Open Access Article
Open Access ArticleOrganobase triggered controlled supramolecular ring opening polymerization and 2D assembly†
Anwesha
Chakraborty‡
a,
Goutam
Ghosh‡
a,
Deep Sankar
Pal
a,
Shinto
Varghese
 b and
Suhrit
Ghosh
b and
Suhrit
Ghosh
 *a
*a
aSchool of Applied and Interdisciplinary Science, 2A and 2B Raja S. C. Mullick Road, Kolkata, India-700032. E-mail: psusg2@iacs.res.in
bTechnical Research Center, Indian Association for the Cultivation of Science, 2A and 2B Raja S. C. Mullick Road, Kolkata, India-700032
First published on 27th June 2019
Abstract
A carboxylic acid appended naphthalene-diimide (NDI) derivative spontaneously aggregates in decane to generate a kinetically controlled product with irregular fibrillar morphology. By fine-tuning the sample preparation conditions, the carboxylic acid group can be trapped by intra-molecular H-bonds with the adjacent imide carbonyl, which retards the spontaneous aggregation. In the presence of a catalytic amount of a non-nucleophilic organic base (DBU or DMAP), the meta-stable monomer exhibits supramolecular polymerization through a thermodynamically controlled pathway involving simultaneous H-bonding and π-stacking and generates ultra-thin 2D nano-sheets. DMAP/DBU helps in ring-opening of the intra-molecularly H-bonded monomer and in situ breeds the free acid, which, beyond a critical concentration, initiates controlled supramolecular ring opening polymerization (SROP) via the chain-growth mechanism. The 2D polymer acts as a macro-initiator for subsequent two cycles of SROP and produces laterally extended ultra-thin nano-sheets.
Introduction
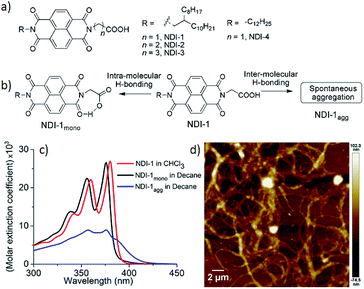
Controlled supramolecular polymerization (CSP)1 has emerged as an active research area in the recent past. CSP emulates controlled/living covalent chain polymerization and endows control over the degree of polymerization, dispersity and successful synthesis of block copolymers. Rather counter-intuitively, supramolecular polymers,2 albeit having an internal order,3 lack the structural precision in the mesoscopic scale. As exemplified in the recent literature, pathway complexity in supramolecular polymerization4 produces distinctly different products from the same building block depending on the conditions. This provides a clue for designing strategies for supramolecular polymerization by the chain growth mechanism. For example, a monomer, under kinetic control, generates polymer P1 while under thermodynamic control produces P2. P2 can be fragmented to form a seed, which guides the pool of monomers, trapped in the P1 state, to polymerize in a chain growth fashion with features similar to controlled/living covalent polymerization. Such possibilities have been successfully demonstrated in crystallization driven living supramolecular polymerization of macromolecules5 and more recently with π-conjugated small molecules.6 However, the seed, which essentially plays a similar role to that of an initiator in covalent chain growth polymerization, is not a discrete molecular entity, rather it is an ill-defined fragment of a pre-formed polymer. To best of our knowledge till date there is only one report6a demonstrating the use of a discrete molecular entity to trigger CSP. We have recently reported CSP of NDI-4 (Fig. 1a) by a seeding approach.7 In methyl-cyclohexane, it showed spontaneous J-aggregation by inter-molecular H-bonding among the carboxylic acid groups. However, by adopting a different solution preparation method, it was possible to isolate a meta-stable dormant state wherein the carboxylic acid groups were engaged in intra-molecular H-bonding with the adjacent imide carbonyl group (Fig. 1b). The seed prepared from the J-aggregate was shown to be effective in initiating CSP of this dormant monomer producing a 1D polymer with tunable length. We envisaged that this particular system might offer an opportunity to explore CSP in the presence of an organobase (similar to organo-catalytic ring opening polymerization of a cyclic lactide or carbonate monomers) instead of a seed, if the organobase could open up the intra-molecularly H-bonded cyclic monomer by being a stronger H-bond acceptor than the imide carbonyl. To test such possibilities, we have synthesized NDI-1 (Fig. 1a) which is structurally similar to previously reported NDI-4 (Fig. 1a), except for the peripheral alkyl chains. As control molecules, NDI-2 and NDI-3 (Fig. 1a) were also studied in which the intra-molecularly H-bonded dormant state is anticipated to be relatively less stable due to larger ring size. In this article we reveal supramolecular ring opening polymerization (SROP) of NDI-1 in the presence of a catalytic amount of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or 4-dimethylaminopyridine (DMAP) producing well defined 2D sheets with controllable dimensions in contrast to the uncontrolled fibrillar network structure produced by the spontaneous aggregation.Results and discussion
