Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Robust Buchwald–Hartwig amination enabled by ball-milling†
Qun
Cao
,
William I.
Nicholson
,
Andrew C.
Jones
and
Duncan L.
Browne
 *
*
School of Chemistry, Cardiff University, Main Building, Park Place, Cardiff CF10 3EQ, UK. E-mail: dlbrowne@cardiff.ac.uk
First published on 18th September 2018
Abstract
An operationally simple mechanochemical method for the Pd catalysed Buchwald–Hartwig amination of arylhalides with secondary amines has been developed using a Pd PEPPSI catalyst system. The system is demonstrated on 30 substrates and applied in the context of a target synthesis. Furthermore, the performance of the reaction under aerobic conditions has been probed under traditional solution and mechanochemical conditions, the observations are discussed herein.
Introduction
The C–N bond is ubiquitous in functional materials, including; pharmaceuticals, agrochemicals, flavours, fragrances and dyes. The Buchwald–Hartwig reaction offers a highly versatile catalytic method to forge aryl C–N bonds and is regularly used in the discovery of new compounds.1 Since its inception the development of the Buchwald–Hartwig reaction has focused on increasing the range of amenable substrates through the design of privileged ligands and pre-catalysts.2 Some of the most studied systems involve phosphine or NHC ligand classes and collectively have led to a catalytic toolkit capable of delivering very broad substrate scopes at low catalyst loadings.1,2 Nonetheless, for several of these systems a potential drawback is the sensitivity of the reaction to aerobic conditions which can lead to variability in reaction outcome. Improvements on this front have been made by Organ and co-workers with the discovery and development of the PEPPSI series of catalysts which feature both an imidazolium based NHC ligand and a ‘throw away’ pyridine component to afford an easy to handle pre-catalyst that renders a highly active catalyst in the reaction.3Recently, ball milling and other automated mechanochemical techniques have been explored as a means to conduct solvent-minimised reactions, with several recent examples demonstrating additional opportunities to synthetic chemists, such as, decreased reaction times, increased selectivity or differing reaction outcomes over solution results.4 Indeed several palladium mediated processes have been explored through ball-milling.5 It is also known that solid-state synthesis by ball-milling can enable (water and/or oxygen) sensitive reactions to be more reliably carried-out.6 This is particularly attractive, not only from the view-point of increased reproducibility but also from the stance of reduced effort and resource required for solvent drying and purification and provision of inert atmospheres during weighing of materials and running the reaction. Thus, we set out to explore the mechanochemical Buchwald–Hartwig reaction to see what the unison of these two processes could offer, our observations are reported herein.7
Results and discussion
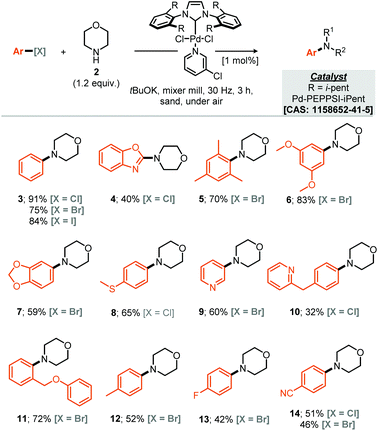
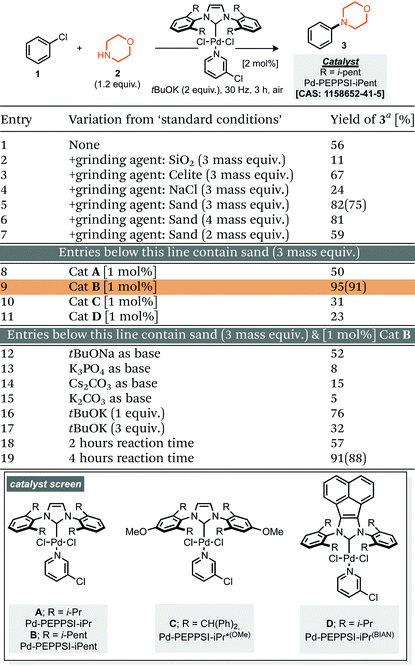
Our studies commenced by evaluating the model coupling of chlorobenzene (1) with morpholine (2) under mixer mill conditions using Pd-PEPPSI-iPent as catalyst (2 mol% loading) and potassium tert-butoxide as base.8 All materials were weighed and then added directly to the milling jar under an air atmosphere, i.e. no precaution was taken, the jars were then milled at 30 Hz for 3 hours. Under these conditions the cross-coupled product (3) was afforded in 56% yield (1H NMR, Table 1, entry 1). With this result in hand we explored refinement of the parameters to further optimise the reaction outcome and commenced with the addition of grinding agents to improve the flowability and mixing of the reaction