A novel alkaline phosphatase assay based on the specific chromogenic interaction between Fe3+ and ascorbic acid 2-phosphate†
Linjie
Wang
a,
Kun
Ye
a,
Jianming
Pan
*a,
Hongwei
Song
b and
Xiangheng
Niu
 *a
*a
aInstitute of Green Chemistry and Chemical Technology, School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: pjm@ujs.edu.cn; niuxiangheng@126.com
bSchool of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, China
First published on 22nd April 2019
Abstract
In this work, a new colorimetric strategy based on the specific chromogenic interaction between Fe3+ and ascorbic acid 2-phosphate was proposed for the highly selective, repeatable, and accurate detection of alkaline phosphatase with extremely simple operation and low cost.
Alkaline phosphatase (ALP), which exists in many human tissues and organs, is an enzyme that can catalyze the dephosphorylation of nucleic acids, proteins, and small molecules. In addition to the dephosphorylation function, it also plays an important role in regulating various cellular processes.1 Considerable studies have demonstrated that the abnormality of ALP activity is closely associated with a variety of diseases, such as bone disease,2 liver dysfunction,3 diabetes,4 and breast and prostate cancers.5,6 In this regard, it has become an important biomarker for the diagnosis and treatment of these diseases. Thus, it is of great significance to develop effective methods for ALP clinical analysis.
So far, there have been a lot of methods, including fluorometric,7–12 colorimetric,13–20 Raman,21 and electrochemical22,23 approaches, explored for the determination of ALP based on its ability to remove the phosphate group from various substrates to get signal readouts. Among these methods, colorimetric analysis has attracted special attention due to its inherent advantage of direct visual readout. Currently, the p-nitrophenyl phosphate (p-NPP) method, which is based on the hydrolysis of colorless p-NPP to yellow p-nitrophenol catalyzed by ALP, is usually employed to detect ALP in clinical diagnosis. Although this approach is simple, the p-NPP substrate is too sensitive to light, and it can also be hydrolyzed in the absence of the enzyme, inevitably resulting in some false positive results. To avoid the problem, several attractive strategies have been proposed for ALP sensing in the past few years.13–15,19,20,24–30 Even so, most of these proposed methods still have some intrinsic deficiencies in terms of repeatability, anti-interference, detection cost, and convenience of operation. It still requires effort to develop novel principles and approaches with good practicability for ALP detection.
Herein, a novel strategy based on the specific chromogenic interaction between Fe3+ and ascorbic acid 2-phosphate (AAP) is proposed for the colorimetric determination of ALP. As illustrated in Scheme 1, Fe3+ is able to specifically coordinate with the colorless AAP substrate to form a brown Fe3+–AAP complex. When ALP is added, it can hydrolyze the AAP substrate to ascorbic acid (AA) and phosphate ion (Pi), both of which cannot lead to any chromogenic reactions with Fe3+. Thus, the presence of ALP hinders the formation of the brown Fe3+–AAP complex. With this new principle, colorimetric detection of ALP with high sensitivity and excellent selectivity was achieved. Due to the prominent stability of the Fe3+–AAP complex formed, the developed ALP assay could provide outstanding repeatability. Accurate detection of the target in human serum samples was also validated, revealing our assay as a promising tool for ALP clinical analysis.