The UV/Vis spectrum of NDI-1 in CHCl3 (Fig. 1c) exhibits sharp absorption bands with a vibronic fine structure which is typical of monomeric dyes.8 However, in a hydrocarbon solvent decane, which is conducive for H-bonding, a broad structure-less absorption band with reduced intensity indicates ill-defined aggregates (NDI-1agg) with a critical aggregation concentration (CAC) of 3 × 10−5 M (Fig. S1†). The AFM image (Fig. 1d) in decane reveals the formation of an irregular fibrillar network for NDI-1agg. Our previously reported method7 allowed isolating the monomeric state of NDI-4 only at a very dilute concentration (1 × 10−5 M). To improve on that we have now introduced a branched alkyl chain into NDI-1 to reduce its propensity for spontaneous aggregation. Indeed, NDI-1mono could be isolated at ten times higher concentration (1 × 10−4 M) than NDI-4mono in decane by slightly modified sample preparation conditions. Instead of directly dissolving NDI-1 in decane, it was dissolved in CHCl3/MeOH and dried to form a thin film. It was then dissolved in CHCl3 and an aliquot was mixed with decane and then the good solvent was selectively removed by evaporation. The UV/Vis spectrum of the so-prepared sample in decane was found to be almost identical to that in CHCl3 (Fig. 1c) except for a blue shift due to the solvatochromic effect which confirmed formation of the monomeric state (NDI-1mono) in decane. The fluorescence spectrum of NDI-1mono in decane appeared similar (Fig. S2†) to that in CHCl3 reconfirming lack of aggregation. In contrast the fluorescence spectrum of NDI-1agg clearly showed a broad excimer peak (Fig. S2†). FT-IR spectra (Fig. S3†) of NDI-1 in CHCl3 showed the O–H stretching band at 3087 cm−1 indicating non-H-bonded COOH. For NDI-1agg, the peak appeared at 2995 cm−1 suggesting intermolecular H-bonding among the COOH groups in the aggregated state.6b Interestingly for NDI-1mono, the OH stretching peak appeared at a different position (3053 cm−1) which is assigned to the intra-molecular H-bonding with the imide carbonyl (Fig. 1b). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band of the carboxylic acid group for NDI-1agg in decane appeared (Fig. S3b†) at 1732 cm−1, matching with that for NDI-1 in solid state indicating inter-molecular H-bonding. However for NDI-1mono in decane, the band appeared at a higher wavenumber (1761 cm−1), indicating the C
O stretching band of the carboxylic acid group for NDI-1agg in decane appeared (Fig. S3b†) at 1732 cm−1, matching with that for NDI-1 in solid state indicating inter-molecular H-bonding. However for NDI-1mono in decane, the band appeared at a higher wavenumber (1761 cm−1), indicating the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the COOH group is not involved in H-bonding as should be the case for the proposed cyclic structure (Fig. 1b). To further support the formation of an intra-molecular H-bonded state in NDI-1mono, control molecules NDI-2 and NDI-3 (Fig. 1a) were studied. In these cases also, the monomeric state could be trapped in decane following a similar sample preparation procedure. But the UV/Vis spectra of NDI-2mono and NDI-3mono were changed to aggregated spectra within 2 h (rt, decane) while the monomeric spectrum of NDI-1mono remained stable at least for 24 h at rt (Fig. S4†).9 This may be attributed to the increase in ring-size and further supports the hypothesis on the intra-molecularly H-bonded structure of the monomeric state. To gain more insight, dispersion-corrected density functional theory (DFT-D3BJ) at the B3LYP/6-311G (d, p) level was employed for the optimization of the intra-molecular H-bonded structures using Gaussian 16 program.10a Non-Covalent Interaction (NCI) analyses were used to get an estimate of the attractive and repulsive interactions.10b The reduced gradient density (RDG) isosurface gives a direct visualization of the real space interactions and the scattering plot of RDG against the sign of the second Hessian eigenvalue times the electron density (sign(λ2)ρ) discriminates the attractive and the repulsive interactions as implemented in Multiwfn 3.6 software.10c The scattering plot (Fig. S5†) showed intra-molecular H-bond strength to be highest in NDI-1 while slightly lower in NDI-2 and considerably low in NDI-3 which corroborate with the experimental results.
