Dissymmetrical tails-regulated helical nanoarchitectonics of amphiphilic ornithines: nanotubes, bundles and twists†
Han-Xiao
Wang
a,
Lifei
Xu
ab,
Xuefeng
Zhu
*a,
Chenlu
Xue
ac,
Li
Zhang
 a and
Minghua
Liu
a and
Minghua
Liu
 *ab
*ab
aBeijing National Laboratory for Molecular Science (BNLMS), CAS Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: zhuxf06@iccas.ac.cn; liumh@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
cCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001, P. R. China
First published on 22nd December 2021
Abstract
How dissymmetrical tails (i.e. tails of different lengths) in one lipid molecule exert an impact on the structure and properties of the resulting assembly is an intriguing issue in both biological and material senses. However, the underlying mechanism that engenders such phenomena is still obscure, which prompted us to unmask it by exploring the self-assembly behaviours of artificial building blocks comprising dissymmetrical tails. Here, a series of Fmoc-protected ornithine lipids with dissymmetrical alkyl tails was designed and the dissymmetry of the two tails was found to hierarchically tune the self-assembled nanostructures from nanotubes to bundles and nanotwists. With the Fmoc-headgroup employed as a chromophorous probe, it was revealed that the alkyl chain dissymmetry controlled the interacting modes of van der Waals interactions between alkyl tails, π–π stacking between Fmoc motifs and hydrogen bonding formed by the three amide bonds in lipid bilayers. The counterbalance between those noncovalent interactions was responsible for such remarkable tuning ability towards self-assembly and emissive behaviours of the lipids, including circularly polarized light emission. This work provides insight into dissymmetrical tails-regulated biological structures and functions of natural lipids, and also sets up a novel strategy of rationally modulating chiral and emissive properties of supramolecular materials, i.e., tunable CPL materials, by exploitation of the tail dissymmetry.
Introduction
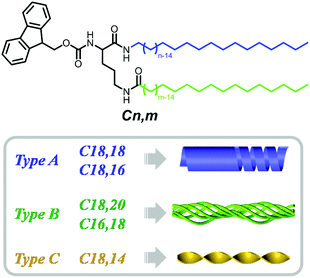
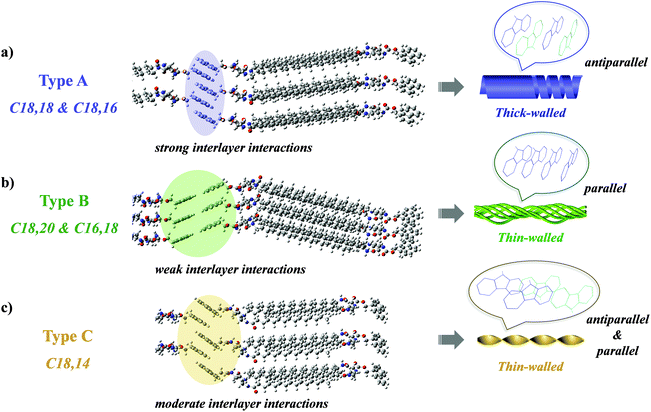
Self-assembly is widely observed in nature and occurs at all scales.1 During self-assembly the component molecules or building blocks come together to form ordered structures through covalent and noncovalent forces, such as π–π stacking, hydrogen bonding, the solvophobic effect, and van der Waals and metal–ligand interactions.2–13 Among various building blocks, the amphiphiles, which simultaneously consist of hydrophilic headgroups and hydrophobic tails, form one category of the most powerful building blocks and could possibly form almost any kind of nanostructure.14–26 In nature, amphiphiles such as phospholipids play a very important role in constructing bio-membranes and control their functions.27–29 Interestingly, natural phospholipids always comprise two dissymmetrical tails to achieve various functions, such as tuning the fluidity and altering the penetration properties of cell membranes.30–33 Inspired by this nature-selected intelligent strategy, artificial phospholipids and other molecules with two dissymmetrical tails have been designed to realize the fine tuning of functional properties.34–38 However, it remains unknown how the two tails in the amphiphile could affect the packing of the functional groups and finally their manner of collective assembly, while shedding light on this issue is of great significance for unravelling the dissymmetrical tails-harnessed biological functions. Herein, we selected Fmoc-protected L-ornithine amphiphiles with dissymmetrical tails to