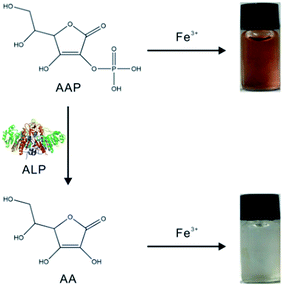 | ||
| Scheme 1 Illustration of the ALP detection strategy based on the specific chromogenic reaction of Fe3+ and AAP. | ||
In fact, there are lots of phosphorylated molecules that can act as the substrate of ALP. As a commonly used substrate, AAP can be hydrolyzed by ALP to the corresponding product AA. The strong reducibility of the product has been widely utilized for ALP sensing.19,27,30–33 Besides, the unique cis-diol structure of AA can also be employed for the determination of ALP.34,35 In this work, we proposed a new ALP sensing strategy based on the specific reaction of AAP and Fe3+. As depicted in Fig. 1A, when Fe3+ with a low concentration (1.33 mM) is employed, it provides a negligible color background. The AAP substrate also shows no notable color. However, when the two species are mixed together (see the Experimental section in ESI†), it is interestingly found that a chromogenic reaction occurs rapidly, resulting in a remarkable UV-vis absorption peak at around 472 nm. Further, the effect of the Fe3+ concentration on the chromogenic reaction was investigated. As depicted in Fig. S1 (ESI),† by controlling the AAP level at 0.33 mM, when the concentration of Fe3+ reached to 0.67 mM, the readout has no change any more, indicating that Fe3+ and AAP have reacted completely. Considering the possible loss of Fe3+ during reaction and the color background provided by Fe3+, an excessive concentration of Fe3+ (1.33 mM) was used in the following study. As a matter of fact, it is not surprising that the AAP substrate can react with Fe3+, because previous studies have indicated that some metal ions (Fe3+, Cu2+, Ce3+, etc.) can coordinate with phosphorylated molecules including pyrophosphate36–39 and monophosphate.40,41 To explore whether other metal ions can react with AAP to produce color changes, several common metal ions including Cu2+ and Ce3+ were tested. As depicted in Fig. S2 (ESI),† neither color changes nor specific UV absorption peaks are observed, indicating that only Fe3+ can induce a remarkable chromogenic reaction with the AAP species. According to previous studies,40,41 it is reasonably speculated that the phosphate group in AAP can coordinate with Fe3+ to form a brown Fe3+–AAP complex (Fig. 1B). Considering that ALP will hydrolyze the AAP substrate to AA and Pi, we also incubated the two products with Fe3+, respectively. It is found that the two products cannot trigger any chromogenic reactions as that observed in the AAP + Fe3+ system (Fig. 1A). This result reveals that Fe3+ can well distinguish the AAP substrate from its corresponding products AA and Pi, providing the crucial precondition for the following ALP colorimetric assay.
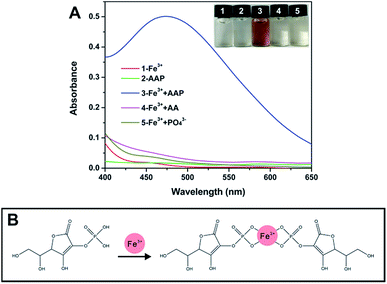 | ||
| Fig. 1 (A) shows the UV-vis spectra of different systems, and the inset exhibits the corresponding photograph. (B) illustrates the possible interaction between AAP and Fe3+. | ||
In addition, the stability of the formed Fe3+–AAP complex under different conditions was investigated. To check the durability of the brown complex over time, we measured its UV-vis spectra at a 2 h interval within 12 h. As depicted in Fig. S3 (ESI),† less than ±1% variations of the absorbance are observed, indicating that the Fe3+–AAP complex is very stable upon time. When it is incubated at different temperatures, the UV-vis absorbance also exhibits no notable changes (Fig. S4, ESI†), demonstrating that the complex is not sensitive to temperature. The outstanding stability will be quite beneficial for ALP analysis in practical conditions.
To optimize the chromogenic reaction of AAP and Fe3+ for the following ALP detection, we further observed the reaction occurring in buffers with different pH values. As depicted in Fig. S5 (ESI),† with the buffer pH increases, the color of the mixture seems to be deeper, while the absorption peak turns to be unidentifiable gradually. When alkaline buffers are employed, no color is observed in the mixture of AAP and Fe3+. As a result, the buffer with pH 3 was selected to be used in the following ALP assay.
It is widely known that ALP can hydrolyze the AAP substrate to AA and Pi. Given that AAP is able to trigger a remarkable chromogenic reaction with Fe3+ while AA and Pi cannot, the above finding may work for the colorimetric sensing of ALP. To exclude the possible interaction between Fe3+ and ALP, we mixed the two species together and recorded the UV-vis spectrum. As verified by Fig. 2A, no notable absorption signal is observed in the system, indicating that the Fe3+ species cannot interact with ALP. As expected, the mixing of AAP and Fe3+ results in a remarkable chromogenic reaction. When a certain amount of ALP is added, the enzyme hydrolyzes the AAP substrate, leading to an obvious absorbance decrease of the mixture of AAP and Fe3+. This contrast makes it possible for the colorimetric detection of ALP. Besides, the chromogenic interaction between AAP and Fe3+ can only be hindered by ALP. When other biological species that possibly coexists with ALP in clinical fluids are added to the mixture of AAP and Fe3+, no UV-vis changes are found compared with the blank group (Fig. 2B). These results reveal that the above principle indeed works for the selective detection of ALP.