O of the COOH group is not involved in H-bonding as should be the case for the proposed cyclic structure (Fig. 1b). To further support the formation of an intra-molecular H-bonded state in NDI-1mono, control molecules NDI-2 and NDI-3 (Fig. 1a) were studied. In these cases also, the monomeric state could be trapped in decane following a similar sample preparation procedure. But the UV/Vis spectra of NDI-2mono and NDI-3mono were changed to aggregated spectra within 2 h (rt, decane) while the monomeric spectrum of NDI-1mono remained stable at least for 24 h at rt (Fig. S4†).9 This may be attributed to the increase in ring-size and further supports the hypothesis on the intra-molecularly H-bonded structure of the monomeric state. To gain more insight, dispersion-corrected density functional theory (DFT-D3BJ) at the B3LYP/6-311G (d, p) level was employed for the optimization of the intra-molecular H-bonded structures using Gaussian 16 program.10a Non-Covalent Interaction (NCI) analyses were used to get an estimate of the attractive and repulsive interactions.10b The reduced gradient density (RDG) isosurface gives a direct visualization of the real space interactions and the scattering plot of RDG against the sign of the second Hessian eigenvalue times the electron density (sign(λ2)ρ) discriminates the attractive and the repulsive interactions as implemented in Multiwfn 3.6 software.10c The scattering plot (Fig. S5†) showed intra-molecular H-bond strength to be highest in NDI-1 while slightly lower in NDI-2 and considerably low in NDI-3 which corroborate with the experimental results.
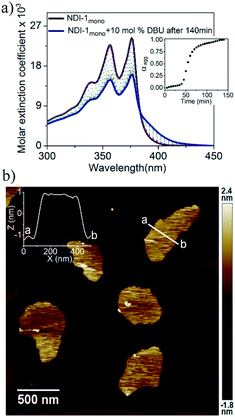
Supramolecular ring opening polymerization (SROP) of NDI-1mono was attempted using a catalytic amount of a non-nucleophilic base DBU which has been extensively used for ring opening polymerization of cyclic lactides.11 A solution of DBU (10 mol%) in decane was added to a freshly prepared solution of NDI-1mono (1 × 10−4 M) in decane and supramolecular polymerization was monitored by UV/Vis spectroscopy (Fig. 2a) at 25 °C. For the initial few minutes, no significant change was noticed, but after about 35 min, the band intensity started decreasing sharply followed by saturation in about 75 min. The mole fraction of the aggregate (αagg) (for calculations see ESI†) vs. time plot (inset-Fig. 2a) revealed an initial lag phase prior to the onset of the supramolecular polymerization. Intriguingly, the UV-Vis spectra of the DBU triggered supramolecular polymer (NDI-1SP) and NDI-1agg were distinctly different. It appears that for NDI-1SP there was a face to face π-stacking with rotational displacement along the long axis8 resulting in the appearance of a low-energy shoulder band unlike the broad structure-less spectrum for NDI-1agg (Fig. 1b).
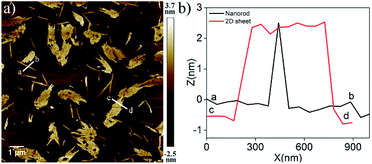
The AFM image of NDI-1SP showed (Fig. 2b) a well defined 2D uniform nanosheet12 morphology with height and diameter in the range of 2.0 nm and 400 nm, respectively, in sharp contrast to the irregular fibrillar network of NDI-1agg (Fig. 1c). Concentration dependent UV/Vis experiments in decane (Fig. 3a) revealed a significant difference in the CAC of NDI-1agg and NDI-1SP.