mixture.9 For this purpose addition of silica gel, Celite, sodium chloride or sand (Table 1, entries 2–7), to the reaction mixture was explored; 3 mass equivalents of sand provided a clear improvement, affording compound 3 in 75% isolated yield. Screening a small range of catalysts at 1 mol% loading (Table 1, entries 8–11) demonstrated that the Pd-PEPPSI-iPent was optimal for this process, with an intriguing improvement in yield observed at this reduced loading (91% isolated yield, Table 1, entry 9). Further experiments explored the species of base, equivalents of base and reaction time, with no further improvements observed (Table 1, entries 12–19). With optimal conditions identified (Table 1, entry 9), application to a small range of 15 aryl halides was investigated, initially this lead to the verification that these conditions were applicable to aryl chlorides, bromides and iodides but also heteroaromatic, electron rich and electron poor substrates, although in the latter case, a simple SNAr mechanism could achieve the same targeted products (Scheme 1). In each case the reaction was conducted under an air atmosphere and afforded the products in moderate to excellent yield (31–91% isolated yields, Scheme 1). Exploring an applicable substrate scope under mechanochemical conditions is important as the robustness of any discovered method can be dependant on the form, density and hardness of the input materials – within the substrate scope presented here the morpholine is a liquid and amongst the aryl halide coupling partners explored were both solid and liquid reagents.| a Yield determined by 1H NMR using mesitylene as internal standard, numbers in parentheses represent isolated yields. |
|---|
|
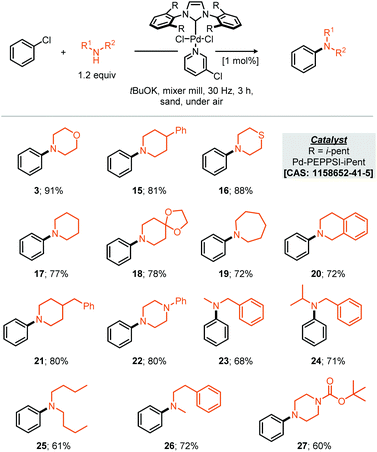
With respect to the reaction scope of the secondary amine component 14 partners were successfully coupled, demonstrating that the reaction is not only limited to the privileged morpholine. Other highly nucleophilic cyclic amines led to good to excellent yields from 91% for the optimised substrate morpholine to 60% for N-Boc protected piperazine (Scheme 2, compounds 3 and 27). Acyclic secondary amines also participated in this reaction affording the resulting cross-coupled products in good to very good yields. For the benzyl acyclic examples, the more sterically hindered N-isopropyl amine afforded essentially the same yield as the N-methyl variant (Scheme 2, compounds 23 & 24).
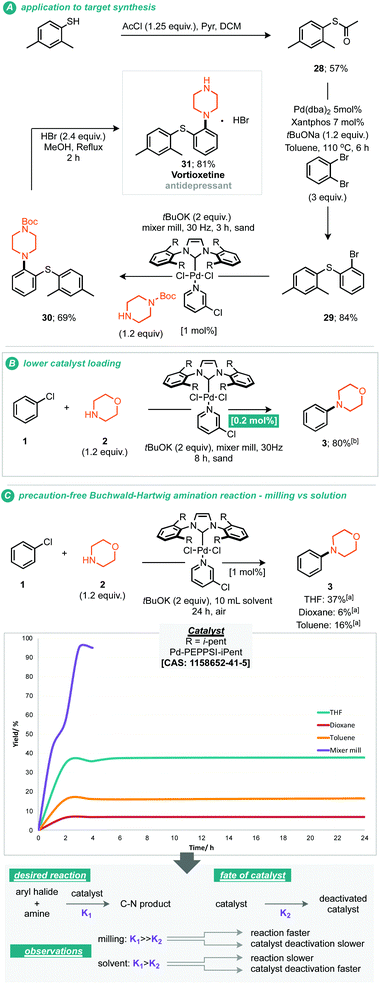
It should be noted that the presently reported conditions were not successfully applied to primary amine coupling reactions, attempted cross coupling of aniline or octylamine with morpholine 3 afforded the corresponding product in less than 5% GC yield. Nonetheless with a proven applicability to a small range of both coupling partners the synthesis of a pharmaceutical (API) was targeted using the mechanochemical Buchwald–Hartwig coupling. Treatment of thio-ether (29) under the optimised reaction conditions with N-Boc piperazine afforded the resultant protected vortioxetine (30) in 69% yield (A, Scheme 3). Notably, this reaction was conducted without any precaution for air or moisture sensitivity. Intrigued by the apparent robustness of the discovered reaction process we explored the reaction parameters at 0.2 mol% catalyst loading; at this catalyst loading a good yield of the desired product was obtained within eight hours (B, Scheme 3). Furthermore, the apparent robustness of these reaction parameters was checked by comparison of the same conditions applied to reactions conducted in solvent and the presence of air, i.e. where careful preparation of the solvent was omitted (C, Scheme 3). Common solvents for the Buchwald–Hartwig amination reaction were chosen for this experiment, including THF, 1,4-dioxane and toluene. In the