investigate their packing modes, in which Fmoc acted as a luminescent pi-functional motif, and two alkyl tails with different chain lengths were introduced to prepare the dissymmetrical lipids Cn,m (Scheme 1). The emissive fluorenyl group in Fmoc could be employed as a probe to detect the arrangement of the amphiphiles. Intriguingly, as shown in Fig. 1, when the length of alkyl chains at the α- and ω-sites in lipids was altered, the morphology of the assemblies formed by these lipids changed remarkably from hollow helical nanotubes for C18,18 and C18,16 (denoted type A), bundles with fine fibrils for C18,20 and C16,18 (denoted type B), to twisted fibrils for C18,14 (denoted type C). A range of spectral characterizations revealed that such morphological tuning could be ascribed to different interacting modes consisting of π–π stacking, hydrogen-bonding and alkyl packing. The results not only contribute to deciphering the role the dissymmetrical tails in natural phospholipids played in tuning their functions, but also provided a new modification strategy to design functional supramolecular materials, especially supramolecular chiral materials and luminescent materials.Results and discussion
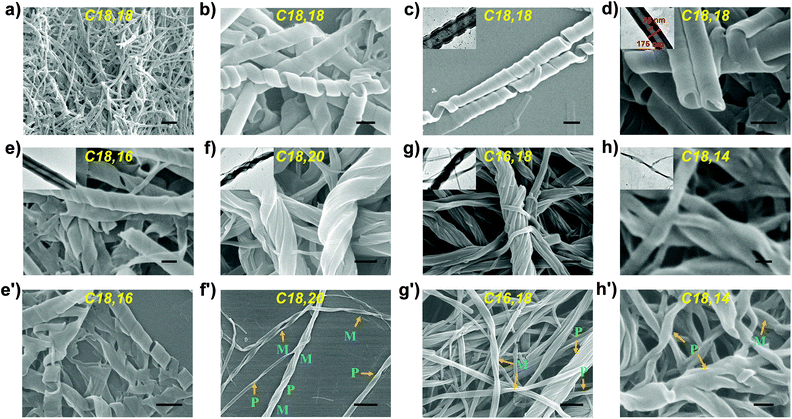
Via a simple route comprising two steps of amide condensation reaction and one step of deprotection, a series of L-ornithine-based lipids Cn,m with alkyl chains of different lengths was synthesized in reasonable overall yields (Scheme 1). It was found that with π-conjugated headgroups, multiple hydrogen bonding sites and long alkyl chains, all the lipids assembled into white opaque supramolecular gels in DMSO through a heating-and-cooling method at a concentration of 1 × 10−2 mol L−1 (Fig. S6†).SEM and TEM characterization showed that lipid C18,18 self-assembled into right-handed helical nanotubes with a uniform helical angle of around 70° (Fig. 1a–d and S8a–d†). It could be observed that the helical nanotubes were formed by the scrolling of nanobelts, which ended up with the sewing up of the helical seams to generate uniform nanotubes (Fig. S9†),39 with outer diameters of 175 nm, inner diameters of 75 nm and a thickness of about 50 nm (Fig. 1d). Lipid C18,16 self-assembled into right-handed helical nanotubes as C18,18 did (Fig. 1e and e′).
Surprisingly, a slight change in the length of the α- and ω-alkyl chains in the lipid could induce a significant morphological transformation. Specifically, adding two extra methylene units in the ω-alkyl chain to yield C18,20 changed the nanotubes formed by C18,18 into bundles (Fig. 1f). Interestingly, lipid C16,18 which had the same total chain length as C18,16, self-assembled into bundles (Fig. 1g), analogous to the nanostructures of the gel prepared from C18,20. Explicitly, with regard to lipids C18,16 and C16,18, by keeping the total length of the two tails constant and only changing the position of the junction, the self-assembly behaviours were dramatically affected. Meanwhile, lipid C18,14 formed the third kind of nanostructure, twisted fibers (Fig. 1h). After scrutiny of the SEM images (Fig. 1f′–h′ and S7†), we found that although one single helical or twisted fiber might display left- or right-handedness on the nanoscale, structures of both P, M or even combined helicities assembled from lipids C16,18, C18,20 or C18,14 could be observed. In short, according to nanostructures of the gels, the lipids with dissymmetrical tails could be categorized into three types: type A, C18,18 and C18,16 (right-handed nanotubes formed by helical belts); type B, C18,20 and C16,18 (helical bundles); and type C, C18,14 (twisted fibrils).