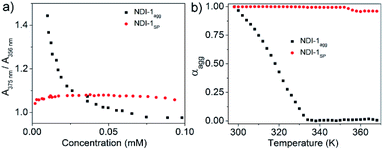 | ||
| Fig. 3 Comparison of the (a) CAC and (b) thermal stability of NDI-1agg (aggregated spontaneously) and NDI-1SP (prepared by CSP of NDI-1mono using 10% DBU). A375 and A356 indicate absorption at 375 and 356 nm, respectively, in the UV/Vis spectra in decane [l = 1 cm; C = 1 × 10−4 M]; αagg at each temperature was calculated from the variable temperature UV/Vis spectra (Fig. S5†). | ||
The ratio of absorption intensities at 375 and 356 nm has been used in the literature to probe π-stacking among the NDI chromophores.8 For NDI-1agg, the ratio sharply increases below a critical concentration (3 × 10−5 M) indicating disassembly while for NDI-1SP, no sign of disassembly was noticed even at 0.002 mM indicating its much lower CAC. Furthermore, variable temperature UV/Vis studies (Fig. 3b, S6†) showed the onset of disassembly for NDI-1agg just above rt and complete melting at ∼60 °C, while for NDI-1SP, no disassembly was noticed up to 95 °C. Therefore it is evident that DBU triggered CSP of NDI-1mono guided the formation of a distinctly different product (NDI-1SP) that not only differed in morphology but also exhibited a much higher stability and lower CAC than NDI-1agg. To examine the generalized applicability of the method, SROP was also attempted using another commonly used organobase DMAP under identical conditions.
In this case, similar changes were noticed in the UV/Vis spectra (Fig. S7†) except that the lag phase was significantly reduced (Table 1). As the supramolecular polymerization was relatively faster in this case, DMAP was used as the base for all subsequent mechanistic investigations. SP was performed with varying amounts of DMAP and it was noticed that with increasing the concentration of the base (2.5 to 10 mol%), the initial lag phase shortened from 70 min to 20 min (Table 1, Fig. S8†). AFM images showed formation of 2D nanosheets in all cases (Fig. 4b, S9†) with consistent height (∼2 nm), similar to that observed for DBU triggered SROP. The size and aspect ratio of the sheets increased gradually with increasing amounts of DMAP (Table 1, Fig. S9†).
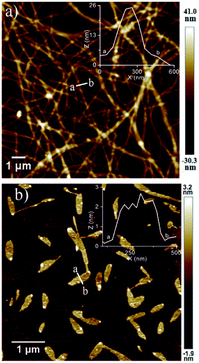
In a control experiment, a mixture of NDI-1 (not NDI-1mono) and DMAP (10 mol%) was dissolved in decane under hot conditions and the solution was allowed to equilibrate at rt for a couple of hours. This particular sample when examined under AFM, revealed a micrometer long entangled fibrillar network (Fig. 4a), in sharp contrast to the distinct 2D sheets obtained by SROP of NDI-1mono with the same amount of base (Fig. 4b). The height of the fibers was found to be >20 nm which is much larger than 2 nm thick nanosheets. Therefore it is confirmed that the mere presence of DMAP was not responsible for the formation of 2D sheets, but trapping the active monomer in the dormant state was essential to realize the base triggered SROP. Not to mention that NDI-1mono alone did not produce 2D sheets either (Fig. S4†) after seven days confirming the essential role played by the organobase in the SROP. It is also noteworthy that NDI-2 or NDI-3, in which case isolation of a stable dormant monomeric state was not possible, also did not produce 2D sheets in the presence of DMAP, rather produced ill-defined aggregates or fibrillar morphology (Fig. S10†). To test whether the methodology is applicable to another monomer, DMAP triggered SROP was tested with NDI-4 which differs from NDI-1 in the structure of the peripheral alkyl chains. By the modified sample preparation method, NDI-4 also was successfully trapped in the monomeric state in decane at C = 10−4 M (Fig. S11a†). In the presence of 10% DMAP, a lag phase followed by supramolecular polymerization was noticed (Fig. S11b†) similar to that observed for NDI-1mono. The AFM image (Fig. S11c†) confirmed formation of 2D sheets identical to that produced by NDI-1mono. It is noteworthy that NDI-4mono when polymerized using the seed (prepared from its spontaneously formed J-aggregate) produced J-aggregates and 1D fiber as evident from the UV/Vis spectrum and AFM image (Fig. S12†).7
The same monomer now when polymerized using DMAP revealed different modes of π-stacking as evident from the absence of any J-band and 2D nanostructure (Fig. S11a–c†), indicating that the DMAP triggered SROP indeed followed a different pathway than seeded supramolecular polymerization.