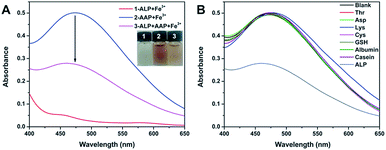 | ||
| Fig. 2 (A) compares the UV-vis spectra of different combinations of Fe3+, AAP, and ALP. (B) records the UV-vis response of the AAP + Fe3+ system with the presence of various biological species. | ||
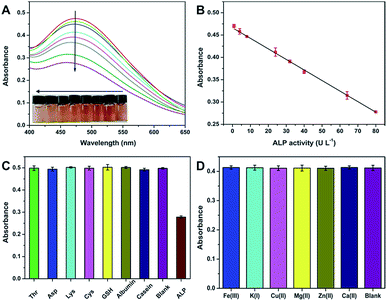
With the above principle, we developed a simple but effective colorimetric assay for ALP analysis. As expected, when increasing ALP is added to the AAP + Fe3+ system, the UV-vis absorption signal decreases correspondingly (Fig. 3A). It is further found that the absorbance at 472 nm is well linear to the ALP activity ranging from 0.8–80 U L−1 (Fig. 3B). The corresponding fitting equation can be described as Abs = 0.4663 − 0.0024[ALP] (U L−1) with a correlation coefficient of 0.9989. According to the signal-to-noise of three (S/N = 3) rule, the limit of detection (LOD) is calculated to be as low as 0.66 U L−1. Given that the ALP activity in normal human serum is in the range of 40–190 U L−1, the sensitivity, linear range, and LOD of our assay are enough for the analysis of the target in blood samples. In comparison with previously reported strategies for ALP sensing, the analytical performance of our method is also comparable in terms of linear range and LOD (Table S1†). More attractively, our strategy exhibits the following merits: (1) compared with fluorescent sensors, the developed colorimetric assay is able to provide comparable performance but only requires simpler equipment. With the inherent superiority of direct visual readout, the colorimetric signal can be read by a smartphone or even naked eyes for quantitative detection;42,43 (2) all the sensing elements required in our strategy can be directly gained from commercial chemicals and reagents, and no synthetic nanomaterials are necessary, not only simplifying the operation but also reducing the detection cost; (3) since the Fe3+–AAP complex formed is quite stable over time and temperature, our method is able to offer prominent repeatability, giving a relative standard deviation (RSD) of only 0.7% for ten parallel measurements. These advantages will endow it with great potential in clinical applications.
According to Fig. 2B, our method can exhibit good selectivity for the colorimetric determination of ALP. To further confirm the conclusion, we tried to evaluate the possible interference from various substances that coexists with ALP in biological fluids. To this end, common species including amino acids (Thr, Asp, Lys, and Cys), polypeptides (GSH), and proteins (albumin and casein) were tested. As depicted in Fig. 3C, these coexisting substances show no notable effects on ALP detection. Since the proposed strategy is not on the basis of any redox reactions, reducing agents such as AA, GSH, and dopamine will also not affect the specific sensing of ALP. Further, possible effects of metal ions existing in serum on the detection of ALP were also studied. As depicted in Fig. 3D, these metal ions with a concentration in the normal range in human serum have almost no effect on the detection of ALP with an activity of 24 U L−1. The excellent selectivity makes the method more promising to be used for ALP analysis.
Inspired by the favorable performance of the developed ALP assay, we tried to use it to detect the target in human serum. The serum samples were diluted 5-fold with deionized water before measurement. Considering that our sensitivity, linear range, and LOD can fully meet the demands for ALP sensing, the proposed method was directly utilized to measure ALP in these samples. The detection results are listed in Table 1. It is found that our detection results are in good agreement with the clinical data obtained by the p-NPP method, providing the relative errors (RE) less than ±10%. This verifies that our method is very accurate and reliable for ALP clinical detection.
| Sample | Detected (U L−1) | Clinical data (U L−1) | RE (%) |
|---|---|---|---|
| 1# | 206.3 ± 5.7 | 204 | +1.1 |
| 2# | 60.7 ± 3.5 | 64 | −5.2 |
| 3# | 64.7 ± 1.5 | 65 | −0.5 |
| 4# | 49.3 ± 1.5 | 46 | +7.2 |
| 5# | 35.3 ± 3.2 | 39 | −9.5 |
Conclusions
In summary, we have proposed a quite simple but very effective ALP assay based on the specific chromogenic interaction between AAP and Fe3+. The method can offer favorable colorimetric readouts for ALP detection. More attractively, all the sensing elements required can be available directly from commercial chemicals and reagents, significantly simplifying the operation and reducing the detection cost. As demonstrated, our assay with high sensitivity, excellent selectivity, and outstanding repeatability can be employed to detect ALP in human serum accurately. Taken together, the developed ALP assay exhibits great promise for clinical applications. Also, the proposed strategy will inspire the development of ALP-linked immunosorbent assays.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Nos. 21605061 and 31601549), the Natural Science Foundation of Jiangsu Province (No. BK20160489), the Open Fund from the Shanghai Key Laboratory of Functional Materials Chemistry (No. SKLFMC201601), the Open Fund from the State Key Laboratory of Bioreactor University, and the Cultivation Project for Excellent Young Teachers of Jiangsu University.References
- R. B. McComb, J. G. N. Bowers and S. Posen, Alkaline Phosphatase, Springer Science & Business Media, 2013 Search PubMed.