To disclose the underlying mechanism of the influence that alkyl chain dissymmetry exerted on the nanostructures, a detailed study of the UV-Vis, FL, CD, CPL, FT-IR and XRD spectra was carried out.
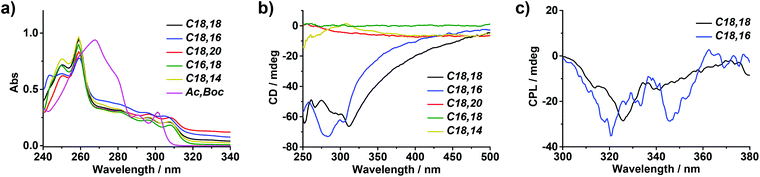
From the UV-Vis spectra in Fig. 2a, it could be inferred that the headgroups were in the aggregated form in all gelated lipids as the absorption peaks exhibited a notable bathochromic shift of the π–π* transition in the fluorenyl groups (290–320 nm) compared with that of Ac,Boc, which could represent the molecular state of the fluorenyl group.40 In the meantime, the absorption bands in assemblies of type A displayed a slight hypochromic shift in comparison with that in assemblies of type B and type C. According to previous literature,41 when the fluorenyl groups adopted antiparallel orientations in the aggregate, their absorption band would appear in regions of higher energy than when adopting parallel orientations, indicating that in type A the headgroups might be packed in an antiparallel manner.
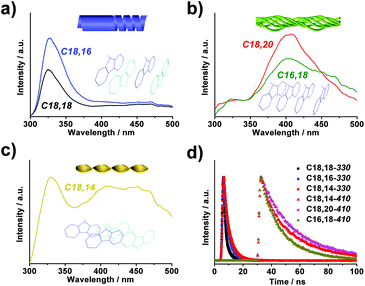
Fluorescence spectra of each type displayed pronounced differences as well. When excited by 265 nm irradiation, the nanotubes (type A) gave an emission around 330 nm (Fig. 3a), which was a clear indication that the fluorenyl motifs were arranged in an antiparallel manner.42–44 In comparison, the bundles (type B) displayed an emission band centered at 408 nm (Fig. 3b), suggesting a stacking mode of the fluorenyl motifs in a parallel manner. The twisted fibrils, on the other hand, exhibited dual emission maxima at 330 nm and 410 nm (Fig. 3c), respectively, revealing the coexistence of antiparallel and parallel modes in the arrangement of fluorenyl motifs. It was further demonstrated that in all the systems, the lifetime of the 330 nm-centered band was 6.5 ns while that of the band around 410 nm was determined to be 32.3 ns (Fig. 3d), confirming that both peaks were fluorescent emission, and that the latter might be ascribed to an excimer emission induced by the parallel packing of fluorenyl groups.40
Moreover, in supramolecular assemblies, the asymmetrical information could be expected to transfer from the chiral center to the chromophores through chiral packing controlled by the molecular chirality, and be detected by means of spectroscopic techniques such as circular dichroism and circularly polarized luminescence, providing unique information on the molecular stacking mode, especially the arrangement concerning the chromophores.45–50 In order to probe the supramolecular chirality of assemblies with different morphologies, the gels were first subjected to circular dichroism (CD) measurements. As shown in Fig. 2b, right-handed nanotubes assembled from C18,18 and C18,16 presented a strong negative Cotton effect in the range of 265–310 nm coinciding with their absorbance bands, while the molecularly dispersed DMSO solution kept silent in the CD spectra (Fig. S10,† blue line), indicating that supramolecular chirality was induced by chiral stacking of the fluorenyl motifs during the assembly process. However, in line with the observation of SEM (Fig. 1f′–h′ and S7†), neither bundles formed by C18,20 and C16,18 nor twisted fibrils by C18,14 showed discernible CD signals (Fig. 2b) which indicated that on the whole the supramolecular chirality was offset by the coexisting P- and M-handed nanostructures.