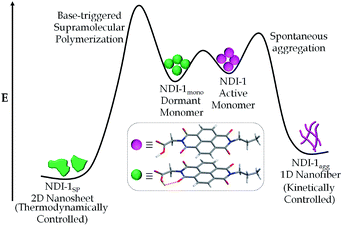
Having established the distinct supramolecular polymerization pathway for NDI-1mono/NDI-4mono in the presence of an organobase, we sought to examine the mechanistic details of the SROP. What role does DBU/DMAP play in the SROP? Is it an initiator or a catalyst? In chain growth polymerization, increasing the initiator/monomer ratio should decrease the degree of polymerization. Therefore the reverse trend (Table 1, Fig. S8†) observed for DMAP triggered SROP of NDI-1mono eliminates the role of DMAP as an initiator. It is proposed (Scheme 1) that DBU/DMAP, being a stronger H-bond acceptor than carbonyl oxygen, opens the intra-molecularly H-bonded ring to produce the intermediate 1 which remains in equilibrium with the free acid. This is evident from the FT-IR spectra (Fig. S13†) which show a characteristic shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency of DMAP indicating its involvement in H-bonding. The lag phase observed in all cases possibly corresponds to the time required to establish the equilibrium and reaching a threshold concentration of the free acid beyond which it is able to form a nucleus (either by associating with itself or with the monomer) that subsequently initiates SROP of NDI-1monovia the chain growth mechanism. The lag phase was found to increase with increasing temperature and at 35 °C, the supramolecular polymerization did not begin even after 6 h (Fig. S14†). This is conceivable because two of the three steps before propagation include non-covalent association which in organic solvent is likely to be driven by enthalpy and thereby should be less pronounced at elevated temperature. DBU being a stronger base than DMAP, the equilibrium remains more shifted towards the intermediate 1 (Scheme 1) and therefore it requires longer time to attain the critical free acid concentration for nucleation and thus the lag phase is longer.13
N stretching frequency of DMAP indicating its involvement in H-bonding. The lag phase observed in all cases possibly corresponds to the time required to establish the equilibrium and reaching a threshold concentration of the free acid beyond which it is able to form a nucleus (either by associating with itself or with the monomer) that subsequently initiates SROP of NDI-1monovia the chain growth mechanism. The lag phase was found to increase with increasing temperature and at 35 °C, the supramolecular polymerization did not begin even after 6 h (Fig. S14†). This is conceivable because two of the three steps before propagation include non-covalent association which in organic solvent is likely to be driven by enthalpy and thereby should be less pronounced at elevated temperature. DBU being a stronger base than DMAP, the equilibrium remains more shifted towards the intermediate 1 (Scheme 1) and therefore it requires longer time to attain the critical free acid concentration for nucleation and thus the lag phase is longer.13
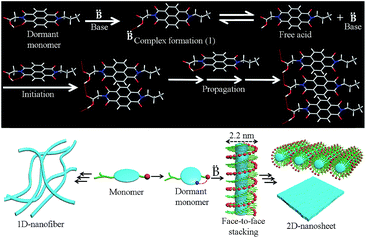 | ||
| Scheme 1 Top – probable mechanistic pathway for organobase triggered SROP of NDI-1mono; bottom – pathway diversity and 2D-assembly of the product produced by SROP. | ||
On the other hand, with increasing concentration of DMAP, it takes less time to generate the required amount of free acid for initiation of SROP and therefore the lag phase shortens. Increase in the size of the sheets with increasing DMAP concentration may be correlated with increase in monomer conversion, which in fact was evident by comparing the UV/Vis spectra of the end products (Fig. S15†) in three different DMAP-triggered SROP. If indeed this mechanism is operating, then the free acid itself should be able to initiate the polymerization. To test this possibility, a solution of NDI-1 (C = 1 × 10−5 M < CAC of NDI-1agg) in decane was added to the NDI-1mono solution and indeed it could initiate the SROP leading to the formation of similar 2D nanosheets (Fig. S16†). It is noteworthy that the CAC of NDI-1SP ≪ NDI-1agg (Fig. 3) and thus nucleation for NDI-1SP at 0.01 mM (<CAC of NDI-1agg) is conceivable.