event, it can be seen that in the absence of any precaution, THF preforms the best, affording the cross-coupled product in 37% yield. It can be seen from the data that the solvent based reaction stalls by the two hour point, potentially through catalyst deactivation, indeed such sensitivity has been reported by Organ and co-workers.10 With these experimental observations we can hypothesise about where such reaction performance may derive. If we crudely consider the overall effectiveness of the reaction being a reflection of the effective rate of the desired product formation (K1) versus the effective rate of catalyst degradation (K2), then clearly in the case of milling, where the reaction is faster than in solution, the differences between K1 and K2 are greater relative to solution (empirically shown in C, Scheme 3). This observation could be due to the rate of the desired reaction being faster under milling conditions, or, because the rate of catalyst degradation is slower, indeed, milling could afford a pathway whereby deactivated catalyst is transformed back into active catalyst by mechanical activation.10 Regarding air and moisture sensitivity, it should be noted that mechanochemical milling jars are not hermetically sealed, but, air ingress is likely to be greatly reduced, thus if the reaction system is able to generate it's own anaerobic conditions within the milling-jar then disruption of those conditions is likely to be slow in comparison to a solution based reaction, such considerations could help prevent catalyst deactivation and contribute towards the observed behaviour.
Conclusion
Herein we report an operationally simple mechanochemical method to carry out a solvent-less Buchwald–Hartwig amination reaction of secondary amines. The reported method is robust and can be achieved without special precaution to preclude air or water from the reaction system. The method has been applied to 30 different substrates including both liquid and solid components and was successfully incorporated into the synthesis of the antidepressant API Vortioxetine. The potential for mechanical activation to offer benefits to catalytic reactions has been briefly described.Notes
Information about the data underpinning the results presented here, including how to access them, can be found in the Cardiff University data catalogue at http://doi.org/10.17035/d.2018.0056464884.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. L. B. is grateful to the EPSRC for a First Grant (D. L. B. & Q. C. EP/P002951/1), the EPSRC U.K. National Mass Spectrometry Facility at Swansea University and the School of Chemistry at Cardiff University for generous support. We thank the Cardiff Catalysis Institute for providing access to the GC equipment used in this study and in particularly the expert technical support from Dr Greg Shaw.Notes and references
-
(a) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534–1544 CrossRef CAS PubMed
; (b) C. Valente, M. Pompeo, M. Sayah and M. G. Organ, Org. Process Res. Dev., 2014, 18, 180–190 CrossRef CAS
; (c) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338–6361 CrossRef CAS
.
-
(a) C. Valente, S. Calimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314–3332 CrossRef CAS PubMed
; (b) J. Nasielski, N. Hadei, G. Achonduh, E. A. B. Kantchev, C. J. O'Brien, A. Lough and M. G. Organ, Chem. – Eur. J., 2010, 16, 10844–10853 CrossRef CAS
; (c) E. A. B. Kantchev, C. J. O'Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768–2813 CrossRef CAS
; (d) G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151–5169 RSC
; (e) S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef CAS
; (f) C. J. O'Brien, E. A. B. Kantchev, G. A. Chass, N. Hadei, A. C. Hopkinson, M. G. Organ, D. H. Setiadi, T.-H. Tang and D.-C. Fang, Tetrahedron, 2005, 61, 9723–9735 CrossRef
.