Circularly polarized luminescence (CPL) spectra provided chiral information about chromophores in the excited state.51–55 As expected, nanotubes assembled from both C18,18 and C18,16 emitted right-handed CPL centered at 330 nm, corresponding to the wavelength of their fluorescence (Fig. 2c). As for bundles from C18,20 and C16,18 and twisted fibrils from C18,14, no CPL signals were detected (Fig. S11†), which was consistent with the results of SEM and CD measurements. On account of the chiral center introduced by the ornithine unit, it was reasoned that in assemblies of type A, the molecular chirality induced asymmetrical packing of the lipids bridged by noncovalent interactions and thus an asymmetrical arrangement of the chromophoric Fmoc segments during the assembly process, leading to the manifestation of CD and CPL signals. In contrast, the transfer from molecular chirality to supramolecular chirality in assemblies of type B and type C was far from effective, which resulted in silence in both CD and CPL spectra.
It had been revealed that the difference in length of the two alkyl tails exerted notable influence on the packing modes of the luminescent fluorenyl headgroups. In order to clarify the molecular arrangement and noncovalent interactions responsible for the above phenomena, FT-IR and XRD measurements were carried out on the gels. As shown in Fig. 4 and Table S1,† peaks at 3290–3300 cm−1, which could be assigned to N–H stretching vibrations, demonstrated the existence of hydrogen bonds involving the N–H bonds on the amide groups.56 Bands centered around 2920 cm−1 and 2850 cm−1 attributed to asymmetric and symmetric CH2 stretching vibrations, respectively, indicated that the alkyl chains were closely packed in the supramolecular assemblies. Apart from the above features in common, there were distinct differences among the three types of lipids. The most evident difference lay in the vibrational region of the amide. For bundles (type B), the amide I band appeared at 1638 cm−1, while for twisted fibrils (type C) and nanotubes (type A), the band shifted to 1639 cm−1 and 1643 cm−1, respectively, and two new peaks emerged at 1690 cm−1 and 1655 cm−1 (type A) and 1686 cm−1 and 1658 cm−1 (type C). The amide II band was observed at 1551 cm−1 for type B, at 1542 cm−1 for type A, and was split into 1551 cm−1 and 1542 cm−1 for type C. The amide III band was detected at 1259 cm−1 for types B and C, but there was an extra shoulder peak at 1249 cm−1 for type A. All these differences suggested that compared to assemblies of type B, in assemblies of type A and C, the hydrogen bonds involving the amide groups were weaker, and additionally the chemical environments surrounding the three amide groups in one lipid molecule were differentiated from each other. Moreover, the peaks ascribed to methylene groups in the tails also displayed obvious differences. To elaborate, in types A and C, a shoulder peak at 1451 cm−1 was detected in addition to the peak at 1468 cm−1, and a new signal at 737 cm−1 appeared apart from the peak at 723 cm−1, which was an indicator that for types A and C, the alkyl chains did not adopt all-trans zigzag conformations as in type B. From the above results from the FT-IR spectra, it could be summarized that in assemblies of types A and C, the hydrogen bonds were weaker and the alkyl chains were in a lower order than those in assemblies of type B. The XRD measurements also revealed molecular arrangement in the supramolecular gels. All the assemblies were determined to adopt lamellar structures with different d-spacing values corresponding to lipids with different tail lengths (Fig. S12†). These results unveiled that the supramolecular assemblies were stabilized by multiple noncovalent interactions including π–π interactions (optical spectra), hydrogen bonds and van der Waals interactions (FT-IR spectra), which jointly contributed to the speculation that in assemblies of different types, distinctive modes of trade-off between these interactions were maintained and finally brought forth diverse nanostructures and optical behaviours.
