dienes (Scheme 3). 2,1-Migratory insertion of the (E)-diene into a Co–H species forms an allylcobalt intermediate I, which turns over with PhSiH3 to release the allylsilane product and regenerate the Co–H species.
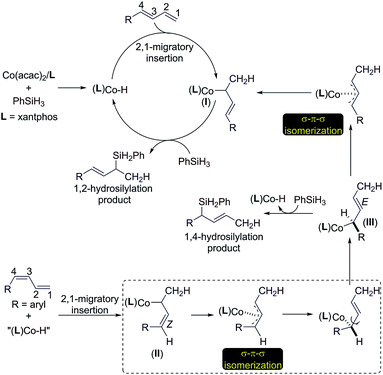 | ||
| Scheme 3 Proposed catalytic pathways for the Co-catalyzed stereoconvergent Markovnikov hydrosilylation of dienes. | ||
For the hydrosilylation of (Z)-dienes, 2,1-migratory insertion of (Z)-dienes occurs to generate an allylcobalt species II, which undergoes σ–π–σ isomerization to form the allylcobalt intermediate III.13 This allylcobalt species reacts with PhSiH3 to give a 1,4-hydrosilylation product. In addition, the allylcobalt species III also undergoes σ–π–σ isomerization to generate the allylcobalt intermediate I, and this explains the observed stereoconvergency of the (Z/E)-diene hydrosilylation. Both allylcobalt species I and III can react with PhSiH3 to generate allylsilane products and the difference in the sterics around the Co–C bonds in these two allylcobalt species may account for the product ratio observed for the reaction listed in Scheme 2A.
After developing this stereoconvergent hydrosilylation reaction, we rationalized that the separation of a (Z)-diene from Z/E-diene mixtures could be achieved if we could identify a cobalt catalyst that can convert only the (E)-isomer of dienes. We tested various cobalt catalysts generated from the combination of Co(acac)2 and bisphosphine ligands for this purpose. To our delight, we found that the cobalt complex from Co(acac)2/binap was active for Markovnikov 1,2-hydrosilylation of (E)-1-phenyl-1,3-diene (Scheme 4A) but did not catalyze the hydrosilylation or the isomerization of (Z)-1-phenyl-1,3-diene (Scheme 4B). Then, we conducted this hydrosilylation reaction with a E/Z-mixture of 1-phenyl-1,3-diene (Scheme 4C). As expected, this reaction afforded (E)-allylsilane 1a in 58% isolated yield and (Z)-1-phenyl-1,3-diene was recovered in 45% isolated yield with a Z/E ratio of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
We subsequently studied cobalt catalysts generated from Co(acac)2 and chiral bisphosphine ligands in order to develop the asymmetric Markovnikov 1,2-hydrosilylation of conjugated dienes.14 After evaluating various chiral phosphine ligands (see ESI† for details), we found that the hydrosilylation of trans-diene with PhSiH3 conducted with Co(acac)2 and (R)-difluorphos proceeded smoothly to afford the corresponding allylsilanes in good yield and good er (eqn (2)).
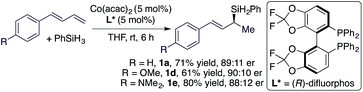 | (2) |
Conclusions
In summary, we have developed the first Co-catalyzed Markovnikov 1,2-hydrosilylation of conjugated dienes with a catalyst generated from Co(acac)2 and xantphos. A broad scope of trans-dienes underwent this Markovnikov hydrosilylation to afford (E)-allylsilanes in high isolated yields and with excellent regioselectivities (b/l = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In addition, (E/Z)-isomeric 1,3-dienes reacted in a stereoconvergent manner to form (E)-allylsilanes with good to excellent regioselectivity (ratios of 1,2/1,4-hydrosilylation up to 99
1). In addition, (E/Z)-isomeric 1,3-dienes reacted in a stereoconvergent manner to form (E)-allylsilanes with good to excellent regioselectivity (ratios of 1,2/1,4-hydrosilylation up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This stereoconvergence resulted from a σ–π–σ isomerization of the allylcobalt intermediate. In particular, we also identified a cobalt catalyst, Co(acac)2/binap, for selectively converting the (E)-isomer of a mixture of (E/Z)-isomers, and this allows the separation of (Z)-dienes from a mixture of (E/Z)-dienes.
1). This stereoconvergence resulted from a σ–π–σ isomerization of the allylcobalt intermediate. In particular, we also identified a cobalt catalyst, Co(acac)2/binap, for selectively converting the (E)-isomer of a mixture of (E/Z)-isomers, and this allows the separation of (Z)-dienes from a mixture of (E/Z)-dienes.