-
(a) C. J. O'Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. C. Hopkinson and M. G. Organ, Chem. – Eur. J., 2006, 12, 4743–4748 CrossRef
; (b) M. Pompeo, J. L. Farmer, R. D. J. Froese and M. G. Organ, Angew. Chem., Int. Ed., 2014, 53, 3223–3226 CrossRef CAS
; (c) K. H. Hoi, S. Calimsiz, R. D. J. Froese, A. C. Hopkinson and M. G. Organ, Chem. – Eur. J., 2011, 17, 3086–3090 CrossRef CAS
.
-
(a) J. S. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC
; (b) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef
; (c) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC
; (d) J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed
; (e) O. Eguaogie, J. S. Vyle, P. F. Conlon, M. A. Gîlea and Y. Liang, Beilstein J. Org. Chem., 2018, 14, 955 CrossRef CAS
; (f) T.-X. Métro, J. Martinez and F. Lamaty, ACS Sustainable Chem. Eng., 2017, 5, 9599 CrossRef
; (g) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC
.
-
(a) Q. Cao, J. L. Howard, E. Wheatley and D. L. Browne, Angew. Chem., Int. Ed., 2018, 57, 11339–11343 CrossRef CAS
; (b) Z.-J. Jiang, Z.-H. Li, J.-B. Yu and W.-K. Su, J. Org. Chem., 2016, 81, 10049–10055 CrossRef CAS
; (c) L. Chen, M. Regan and J. Mack, ACS Catal., 2016, 6, 868–872 CrossRef CAS
; (d) S.-J. Lou, Y.-J. Mao, D.-Q. Xu, J.-Q. He, Q. Chen and Z.-Y. Xu, ACS Catal., 2016, 6, 3890–3894 CrossRef CAS
; (e) K.-Y. Jia, J.-B. Yu, Z.-J. Jiang and W.-K. Su, J. Org. Chem., 2016, 81, 6049–6055 CrossRef CAS
.
- D. C. Waddell, T. D. Clark and J. Mack, Tetrahedron Lett., 2012, 53, 4510–4513 CrossRef CAS
.
- During the preparation of this manuscript we became aware of a closely related report: Q.-L. Shao, Z.-J. Jiang and W.-K. Su, Tetrahedron Lett., 2018, 59, 2277–2280 CrossRef CAS
.
- For examples of Pd-PEPPSI catalysed cross coupling reactions using tBuOK as base see:
(a) M. G. Organ, M. Abdel-Hadi, S. Avola, I. Bubovyk, N. Hadei, E. A. B. Kantchev, C. O'Brien, M. Sayah and C. Valente, Chem. – Eur. J., 2008, 14, 2443–2452 CrossRef CAS
; (b) K. H. Hoi, J. A. Coggan and M. G. Organ, Chem. – Eur. J., 2013, 19, 843–845 CrossRef CAS
; (c) M. G. Organ, S. Calimsiz, M. Sayah, K. H. Hoi and A. J. Lough, Angew. Chem., Int. Ed., 2009, 48, 2383–2387 CrossRef CAS
; (d) S. Meiries, G. L. Duc, A. Chartoire, A. Collado, K. Speck, K. S. A. Arachchige, A. M. Z. Slawin and S. P. Nolan, Chem. – Eur. J., 2013, 19, 17358–17368 CrossRef CAS
.
-
(a) J.-L. Do, C. Mottillo, D. Tan, V. Štrukil and T. Friščić, J. Am. Chem. Soc., 2015, 137, 2476–2479 CrossRef CAS
; (b) B. P. Hutchings, D. E. Crawford, L. Gao, P. Hu and S. L. James, Angew. Chem., Int. Ed., 2017, 56, 15252–15256 CrossRef CAS
; (c) J. L. Howard, W. Nicholson, Y. Sagatov and D. L. Browne, Beilstein J. Org. Chem., 2017, 13, 1950–1956 CrossRef CAS
; (d) J. L. Howard, Y. Sagatov and D. L. Browne, Tetrahedron, 2018, 74, 3118–3123 CrossRef CAS
; (e) J. L. Howard, Y. Sagatov, L. Repusseau, C. Schotten and D. L. Browne, Green Chem., 2017, 19, 2798–2802 RSC
.
- C. Valente, M. Pompeo, M. Sayah and M. G. Organ, Org. Process Res. Dev., 2013, 18, 180–190 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ob01781f |
| This journal is © The Royal Society of Chemistry 2019 |