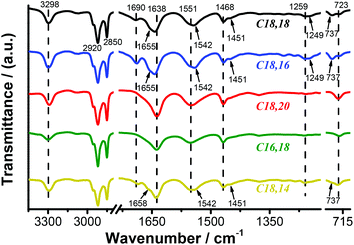 | ||
| Fig. 4 FT-IR spectra of self-assembled nanostructures in DMSO (1 × 10−2 mol L−1) of lipids C18,18 (black line), C18,16 (blue line), C18,20 (red line), C16,18 (green line) and C18,14 (yellow line). | ||
As has been analyzed above, we proposed a mechanism through which the alkyl chain-tuned morphology and emission were realized, in which the values of n–m in Cn,m played a decisive role (Scheme 2). In the two tails of each lipid, the one denoted Cn (the blue-coloured tail in Scheme 1) was linked to the chiral center via an amide group with the alkyl chain adjacent to the amino moiety, while the one denoted Cm (the green-coloured tail in Scheme 1) was linked to the chiral center via a (CH2)3 spacer and an amide group with an inversed connectivity, i.e., the alkyl chain lying adjacent to the carbonyl moiety. This dissymmetry57 combined with the value of n–m led to marked differences in the assembly behaviours. In the polar solvent DMSO, the lipids first assembled into a bilayer structure with the alkyl chains pointing inward and interdigitating. In assemblies of type A where n–m = 0 or 2,34,58 the two tails could be adjusted into a nearly flush state, which thus resulted in an appropriate mode in which the headgroups in every pair of neighbouring bilayers interacted with each other to strengthen the interlayer interactions (Scheme 2a). In consequence, the fluorenyl groups in the lipid were arranged in an antiparallel fashion and gave out an emission at 330 nm. Moreover, the interlayer π–π interactions between the fluorenyl groups prevailed over the intralayer multiple hydrogen bonding and van der Waals interactions and thus weakened them, which could be evidenced by the FT-IR spectra (Fig. 4). With the help of interlayer π–π interactions, the bilayers were steadily stacked successively and finally assembled into right-handed nanotubes, the wall of which was calculated to be composed of approximately 10 lipid bilayers (the thickness was about 50 nm, as stated above, and the d-spacing value was 5.19 nm, as determined by XRD, Fig. S12†). It could be justified that ca. 10 bilayers resulted in a relatively thick wall which was liable to curve in a helical manner and then tighten to afford seamless nanotubes.59 During the assembly process, the molecular chirality was effectively transferred to the supramolecular level by virtue of strong interlayer interactions and manifested itself in the CD and CPL spectra (Fig. 2b and c). On the other hand, for lipids of type B, in which n–m = −2, one of the tails was notably longer than the other one. So to attain higher ordering in the assemblies, the alkyl chains adopted all-trans zigzag conformations and the amide groups packed in such a manner that they formed strong and uniform intermolecular hydrogen bonds, as exhibited in the FT-IR spectra. Consequently, the orientation of the headgroups was restricted by the aggregated tails, which hindered the adaptation of the Fmoc groups from taking an active part in interlayer interactions (Scheme 2b). Therefore, the fluorenyl moieties were packed in a parallel fashion, supported by the emission at 408 nm. For lipids of type C (n–m = 4), the two tails were matched to a medium extent, providing moderate adaptability for the headgroups to participate in partial interlayer interactions (Scheme 2c), which thus led to the concurrence of parallel and antiparallel packing modes of the fluorenyl moieties, as demonstrated by the appearance of both 330 nm and 410 nm emission bands. From TEM images (Fig. 1f–h, Fig. S8g–l†), it could be spotted that the substructures that formed the bundles (type B) and twisted fibrils (type C) were quite thin, indicating that the bilayers of the lipids were not bound as firmly as those in assemblies of type A, and were apt to adopt saddle-like curvature to form bundles or nanotwists.59 Furthermore, the lack of strong interlayer interactions and efficient layer-by-layer accumulation of the chirality resulted in the absence of supramolecular chirality, witnessed by the aforementioned presence of both P- and M-handed nanostructures and silence in the CD and CPL spectra in assemblies of C18,20, C16,18 and C18,14. Consequently, in a cooperation between dissymmetry in the spacers and variations in the length of the alkyl chains in the two tails, the nanoscale morphologies, supramolecular chirality and fluorescent properties could be effectively modulated, which validated the feasibility of a dissymmetrical-tails strategy in effectively tuning the self-assembly behaviour of lipids.