- R. Furman, J. J. Nicholas and L. Jivoff, J. Bone Jt. Surg., 1970, 52, 1131–1137 CrossRef CAS.
- J. J. Reichling and M. M. Kaplan, Dig. Dis. Sci., 1988, 33, 1601–1614 CrossRef CAS PubMed.
- F. Lumachi, V. Camozzi, V. Tombolan and G. Luisetto, Ann. N. Y. Acad. Sci., 2009, 1173, E64–E67 CrossRef CAS PubMed.
- J. A. Lorente, H. Valenzuela, J. Morote and A. Gelabert, Eur. J. Nucl. Med., 1999, 26, 625–632 CrossRef CAS.
- A. Keshaviah, S. Dellapasqua, N. Rotmensz, J. Lindtner, D. Crivellari, J. Collins, M. Colleoni, B. Thürlimann, C. Mendiola, S. Aebi, K. N. Price, O. Pagani, E. Simoncini, M. C. Gertsch, R. D. Gelber, A. S. Coates and A. Goldhirsch, Ann. Oncol., 2007, 18, 701–708 CrossRef CAS PubMed.
- C. X. Chen, Q. Yuan, P. J. Ni, Y. Y. Jiang, Z. L. Zhao and Y. Z. Lu, Analyst, 2018, 143, 3821–3828 RSC.
- J. J. Deng, P. Yu, Y. X. Wang and L. Q. Mao, Anal. Chem., 2015, 87, 3080–3086 CrossRef CAS PubMed.
- G. L. Li, H. L. Fu, X. J. Chen, P. W. Gong, G. Chen, L. Xia, H. Wang, J. M. You and Y. N. Wu, Anal. Chem., 2016, 88, 2720–2726 CrossRef CAS PubMed.
- E. K. Lim, J. O. Keem, H. S. Yun, J. Jung and B. H. Chung, Chem. Commun., 2015, 51, 3270–3272 RSC.
- S. Y. Liu, X. Y. Wang, S. Pang, W. D. Na, X. Yan and X. G. Su, Anal. Chim. Acta, 2014, 827, 103–110 CrossRef CAS PubMed.
- C. H. Wang, G. E. Tang and H. L. Tan, J. Mater. Chem. B, 2018, 6, 7614–7620 RSC.
- J. Sun, J. H. Zhao, X. F. Bao, Q. F. Wang and X. R. Yang, Anal. Chem., 2018, 90, 6339–6345 CrossRef CAS PubMed.
- Y. Shi, M. Yang, L. Liu, Y. J. Pang, Y. J. Long and H. Z. Zheng, Sens. Actuators, B, 2018, 275, 43–49 CrossRef CAS.
- L. Y. Jin, Y. M. Dong, X. M. Wu, G. X. Cao and G. L. Wang, Anal. Chem., 2015, 87, 10429–10436 CrossRef CAS PubMed.
- C. M. Li, S. J. Zhen, J. Wang, Y. F. Li and C. Z. Huang, Biosens. Bioelectron., 2013, 43, 366–371 CrossRef CAS PubMed.
- H. P. Jiao, J. Chen, W. Y. Li, F. Y. Wang, H. P. Zhou, Y. X. Li and C. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 1979–1985 CrossRef CAS PubMed.
- J. J. Yang, L. Zheng, Y. Wang, W. Li, J. L. Zhang, J. J. Gu and Y. Fu, Biosens. Bioelectron., 2016, 77, 549–556 CrossRef CAS PubMed.
- A. Hayat, G. Bulbul and S. Andreescu, Biosens. Bioelectron., 2014, 56, 334–339 CrossRef CAS PubMed.
- Z. Q. Gao, K. C. Deng, X. D. Wang, M. Miró and D. P. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 18243–18250 CrossRef CAS PubMed.
- C. M. Ruan, W. Wang and B. H. Gu, Anal. Chem., 2006, 78, 3379–3384 CrossRef CAS PubMed.
- S. Goggins, C. Naz, B. J. Marsh and C. G. Frost, Chem. Commun., 2015, 51, 561–564 RSC.
- P. Miao, L. M. Ning, X. X. Li, Y. Q. Shu and G. X. Li, Biosens. Bioelectron., 2011, 27, 178–182 CrossRef CAS PubMed.
- Z. W. Tang, H. F. Zhang, C. B. Ma, P. Gu, G. H. Zhang, K. F. Wu, M. J. Chen and K. M. Wang, Microchim. Acta, 2018, 185, 109 CrossRef PubMed.
- C. H. Wang, J. Gao, Y. L. Cao and H. L. Tan, Anal. Chim. Acta, 2018, 1004, 74–81 CrossRef CAS PubMed.
- D. M. Shi, Y. Sun, L. Lin, C. J. Shi, G. F. Wang and X. J. Zhang, Analyst, 2016, 141, 5549–5554 RSC.
- Q. Hu, B. J. Zhou, F. Li, J. M. Kong and X. J. Zhang, Chem.–Asian J., 2016, 11, 3040–3045 CrossRef CAS PubMed.
- T. T. Wu, W. L. Hou, Z. Y. Ma, M. L. Liu, X. Y. Liu, Y. Y. Zhang and S. Z. Yao, Microchim. Acta, 2019, 186, 123 CrossRef PubMed.
- H. W. Song, H. Y. Wang, X. Li, Y. X. Peng, J. M. Pan and X. H. Niu, Anal. Chim. Acta, 2018, 1044, 154–161 CrossRef CAS PubMed.
- H. W. Song, Z. B. Li, Y. X. Peng, X. Li, X. C. Xu, J. M. Pan and X. H. Niu, Analyst, 2019, 144, 2416–2422 RSC.
- T. Xiao, J. Sun, J. H. Zhao, S. Wang, G. Y. Liu and X. R. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 6560–6569 CrossRef CAS PubMed.
- S. G. Liu, L. Han, N. Li, N. Xiao, Y. J. Ju, N. B. Li and H. Q. Luo, J. Mater. Chem. B, 2018, 6, 2843–2850 RSC.
- D. W. Yang, Z. Z. Guo, Y. G. Tang and P. Miao, ACS Appl. Nano Mater., 2018, 1, 168–174 CrossRef CAS.
- D. Zhao, J. Li, C. Y. Peng, S. Y. Zhu, J. Sun and X. R. Yang, Anal. Chem., 2019, 91, 2978–2984 CrossRef CAS PubMed.
- P. J. Ni, J. F. Xie, C. X. Chen, Y. Y. Jiang, Y. Z. Lu and X. Hu, Microchim. Acta, 2019, 186, 202 CrossRef PubMed.
- L. L. Zhang, J. J. Zhao, M. Duan, H. Zhang, J. H. Jiang and R. Q. Yu, Anal. Chem., 2013, 85, 3797–3801 CrossRef CAS PubMed.
- A. Z. Xu, L. Zhang, H. H. Zeng, R. P. Liang and J. D. Qiu, Microchim. Acta, 2018, 185, 288 CrossRef PubMed.
- X. C. Xu, X. B. Zou, S. W. Wu, L. J. Wang, X. H. Niu, X. Li, J. M. Pan, H. L. Zhao and M. B. Lan, Anal. Chim. Acta, 2019, 1053, 89–97 CrossRef CAS PubMed.
- S. P. Wu, Y. P. Chen and Y. M. Sung, Analyst, 2011, 136, 1887–1891 RSC.
- H. Liang, F. F. Lin, Z. J. Zhang, B. W. Liu, S. H. Jiang, Q. P. Yuan and J. W. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 1352–1360 CrossRef CAS PubMed.
- H. Liang, B. W. Liu, Q. P. Yuan and J. W. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 15615–15622 CrossRef CAS PubMed.
- W. C. Zhang, X. H. Niu, X. Li, Y. F. He, H. W. Song, Y. X. Peng, J. M. Pan, F. X. Qiu, H. L. Zhao and M. B. Lan, Sens. Actuators, B, 2018, 265, 412–420 CrossRef CAS.
- W. C. Zhang, X. Li, X. C. Xu, Y. F. He, F. X. Qiu, J. M. Pan and X. H. Niu, J. Mater. Chem. B, 2019, 7, 233–239 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Fig. S1–S5, and Table S1. See DOI: 10.1039/c9ay00643e |
| This journal is © The Royal Society of Chemistry 2019 |