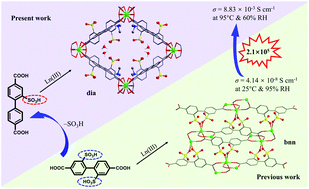
Porous crystalline metal–organic frameworks (MOFs) bearing sulfonic groups (–SO3H) are receiving increasing attention as solid-state proton conductors because the –SO3H group can not only enhance the proton concentration, but also form hydrogen bonding networks for high proton conductivity. A large number of 1,4-phenyldicarboxylic acids or biphenyldicarboxylic acids bearing two –SO3H groups have been applied for the synthesis of proton-conducting MOFs. Surprisingly, 4,4′-biphenyldicarboxylic acid bearing one –SO3H group has never been explored for the construction of proton-conducting materials. Herein, we first designed and synthesized 2-sulfonyl-4,4′-biphenyldicarboxylic acid (H3L). By applying this ligand to react with lanthanide salts, a series of three-dimensional MOFs, (Me2NH2)2(H3O)[LnL2]·8H2O (Ln = Eu (1), Gd (2), Tb (3)) have been prepared. Due to the presence of the uncoordinated –SO3H group and the encapsulation of high concentrations of dimethylammonium and hydronium cations in the cavity, the MOFs 1–3 show a high proton conductivity (8.83 × 10−3 S cm−1) at 95 °C and 60% relative humidity (RH). More importantly, this high proton conductivity can be maintained over 72 hours without any significant decrease at low RH.