Conclusions
In summary, a series of Fmoc-modified ornithine lipids with variations in alkyl chain length was designed and synthesized. By virtue of the intrinsically dissymmetrical structure of the ornithine framework and variation in the length of the two appended tails, three types of assemblies were established which exhibited distinctive nanostructures, including nanotubes with right-handedness, bundles and nanotwists. By employing the fluorenyl headgroup as a fluorescent probe, it was disclosed that in assemblies of types A and B, the headgroups were arranged in antiparallel and parallel manners, respectively, while in assemblies of type C, both manners coexisted. By virtue of comprehensively analyzing the results of UV-Vis, FL, CD, CPL, FT-IR and XRD, it could be concluded that the varied self-assembly behaviours were induced by differentiated dissymmetry of the lipid tails, which subsequently led to different interacting modes affected by a counterbalance between interlayer or intralayer π–π stacking versus hydrogen bonds and van der Waals interactions. Regarding the current paucity of relevant intrinsic mechanisms, this study provides insight into the modulation of assembly behaviours by dissymmetrical structures of lipids in biological systems, and additionally offers a new strategy to rationally design supramolecular chiral luminescent materials tailored by tail dissymmetry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21890734, 21890730) and Key Research Program of Frontier Sciences, CAS (QYZDJSSW-SLH044).Notes and references
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- Z. Li, J. Ma, N. S. Lee and K. L. Wooley, J. Am. Chem. Soc., 2011, 133, 1228–1231 CrossRef CAS PubMed.
- C. G. Claessens and J. F. Stoddart, J. Phys. Org. Chem., 1997, 10, 254–272 CrossRef CAS.
- T. Kawasaki, M. Tokuhiro, N. Kimizuka and T. Kunitake, J. Am. Chem. Soc., 2001, 123, 6792–6800 CrossRef CAS PubMed.
- H. Matsukizono, K. Kuroiwa and N. Kimizuka, J. Am. Chem. Soc., 2008, 130, 5622–5623 CrossRef CAS PubMed.
- C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed.
- C. Oliveras-Gonzalez, F. Di Meo, A. Gonzalez-Campo, D. Beljonne, P. Norman, M. Simon-Sorbed, M. Linares and D. B. Amabilino, J. Am. Chem. Soc., 2015, 137, 15795–15808 CrossRef CAS PubMed.
- B. J. Cafferty, I. Gallego, M. C. Chen, K. I. Farley, R. Eritja and N. V. Hud, J. Am. Chem. Soc., 2013, 135, 2447–2450 CrossRef CAS PubMed.
- H. Katagiri, Y. Tanaka, Y. Furusho and E. Yashima, Angew. Chem., Int. Ed., 2007, 46, 2435–2439 CrossRef CAS PubMed.
- Y. Tu, Z. Ji, X. Yang, X. Wan and Q.-F. Zhou, Macromol. Rapid Commun., 2014, 35, 1795–1800 CrossRef CAS PubMed.
- R. Wang, X. Wan and J. Zhang, Chem. Commun., 2019, 55, 4711–4714 RSC.
- A. K. Patterson and D. K. Smith, Chem. Commun., 2020, 56, 11046–11049 RSC.
- C. C. Piras, P. Slavik and D. K. Smith, Angew. Chem., Int. Ed., 2020, 59, 853–859 CrossRef CAS PubMed.
- D. Berthier, T. Buffeteau, J. M. Leger, R. Oda and I. Huc, J. Am. Chem. Soc., 2002, 124, 13486–13494 CrossRef CAS PubMed.
- A. Brizard, C. Aime, T. Labrot, I. Huc, D. Berthier, F. Artzner, B. Desbat and R. Oda, J. Am. Chem. Soc., 2007, 129, 3754–3762 CrossRef CAS PubMed.
- T. Nakanishi, K. Ariga, T. Michinobu, K. Yoshida, H. Takahashi, T. Teranishi, H. Moehwald and D. G. Kurth, Small, 2007, 3, 2019–2023 CrossRef CAS PubMed.