Interestingly in this case, no lag phase was noticed (Fig. S16†) which further supports the hypothesis that the time taken to reach a critical concentration of the free acid for nucleation is probably responsible for the observed lag phase in DMAP/DBU triggered supramolecular polymerization. From the sharp difference in the UV/Vis spectra and AFM images of NDI-1agg and NDI-1SP, it is clear that they represent distinctly different structures. Interestingly, when the NDI-1agg solution was heated up to 90 °C and cooled slowly (1 K min−1), the UV/Vis spectrum (Fig. S17†) did not look similar to the initial spectrum, rather it exhibited similar features to those observed for the SROP suggesting that NDI-1agg is a kinetically controlled product whereas the thermodynamically controlled product was formed during SROP (Scheme 2).
This is further evident by the contrasting melting curves of NDI-1agg and NDI-1SP (Fig. 3). It is believed that such pathway diversity originates from different possible H-bonding motifs of the COOH group14 in NDI-1 which includes (i) syn–syn catemer type extended chain or (ii) dimerization followed by π-stacking in the orthogonal direction or (iii) simultaneous π-stacking and H-bonding by stacking of the monomer units on top of each. Previous reports from our group and others15 showed prominent J-aggregation in the case of syn–syn catemer type H-bonding driven assembly of structurally similar building blocks.
Therefore, the lack of such a prominent J-band in the UV/Vis spectra of either NDI-1agg or NDI-1SP eliminates the possibility of packing in mode (i). Important to note that the height of the nano-sheets is slightly higher than the length of NDI-1 but lower than its dimer length which supports the packing in mode (iii) for SROP. It is believed that DAMP triggered SROP leads to the formation of a 1D structure by simultaneous π-stacking and H-bonding, which by lateral clustering produces 2D sheets. In fact, during the AFM imaging of a sample before completion of the polymerization, co-existence of such 1D rod-like structures and incompletely grown 2D sheets could be seen (Fig. 5). Interestingly, the heights of the individual rods and the 2D sheets were found to be exactly identical which supports the fact that the sheets are indeed built by the assembly of these rods. On the other hand, for NDI-1agg, mode (ii) appears to be mainly operating in which case less stability is conceivable as in this mode H-bonding and π-stacking do not enjoy a synergistic effect. The difference in the mode of H-bonding between NDI-1agg and NDI-1sp was evident from the FT-IR spectra (Fig. S18†) which showed a clear difference in the position of the peak corresponding to the O–H stretching of the carboxylic acid group.
Finally the living character of the 2D supramolecular polymer generated by SROP of NDI-1mono was investigated by UV/Vis spectroscopy and AFM.6b,d,g Initially a fixed quantity of DMAP (2.5 mol%) was added to NDI-1mono solution (1.5 ml, C = 1 × 10−4 M) which triggered SROP after a lag phase (Fig. 6, S19†) and was completed in ∼3 h. AFM images (Fig. 7a) showed formation of uniform nanosheets with a height and width of approximately 2 nm and 150 nm, respectively.
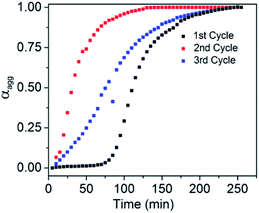 | ||
| Fig. 6 Variation of αaggvs. time for living SROP; 2.5% DMAP was used in the first cycle while NDI-1SP (NDI-1mono/NDI-1SP = 34) was used in the 2nd and 3rd cycles. | ||
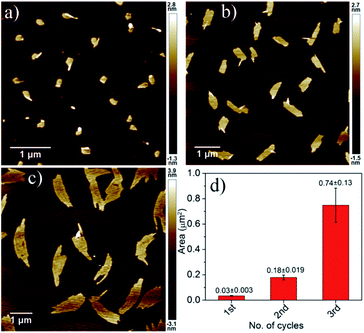 | ||
| Fig. 7 AFM images of NDI-1SP after (a) first, (b) second and (c) third cycles of living SROP and (d) their average area. | ||
An aliquot of the so-prepared NDI-1sp solution was transferred to a cuvette containing another batch of freshly prepared NDI-1mono solution (NDI-1mono/NDI-1sp = 34) while polymerization started immediately without any lag phase and the reaction was over within 2 h (Fig. 6). The AFM image showed (Fig. 7b) significant growth of the nano-sheets (height ∼2 nm, width/area ∼305 nm/0.18 μm2) indicating that the initially formed sheets indeed served the role of a macro-initiator for subsequent SROP and chain extension.