Experimental details
General procedures for stereoconvergent hydrosilylation of (E/Z)-dienes
In an Ar-filled glovebox, a mixture of Co(acac)2 (4.0 μmol) and xantphos (4.0 μmol) in THF (1 mL) was added into a 4 mL screw-capped vial containing a magnetic stirring bar. The resulting mixture was stirred for 2 min prior to adding phenylsilane (0.500 mmol) and (E/Z)-1,3-dienes (0.400 mmol) successively. The vial was removed from the glove box, and the mixture was stirred at room temperature for 6 hours. After that, the crude reaction mixture was concentrated under vacuum and the residue was purified by flash column chromatography using a mixture of ethyl acetate and hexane as an eluent. The conditions for the flash chromatography and the data for the characterization of the products are listed in the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National University of Singapore (R-143-000-614-133) and a grant from the NUS Young Investigator Award (R-143-000-630-133).Notes and references
- (a) Hydrosilylation: A Comprehensive Review on Recent Advances, ed. B. Marciniec, Springer, Berlin, 2009 Search PubMed; (b) Y. Nakajima and S. Shimada, RSC Adv., 2015, 5, 20603 RSC.
- For recent examples of allylsilane syntheses, see: (a) R. Moser, T. Nishikata and B. H. Lipshutz, Org. Lett., 2010, 12, 28 CrossRef CAS PubMed; (b) N. Selander, J. R. Paasch and K. Szabó, J. Am. Chem. Soc., 2011, 133, 409 CrossRef CAS PubMed; (c) H. Ito, Y. Horita and M. Sawamura, Adv. Synth. Catal., 2012, 354, 813 CrossRef CAS; (d) Z. D. Miller, W. Li, T. R. Belderrain and J. Montgomery, J. Am. Chem. Soc., 2013, 135, 15282 CrossRef CAS PubMed; (e) J. R. McAtee, G. P. A. Yap and D. A. Watson, J. Am. Chem. Soc., 2014, 136, 10166 CrossRef CAS PubMed; (f) S. Asako, S. Ishikawa and K. Takai, ACS Catal., 2016, 6, 3387 CrossRef CAS.
- (a) M. F. Lappert, T. A. Nile and S. J. Takahashi, Organomet. Chem., 1974, 72, 425 CrossRef CAS; (b) A. J. Cornish, M. F. Lappert and T. A. Nile, J. Organomet. Chem., 1977, 136, 73 CrossRef CAS; (c) G. Hilt, S. Lüers and F. Schmidt, Synthesis, 2004, 2004, 634 CrossRef; (d) J. Y. Wu, B. N. Stanzl and T. Ritter, J. Am. Chem. Soc., 2010, 132, 13214 CrossRef CAS PubMed.
- For recent examples: (a) Z. Mo, Y. Liu and L. Deng, Angew. Chem., Int. Ed., 2013, 52, 10845 CrossRef CAS PubMed; (b) C. Chen, M. B. Hecht, A. Kavara, W. W. Brennessel, B. Q. Mercado, D. J. Weix and P. L. Holland, J. Am. Chem. Soc., 2015, 137, 13244 CrossRef CAS PubMed; (c) A. D. Ibrahim, S. W. Entsminger, L. Zhu and A. R. Fout, ACS Catal., 2016, 6, 3589 CrossRef CAS; (d) D. Noda, A. Tahara, Y. Sunada and H. Nagashima, J. Am. Chem. Soc., 2016, 138, 2480 CrossRef CAS PubMed; (e) C. H. Schuster, T. Diao, I. Pappas and P. J. Chirik, ACS Catal., 2016, 6, 2632 CrossRef CAS; (f) C. Wang, W. J. Teo and S. Ge, ACS Catal., 2017, 7, 855 CrossRef CAS; (g) Y. Liu and L. Deng, J. Am. Chem. Soc., 2017, 139, 1798 CrossRef CAS PubMed; (h) J. H. Docherty, J. Peng, A. P. Dominey and S. P. Thomas, Nat. Chem., 2017, 9, 595 CrossRef CAS PubMed.
- (a) Z. Mo, J. Xiao, Y. Gao and L. Deng, J. Am. Chem. Soc., 2014, 136, 17414 CrossRef CAS PubMed; (b) K. H. Huang and M. Isobe, Eur. J. Org. Chem., 2014, 2014, 4733 CrossRef CAS; (c) J. Guo and Z. Lu, Angew. Chem., Int. Ed., 2016, 55, 10835 CrossRef CAS PubMed; (d) Z. Zuo, J. Yang and Z. Huang, Angew. Chem., Int. Ed., 2016, 55, 10839 CrossRef CAS PubMed; (e) A. Rivera-Hernández, B. J. Fallon, S. Ventre, C. Simon, M. H. Tremblay, G. Gontard, E. Derat, M. Amatore, C. Aubert and M. Petit, Org. Lett., 2016, 18, 4242 CrossRef PubMed; (f) W. J. Teo, C. Wang, Y. W. Tan and S. Ge, Angew. Chem., Int. Ed., 2017, 56, 4328 CrossRef CAS PubMed; (g) J. Guo, X. Shen and Z. Lu, Angew. Chem., Int. Ed., 2017, 56, 615 CrossRef CAS PubMed.
- For recent reviews: (a) J. Sun and L. Deng, ACS Catal., 2016, 6, 290 CrossRef CAS; (b) X. Du and Z. Huang, ACS Catal., 2017, 7, 1227 CrossRef CAS.
- (a) B. D. Karstedt, Platinum Complexes of Unsaturated Siloxanes and Platinum Containing Organopolysiloxanes, US Pat., 3775452A, 1973; (b) L. N. Lewis, J. Stein, Y. Gao, R. E. Colborn and G. Hutchins, Platinum Met. Rev., 1997, 41, 66 CAS; (c) I. E. Markó, S. Stérin, O. Buisine, G. Mignani, P. Branlard, B. Tinant and J.-P. Declercq, Science, 2002, 298, 204 CrossRef PubMed; (d) I. E. Markó, S. Stérin, O. Buisine, G. Berthon, G. Michaud, B. Tinant and J. P. Declercq, Adv. Synth. Catal., 2004, 346, 1429 CrossRef; (e) B. Marciniec, K. Posała, I. Kownacki, M. Kubicki and R. Taylor, ChemCatChem, 2012, 4, 1935 CrossRef CAS; (f) J. C. Bernhammer and H. V. Huynh, Organometallics, 2014, 33, 172 CrossRef CAS.
- B. Raya, S. Jing, V. Balasanthiran and T. V. RajanBabu, ACS Catal., 2017, 7, 2275 CrossRef CAS PubMed.
- S. E. Parker, J. Börgel and T. Ritter, J. Am. Chem. Soc., 2014, 136, 4857 CrossRef CAS PubMed.
- (a) C. Wang, C. Wu and S. Ge, ACS Catal., 2016, 6, 7585 CrossRef CAS; (b) S. Yu, C. Wu and S. Ge, J. Am. Chem. Soc., 2017, 139, 6526 CrossRef CAS PubMed.
- A similar difference in the reaction rates for (E/Z)-isomers of dienes was observed in a Co-catalyzed vinylation of conjugated dienes, see: Y. N. Timsina, S. Biswas and T. V. RajanBabu, J. Am. Chem. Soc., 2014, 136, 6215 CrossRef CAS PubMed.
- For examples of Co-catalyzed Z/E-isomerization of alkenyl compounds, see: (a) C. Chen, T. R. Dugan, W. W. Brennessel, D. J. Weix and P. J. Holland, J. Am. Chem. Soc., 2014, 136, 945 CrossRef CAS PubMed; (b) S. Fu, N. Y. Chen, X. Liu, Z. Shao, S. P. Luo and Q. Liu, J. Am. Chem. Soc., 2016, 138, 8588 CrossRef CAS PubMed.
- (a) J. Kwiatek and J. K. Seyler, J. Organomet. Chem., 1965, 3, 421 CrossRef CAS; (b) M. G. Burnett, P. J. Connolly and C. Kemball, J. Chem. Soc. A, 1968, 991 RSC; (c) I. Ojima and M. Kumagai, J. Organomet. Chem., 1978, 157, 359 CrossRef CAS; (d) R. K. Sharma and T. V. RajanBabu, J. Am. Chem. Soc., 2010, 132, 3295 CrossRef CAS PubMed.
- Asymmetric Markovnikov 1,2-hydrosilylation of conjugated dienes was not reported previously. For examples of asymmetric 1,4-hydrosilylation of conjugated dienes, see: (a) T. Hayashi, Y. Matsumoto, I. Morikawa and Y. Ito, Tetrahedron: Asymmetry, 1990, 1, 151 CrossRef CAS; (b) H. Ohmura, H. Matsuhashi, M. Tanaka, M. Kuroboshi, T. Hiyama, Y. Hatanaka and K. I. Goda, J. Organomet. Chem., 1995, 499, 167 CrossRef CAS; (c) J. W. Han and T. Hayashi, Tetrahedron: Asymmetry, 2010, 21, 2193 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc04002d |
| This journal is © The Royal Society of Chemistry 2018 |