- P. Iavicoli, H. Xu, L. N. Feldborg, M. Linares, M. Paradinas, S. Stafstrom, C. Ocal, B. L. Nieto-Ortega, J. Casado, J. T. L. Navarrete, R. Lazzaroni, S. De Feyter and D. B. Amabilino, J. Am. Chem. Soc., 2010, 132, 9350–9362 CrossRef CAS PubMed.
- X. Cai, J. Du, L. Zhang, Y. Li, B. Li, H. Li and Y. Yang, Chem. Commun., 2019, 55, 12176–12179 RSC.
- I. Danila, F. Riobe, F. Piron, J. Puigmarti-Luis, J. D. Wallis, M. Linares, H. Agren, D. Beljonne, D. B. Amabilino and N. Avarvari, J. Am. Chem. Soc., 2011, 133, 8344–8353 CrossRef CAS PubMed.
- K. Kuroiwa, M. Yoshida, S. Masaoka, K. Kaneko, K. Sakai and N. Kimizuka, Angew. Chem., Int. Ed., 2012, 51, 656–659 CrossRef CAS PubMed.
- M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2013, 15, 10580–10611 RSC.
- S. Fleming and R. V. Ulijn, Chem. Soc. Rev., 2014, 43, 8150–8177 RSC.
- D. B. Amabilino, D. K. Smith and J. W. Steed, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC.
- M. T. Jeena, L. Palanikumar, E. M. Go, I. Kim, M. G. Kang, S. Lee, S. Park, H. Choi, C. Kim, S.-M. Jin, S. C. Bae, H. W. Rhee, E. Lee, S. K. Kwak and J.-H. Ryu, Nat. Commun., 2017, 8, 26 CrossRef CAS PubMed.
- M. F. J. Mabesoone, A. J. Markvoort, M. Banno, T. Yamaguchi, F. Helmich, Y. Naito, E. Yashima, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 7810–7819 CrossRef CAS PubMed.
- T. Miao, X. Cheng, H. Ma, Z. He, Z. Zhang, N. Zhou, W. Zhang and X. Zhu, Angew. Chem., Int. Ed., 2021, 60, 18566–18571 CrossRef CAS PubMed.
- F. Hirata and J. Axelrod, Science, 1980, 209, 1082–1090 CrossRef CAS PubMed.
- Y. Nishizuka, Science, 1992, 258, 607–614 CrossRef CAS PubMed.
- M. A. Lemmon, Nat. Rev. Mol. Cell Biol., 2008, 9, 99–111 CrossRef CAS PubMed.
- N. Dan, Biochim. Biophys. Acta, Biomembr., 2007, 1768, 2393–2399 CrossRef CAS PubMed.
- S. Leekumjorn and A. K. Sum, J. Phys. Chem. B, 2007, 111, 6026–6033 CrossRef CAS PubMed.
- T. Shimanouchi, H. Ishii, N. Yoshimoto, H. Umakoshi and R. Kuboi, Colloids Surf., B, 2009, 73, 156–160 CrossRef CAS PubMed.
- Y. G. Smirnova, S.-J. Marrink, R. Lipowsky and V. Knecht, J. Am. Chem. Soc., 2010, 132, 6710–6718 CrossRef CAS PubMed.
- R. Oda, I. Huc and S. J. Candau, Chem. Commun., 1997, 21, 2105–2106 RSC.
- D. Mirjanian, A. N. Dickey, J. H. Hoh, T. B. Woolf and M. J. Stevens, J. Phys. Chem. B, 2010, 114, 11061–11068 CrossRef CAS PubMed.
- J. Xu, G. Wu, Z. Wang and X. Zhang, Langmuir, 2013, 29, 10959–10963 CrossRef CAS PubMed.
- H. F. D. Almeida, M. G. Freire, A. M. Fernandes, J. A. Lopes-da-Silva, P. Morgado, K. Shimizu, E. J. M. Filipe, J. N. C. Lopes, L. M. N. B. F. Santos and J. A. P. Coutinho, Langmuir, 2014, 30, 6408–6418 CrossRef CAS PubMed.