It is important to note that the prominent lag phase that is observed in the first cycle is absent in the second cycle indicating that unlike DMAP (during first cycle), initially formed nano-sheets acted as an initiator. In fact the nano-sheet produced after 2nd cycle was also found to be active for initiating the SROP in the 3rd cycle (again without any lag phase) resulting in further chain extension. The AFM image showed (Fig. 7c) formation of much larger sheets (height ∼2 nm, width/area ∼540 nm/0.74 μm2) without compromising with the uniformity of the structure.
Conclusions
Overall we have demonstrated an unprecedented example of controlled supramolecular ring opening polymerization, triggered by a well-known organic base. Although pathway complexity and seeded supramolecular polymerization have been fairly well known now, in most examples neither the initiator (seed) nor the starting material (kinetically controlled aggregate) is a molecularly defined entity. In a significant step forward, both are discrete molecules in the present design. That opens up future opportunities for extending the design to wide ranging p- and n-type semiconductors of different electronic properties and also synthesis of supramolecular copolymers with segregated blocks of two different semiconductors.16 Such structures should be of significant importance, particularly considering the rarely reported (in the context of supramolecular assembly of π-systems) ultra-thin 2D morphology12 which is in the limelight because of the unusual physical properties resulting from the quantum size effect of the ultra-thin structure.17 Further investigation on the mechanistic details, possibility of employing other H-bond donor/acceptor molecules for SROP,18 general applicability of the methodology for other π-systems and understanding the impact of 2D-nanostructure on photo-physical properties19 are underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AC thanks the DST-Inspire Program for a research fellowship. GG thanks IACS for a research fellowship. SV thanks the TRC, IACS for funding. SG thanks DST, India, for funding (SwarnaJayanti Fellowship : DST/SJF/CSA-01/2-14-15).Notes and references
- (a) F. Würthner, Nat. Chem., 2014, 6, 171 CrossRef PubMed; (b) R. D. Mukhopadhyay and A. Ajayaghosh, Science, 2015, 349, 241 CrossRef CAS PubMed; (c) D. van der Zwaag, T. F. A de Greef and E. W. Meijer, Angew. Chem., Int. Ed., 2015, 54, 8334 CrossRef CAS PubMed; (d) B. Adelizzi, N. J. V. Zee, L. N. J. de Windt, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2019, 141, 6110 CrossRef CAS PubMed.
- (a) L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 12, 4071 CrossRef; (b) Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC; (c) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed; (d) E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 4, 2414 CrossRef PubMed; (e) L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed; (f) C. Rest, R. Kandanellia and G. Fernández, Chem. Soc. Rev., 2015, 44, 2543 RSC.
- T. Aida, E. W. Meijer and S. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed.
- P. A. Korevaar, T. F. A. de Greef and E. W. Meijer, Chem. Mater., 2014, 26, 576 CrossRef CAS.
- (a) C. E. Boott, J. Gwyther, R. L. Harniman, D. W. Hayward and I. Manners, Nat. Chem., 2017, 9, 785 CrossRef CAS PubMed; (b) N. Petzetakis, A. P Dove and R. K. O'Reilly, Chem. Sci., 2011, 2, 955 RSC.
- (a) J. Kang, D. Miyajima, T. Mori, Y. Inoue, Y. Itoh and T. Aida, Science, 2015, 347, 646 CrossRef CAS PubMed; (b) S. Ogi, K. Sugiyasu, S. Manna, S. Samitsu and M. Takeuchi, Nat. Chem., 2014, 6, 188 CrossRef CAS PubMed; (c) S. Ogi, V. Stepanenko, K. Sugiyasu, M. Takeuchi and F. Würthner, J. Am. Chem. Soc., 2015, 137, 3300 CrossRef CAS PubMed; (d) A. Pal, M. Malakoutikhah, G. Leonetti, M. Tezcan, M. Colomb-Delsuc, V. D. Nguyen, J. van der Gucht and S. Otto, Angew. Chem., Int. Ed., 2015, 54, 7852 CrossRef CAS PubMed; (e) S. Ogi, T. Fukui, M. L. Jue, M. Takeuchi and K. Sugiyasu, Angew. Chem., Int. Ed., 2014, 53, 14363 CrossRef CAS PubMed; (f) W. Wagner, M. Wehner, V. Stepanenko, S. Ogi and F. Würthner, Angew. Chem., Int. Ed., 2017, 56, 16008 CrossRef CAS PubMed; (g) S. Ogi, K. Matsumoto and S. Yamaguchi, Angew. Chem., Int. Ed., 2018, 57, 2339 CrossRef CAS PubMed; (h) E. E. Greciano, B. Matarranz and L. Sánchez, Angew. Chem., Int. Ed., 2018, 57, 4697 CrossRef CAS PubMed; (i) J. S. Valera, R. Gómez and L. Sánchez, Angew. Chem., Int. Ed., 2019, 58, 510 CrossRef CAS PubMed; (j) E. E. Greciano and L. Sánchez, Chem.–Eur. J., 2016, 22, 13724 CrossRef CAS PubMed; (k) J. S. Valera, R. Gómez and L. Sánchez, Small, 2018, 14, 1702437 CrossRef PubMed.