- S. Khanal, R. J. Brea, M. D. Burkart and N. K. Devaraj, J. Am. Chem. Soc., 2021, 143, 8533–8537 CrossRef CAS PubMed.
- X. Zhu, Y. Li, P. Duan and M. Liu, Chem. – Eur. J., 2010, 16, 8034–8040 CrossRef CAS PubMed.
- T. Nakano and T. Yade, J. Am. Chem. Soc., 2003, 125, 15474–15484 CrossRef CAS PubMed.
- D. Schweitzer and M. W. Haenel, Chem. Ber., 1985, 118, 163–175 CrossRef CAS.
- Y. Zhang, H. Gu, Z. Yang and B. Xu, J. Am. Chem. Soc., 2003, 125, 13680–13681 CrossRef CAS PubMed.
- Z. Yang, H. Gu, D. Fu, P. Gao, J. K. Lam and B. Xu, Adv. Mater., 2004, 16, 1440–1444 CrossRef CAS.
- V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611–614 CrossRef CAS.
- L. Perez-Garcia and D. B. Amabilino, Chem. Soc. Rev., 2002, 31, 342–356 RSC.
- L. Perez-Garcia and D. B. Amabilino, Chem. Soc. Rev., 2007, 36, 941–967 RSC.
- M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304–7397 CrossRef CAS PubMed.
- Z. Zhao, S. Wang, X. Ye, J. Zhang and X. Wan, ACS Macro Lett., 2017, 6, 205–209 CrossRef CAS.
- K. Ariga, T. Mori, T. Kitao and T. Uemura, Adv. Mater., 2020, 32, 1905657 CrossRef CAS PubMed.
- S. Cai, J. Chen, S. Wang, J. Zhang and X. Wan, Angew. Chem., Int. Ed., 2021, 60, 9686–9692 CrossRef CAS PubMed.
- W. Chen, K. Ma, P. Duan, G. Ouyang, X. Zhu, L. Zhang and M. Liu, Nanoscale, 2020, 12, 19497–19515 RSC.
- P. Liu, W. Chen, Y. Okazaki, Y. Battie, L. Brocard, M. Decossas, E. Pouget, P. Muller-Buschbaum, B. Kauffmann, S. Pathan, T. Sagawa and R. Oda, Nano Lett., 2020, 20, 8453–8460 CrossRef CAS PubMed.
- N. Ryu, T. Kawaguchi, H. Yanagita, Y. Okazaki, T. Buffeteau, K. Yoshida, T. Shirosaki, S. Nagaoka, M. Takafuji, H. Ihara and R. Oda, Chem. Commun., 2020, 56, 7241–7244 RSC.
- T. Harada, H. Yanagita, N. Ryu, Y. Okazaki, Y. Kuwahara, M. Takafuji, S. Nagaoka, H. Ihara and R. Oda, Chem. Commun., 2021, 57, 4392–4395 RSC.
- S. Wang, D. Hu, X. Guan, S. Cai, G. Shi, Z. Shuai, J. Zhang, Q. Peng and X. Wan, Angew. Chem., Int. Ed., 2021, 60, 21918–21926 CrossRef CAS PubMed.
- Y. Zhang, X. Cheng, X. Jiang, J. J. Urban, C. H. Lau, S. Liu and L. Shao, Mater. Today, 2020, 36, 40–47 CrossRef CAS.
- P. J. M. Stals, J. C. Everts, R. de Bruijn, I. A. W. Filot, M. M. J. Smulders, R. Martín-Rapún, E. A. Pidko, T. F. A. de Greef, A. R. A. Palmans and E. W. Meijer, Chem. – Eur. J., 2010, 16, 810–821 CrossRef CAS PubMed.
- X. Zhu, Y. Jiang, D. Yang, L. Zhang, Y. Li and M. Liu, Chem. Sci., 2019, 10, 3873–3880 RSC.
- A. Brizard, C. Aimé, T. Labrot, I. Huc, D. Berthier, F. Artzner, B. Desbat and R. Oda, J. Am. Chem. Soc., 2007, 129, 3754–3762 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr07538a |
| This journal is © The Royal Society of Chemistry 2022 |