- D. S. Pal, H. Kar and S. Ghosh, Chem. Commun., 2018, 54, 928 RSC.
- (a) M. A. Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685 CrossRef CAS PubMed; (b) A. Das and S. Ghosh, Chem. Commun., 2016, 52, 6860 RSC.
- The fate of NDI-1mono in decane was monitored for seven days by UV/Vis spectroscopy and AFM (Fig. S4†) in the absence of any added organobase. The monomeric state was stable for 24 h, but subsequently some precipitation was noticed which was also evident by the increase in the baseline intensity of the UV/Vis spectra due to scattering. The AFM image of the sample prepared after seven days showed the presence of heterogeneous spherical particles.
- (a) M. J. Frisch, et. al., Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed; (b) E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498 CrossRef CAS PubMed; (c) T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580 CrossRef CAS PubMed.
- N. E. Kamber, W. Jeong and R. M. Waymouth, Chem. Rev., 2007, 107, 5813 CrossRef CAS PubMed.
- (a) T. Fukui, S. Kawai, S. Fujinuma, Y. Matsushita, T. Yasuda, T. Sakurai, S. Seki, M. Takeuchi and K. Sugiyasu, Nat. Chem., 2017, 9, 493 CrossRef CAS PubMed; (b) C. E. Boott, A. Nazemi and I. Manners, Angew. Chem., Int. Ed., 2015, 54, 13876 CrossRef CAS PubMed; (c) M. Vybornyi, A. Rudnev and R. Häner, Chem. Mater., 2015, 27, 1426 CrossRef CAS; (d) M. Pfeffermann, R. Dong, R. Graf, W. Zajaczkowski, T. Gorelik, W. Pisula, A. Narita, K. Müllen and X. Feng, J. Am. Chem. Soc., 2015, 137, 14525 CrossRef CAS PubMed; (e) S. Ghosh, D. S. Philips, S. Saeki and A. Ajayaghosh, Adv. Mater., 2017, 29, 1605408 CrossRef PubMed.
- D. Vacognea Charlotte and H. Schlaad, Chem. Commun., 2015, 51, 15645 RSC.
- J. N Moorthy, J. Indian Inst. Sci., 2008, 88, 131 Search PubMed.
- (a) M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Chem.–Eur. J., 2014, 20, 760 CrossRef CAS PubMed; (b) F. Salerno, J. A. Berrocal, A. T. Haedler, F. Zinna, E. W. Meijer and L. D. Bari, J. Mater. Chem. C, 2017, 5, 3609 RSC.
- B. Adelizzi, A. Aloi, A. J. Markvoort, H. M. M. T. Eikelder, I. K. Voets, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 7168 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoslev, Nat. Mater., 2006, 6, 183 CrossRef PubMed.
- Preliminary investigations were carried out to examine the possible role of H-bonding donors such as TFA or pyrrole in initiating SROP. In the presence of TFA, no sign of supramolecular polymerization was noticed (Fig. S20†) after 6 h. But pyrrole could initiate supramolecular polymerization with relatively shorter lag phase producing 2D sheets (Fig. S21†).
- S. K. Park, J. H. Kim and S. Y. Park, Adv. Mater., 2018, 30, 1704759 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of NDI – derivatives, dormant monomer preparation, AFM and some additional experimental observations. See DOI: 10.1039/c9sc01972c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |