Fluorosulfonyl radicals: new horizons for the synthesis of sulfonyl fluorides
Fu-Sheng
He
*a,
Yuqing
Li
a and
Jie
Wu
 *abc
*abc
aSchool of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou 318000, China. E-mail: hefs@tzc.edu.cn; jie_wu@fudan.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 18th August 2022
Abstract
Sulfonyl fluorides have found widespread applications in organic synthesis, chemical biology, drug discovery and materials science. Compared with current methods, direct fluorosulfonylation with fluorosulfonyl radicals has emerged as a concise and efficient approach for producing sulfonyl fluorides. This highlight provides an overview of recent advances in the generation of fluorosulfonyl radicals from different precursors and their participation in the synthesis of diverse functionalized sulfonyl fluorides.
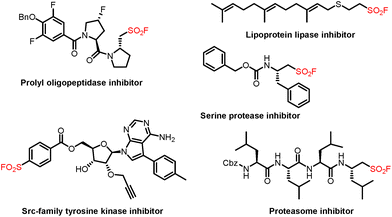
Sulfonyl fluoride is a prevalent structural unit found in biologically active molecules and versatile synthetic intermediates in modern organic synthesis (Fig. 1).1 Since the concept of Sulfur(VI) Fluoride Exchange (SuFEx) was pioneered by Sharpless and co-workers as the next generation of click chemistry in 2014,2 sulfonyl fluorides have received considerable attention and continuous interest from the communities of synthetic chemistry and medicinal chemistry. Generally, strategies for the construction of these frameworks mainly focus on the fluoride–chloride exchange from the corresponding sulfonyl chlorides.3 Other methods, including conjugate addition,4 electrophilic fluorination of thiols,5 anodic oxidative fluorination,6 and SO2 insertion/fluorination,7 for the preparation of sulfonyl fluorides have also been reported. Compared with these methods via S–F bond formation, direct fluorosulfonylation by using FSO2-containing reagents represents a concise and effective approach for C–SO2F bond formation. In this case, the reported fluorosulfonylating reagents such as sulfuryl fluoride gas (SO2F2)2 and other solid reagents (FDIT and AISF)8 have been widely utilized as the synthetic equivalents of electrophilic “FSO2+” synthons to access sulfonyl fluorides. In contrast, fluorosulfonylation of the corresponding fluorosulfonyl radical (FSO2˙) is more challenging and remains less well explored.
As one of the sulfur-centered radicals, the highly active fluorosulfonyl radical has been ignored for a long time owing to its instability and difficulty of generation. It was not until 2013 that Zeng, Beckers, and co-workers first observed the formation of the fluorosulfonyl radical by the flash vacuum pyrolysis of fluorosulfonyl azide.9 Inspired by this observation, many efforts have been devoted to the development of suitable fluorosulfonyl radical precursors for the direct radical fluorosulfonylation reactions. In this highlight article, we focus on the most recent advances in the fluorosulfonyl radical-involved transformations, with emphasis on the synthesis of sulfonyl fluorides.
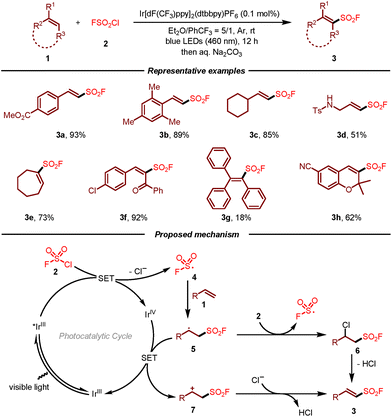
The first radical fluorosulfonylation of alkenes using FSO2Cl as the fluorosulfonyl radical precursor was reported by Liao and co-workers in 2020 (Scheme 1).10,11 This photoredox-catalyzed reaction shows a broad substrate scope and excellent functional group compatibility, providing a novel and facile approach for the synthesis of alkenyl sulfonyl fluorides. Notably, the scope of alkenes could be expanded to cyclic, di- and tri-substituted olefins, giving rise to multi-substituted vinyl sulfonyl fluorides that are challenging to be synthesized using established cross-coupling methods. Moreover, the late-stage fluorosulfonylation of natural products including oleic ester, cinnamate, chromene, estrone, pregnenolone, and cholesterol also demonstrated the superiority of this approach. The reaction mechanism for this visible-light-induced radical fluorosulfonylation of alkenes 1 with FSO2Cl 2 was supported by the experimental and DFT calculation studies. Initially, single-electron reduction of FSO2Cl by the excited Ir(III)* would occur to generate fluorosulfonyl radical 4, which then attacked the double bond of alkene 1 leading to carbon radical intermediate 5. In the case of a radical chain process, the radical would react with FSO2Cl to form chlorinated intermediate 6. Following the loss of HCl, fluorosulfonylation product 3 would be afforded. On the other hand, a redox pathway that involved a single-electron oxidation/deprotonation process could not be excluded.
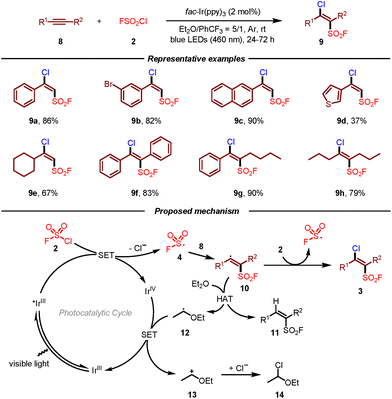
However, the above radical fluorosulfonylation of multi-substituted alkenes usually suffered from side reactions, low reaction efficiency, and low Z/E selectivity. To address these issues, the same group subsequently developed a photoredox-catalyzed radical chloro-fluorosulfonylation of alkynes for the construction of β-chloro alkenylsulfonyl fluorides (BCASF) (Scheme 2).12 Under the optimal reaction conditions, both terminal and internal alkynes reacted smoothly in this transformation. More importantly, this protocol provided a novel and powerful class of sulfonyl fluoride hubs, which could be converted to a series of functionalized alkenylsulfonyl fluorides. As a result, BCASF molecules could undergo different types of transformations at the β-chloro site while maintaining the sulfonyl fluoride group intact, such as Suzuki coupling, Sonogashira coupling, selective reduction, and nucleophilic substitution with nitrogen, oxygen, and sulfur nucleophiles. Furthermore, this versatile platform could also be applied in the late-stage modification of peptides and drugs. Similar to their previous work, it was postulated that fluorosulfonyl radical 4 could be first generated from FSO2Cl 2 by single-electron reduction. Then, addition of fluorosulfonyl radical 4 to alkyne 8 produced vinyl radical intermediate 10, which could attack FSO2Cl 2 to deliver the target product 3 and initiate the radical chain process. Meanwhile, vinyl radical 10 might undergo hydrogen-atom transfer from Et2O leading to alkenylsulfonyl fluoride 11 and radical 12. A single-electron transfer of the oxidized Ir(IV) by ether-derived radical 12 would give rise to carbocation intermediate 13 and regenerate the Ir(III) photocatalyst. Finally, GC-MS detection of α-chlorinated diethyl ether 14 suggested the addition of the chloride anion to the carbocation.
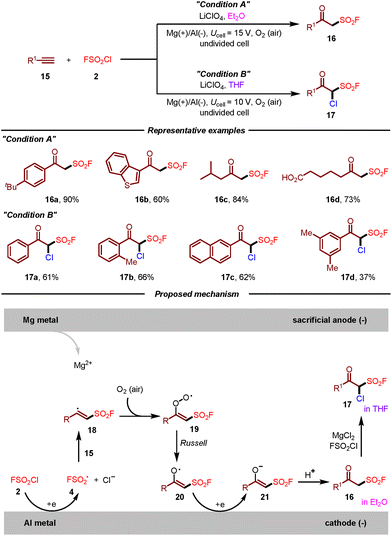
In the same year, Liao, Huang, and co-workers disclosed a practical synthesis of β-keto sulfonyl fluorides by electrochemical oxo-fluorosulfonylation of alkynes with FSO2Cl (Scheme 3).13 For the first time, the fluorosulfonyl radical could be generated under electrochemical conditions. This strategy featured mild reaction conditions, broad substrate scope, air as the oxidant and high chemoselectivity. Remarkably, the divergent synthesis of β-keto sulfonyl fluorides and α-chloro β-keto sulfonyl fluorides from the same starting materials was achieved simply by the variation of solvents. Additionally, some β-keto sulfonyl fluoride derivatives showed potent activities toward Bursaphelenchus xylophilus and Colletotrichum gloeosporioides. Regarding the reaction mechanism, it was suggested that cathodic reduction of FSO2Cl 2 would produce fluorosulfonyl radical 4, which then attacked the triple bond of alkyne 15 leading to vinyl radical 18. Vinyl radical 18 would react with O2 to generate peroxy radical 19, which could be converted to radical 20 according to the Russell mechanism. Subsequently, radical 20 undergoes a cathodic reduction and protonation sequence to furnish β-keto sulfonyl fluoride 16. In the case of THF as the solvent, α-chloro β-keto sulfonyl fluoride 17 could be obtained through electrophilic chlorination of 16 with MgCl2 and FSO2Cl. However, the current electrochemical approach was exclusively limited to employing a sacrificial magnesium anode.
After that, the same group developed an electrochemical radical fluorosulfonylation of vinyl triflates with FSO2Cl by using inexpensive graphite felt as electrodes (Scheme 4).14 As a result, a broad spectrum of readily available vinyl triflates were well tolerated, giving rise to various β-keto sulfonyl fluoride derivatives in moderate to good yields. Moreover, this metal-free electrosynthetic approach was amenable to be scaled up. On the basis of the results of control experiments, a plausible mechanism for this transformation was proposed. Initially, fluorosulfonyl radical 4 would be generated from FSO2Cl 2 by cathodic reduction, which would undergo addition to vinyl triflate 22 giving rise to radical 25. Following β-fragmentation, the final product 24 would be produced with the generation of CF3SO2-radical 26. This F3SO2-radical 26 would release SO2 leading to CF3 radical 27, which would undergo hydrogen atom transfer from diethyl ether to afford HCF328.
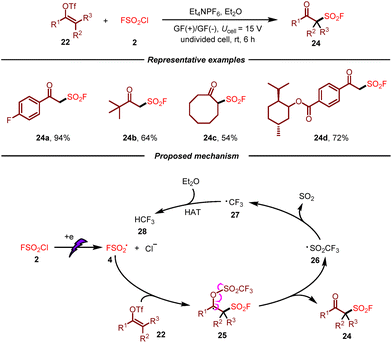 | ||
| Scheme 4 Electrochemical synthesis of β-keto sulfonyl fluorides via radical fluorosulfonylation of vinyl triflates. | ||
Due to the inherent characteristics of gaseous FSO2Cl, including inconvenience in storage and handling as well as moisture sensitiveness, the development of new and practical fluorosulfonyl radical precursors to access sulfonyl fluorides undoubtedly will be more appealing. In this context, Liao and co-workers recently introduced solid state 1-fluorosulfonyl benzoimidazolium triflate (FABI) salts 30 as a convenient and effective redox-active fluorosulfonyl radical precursor (Scheme 5).15 Compared with the known FSO2Cl, this reagent was bench-stable and easy to handle, enabling the radical fluorosulfonylation of alkenes with high efficiency. A wide range of electron-rich, cyclic, di- and trisubstituted olefins were compatible with FABI, which fully demonstrated its superiority over FSO2Cl. Additionally, the photoredox-catalyzed alkoxy-fluorosulfonylation of alkenes with FABI was also developed. By employing suitable alcohols and acids as nucleophiles, the corresponding β-alkoxyl sulfonyl fluorides could be obtained in moderate to good yields. On the basis of the experimental results, a plausible mechanism for this reaction was proposed as shown in Scheme 5. It was reasoned that the redox-active radical precursor 30 underwent single electron transfer by the excited Ir(III)* to give neutral radical 34, which then converted to fluorosulfonyl radical 4 through a homolytic cleavage of the N–S bond. Addition of fluorosulfonyl radical 4 to alkene substrate 29 would produce a new carbon radical 35, which underwent single electron oxidation by Ir(IV) to form carbon cation intermediate 36 and regenerate ground-state Ir(III) to close the photocatalytic cycle. Ultimately, the carbon cation could either be deprotonated or trapped by oxygen nucleophiles to furnish the corresponding product.
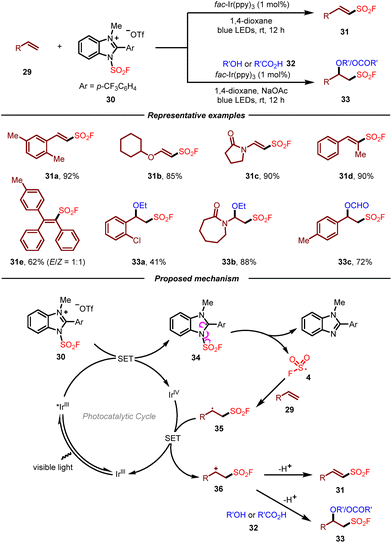 | ||
| Scheme 5 Photoredox-catalyzed radical fluorosulfonylation of alkenes with a benzoimidazolium-based fluorosulfonyl radical precursor. | ||
At the same time, Wang and co-workers reported the same air- and bench-stable benzoimidazolium fluorosulfonate reagent from their previous work on the chemistry of imidazolium sulfonate cationic salts,16 which successfully applied to the radical fluorosulfonylation of unsaturated hydrocarbons (Scheme 6).17 Under suitable optimized photocatalytic conditions, this redox-active fluorosulfonyl radical precursor could react with various alkenes smoothly, providing alkenyl sulfonyl fluoride, alkylsulfonyl fluoride, and migratory fluorosulfonylating products in good yields with high stereoselectivity. This operationally simple protocol enabled the efficient introduction of the FSO2 group into a wide range of complex molecules derived from natural products and drugs. The combination of radical trapping and radical clock experiments demonstrated the generation of the fluorosulfonyl radical during the reaction.
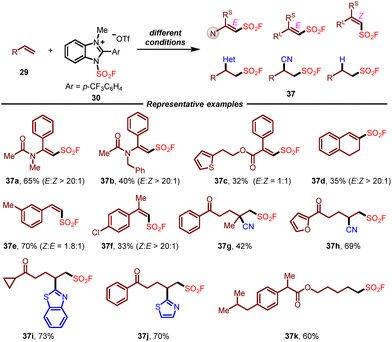 | ||
| Scheme 6 Synthesis of alkenyl and alkyl sulfonyl fluorides with a benzoimidazolium-based fluorosulfonyl radical precursor. | ||
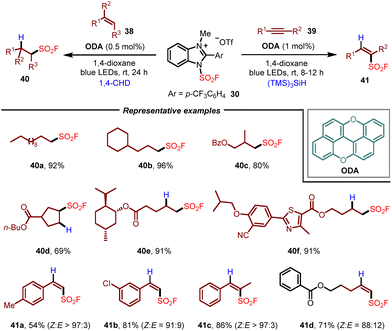
Soon after, using the same 1-fluorosulfonyl benzoimidazolium triflate salt as the fluorosulfonyl radical precursor, the Liao group further developed a radical hydro-fluorosulfonylation of alkenes by employing oxygen-doped anthanthrene (ODA) as the photocatalyst and 1,4-cyclohexadiene (1,4-CHD) as a hydrogen donor (Scheme 7).18 This method enabled the synthesis of various aliphatic sulfonyl fluorides 40 under mild and metal-free conditions, which could be applied to the late-stage functionalization of natural products, peptides and drugs. Moreover, with (TMS)3SiH as a hydrogen donor, the application of this photocatalytic system to the radical hydro-fluorosulfonylation of alkynes was also demonstrated, allowing the formation of alkenylsulfonyl fluorides 41 with high Z-selectivity.
Given the similar bifunctional radical reactivity between alkynyl sulfonyl fluorides and alkynyl triflones, Studer and co-workers recently disclosed an efficient transition-metal-free radical 1,2-difunctionalization of unactivated alkenes to access various aliphatic β-alkynylfluorosulfonylalkanes (Scheme 8).19 Notably, alkynyl sulfonyl fluorides could not only be used as highly valuable bifunctional radical trapping reagents but also as the fluorosulfonyl radical precursor. The successful diversification of products with SuFEx click chemistry to sulfonates and sulfonamides also highlighted the practicality of this method. Mechanistically, it was proposed that the reaction was initiated by AIBN to give AIBN-derived cyanopropyl radical 45. Following the addition to alkyne 43 and radical fragmentation sequence, fluorosulfonyl radical 4 would be generated, which would undergo addition to alkene 42 leading to radical intermediate 46. This radical intermediate 46 could be trapped by alkynyl sulfonyl fluoride 43 giving rise to vinyl radical 47, which underwent β-fragmentation to provide the desired product 44 with the regeneration of fluorosulfonyl radical 4.
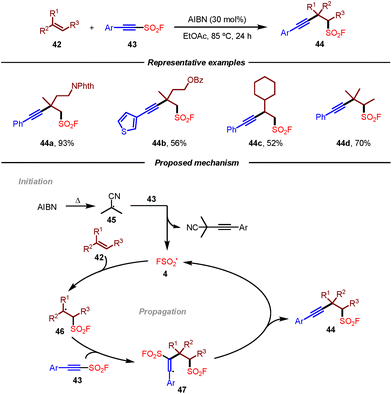 | ||
| Scheme 8 Radical difunctionalization of unactivated alkenes to access β-alkynyl-fluorosulfonylalkanes. | ||
Very recently, Li, Su, and co-workers developed a photocatalytic procedure for the preparation of β-keto sulfonyl fluorides from vinyl fluorosulfates (Scheme 9).20 This methodology featured sustainable conditions and a broad substrate scope, affording aromatic β-keto sulfonyl fluorides in moderate to good yields by using Ir[dF(CF3)ppy]2(dtbbpy)(PF6) (1 mol%) as a photocatalyst under irradiation of 3 W blue LEDs. According to the mechanistic results, two plausible pathways for this photocatalytic transformation of vinyl fluorosulfates were proposed as shown in Scheme 9. In path A, the Ir(III) complex was excited under visible light irradiation to form Ir(III)*, which would then undergo energy transfer with vinyl fluorosulfate 48 to generate enol radical 50 and fluorosulfonyl radical 4. Subsequently, the corresponding product 49 would be produced through recombination of these two radical species. The authors also suggested another plausible pathway through which the excited Ir(III)* and vinyl fluorosulfate 48 would undergo SET to afford Ir(IV) and a radical anion intermediate 51. Following radical fragmentation/reconstruction and SET oxidation, product 49 would be formed (path B).
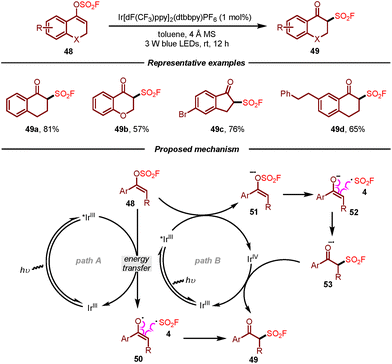 | ||
| Scheme 9 Synthesis of β-keto sulfonyl fluorides via photocatalytic transformation of vinyl fluorosulfates. | ||
The direct fluorosulfonylation process involving the fluorosulfonyl radical has appeared as an attractive strategy for the rapid assembly of sulfonyl fluorides. In this highlight, the most recent progress in this aspect by utilizing different fluorosulfonyl radical precursors is discussed. Specifically, four types of radical fluorosulfonylating reagents including FSO2Cl, benzoimidazolium fluorosulfonates, alkynyl sulfonyl fluorides and vinyl fluorosulfates have been developed and applied to the efficient construction of diverse functionalized sulfonyl fluorides. These achievements would offer new opportunities for SuFEx click chemistry, accelerating the applications of sulfonyl fluorides in chemical biology and drug discovery. There are still some challenges remaining in this field. First, the substrate scope of radical acceptors in the above-mentioned reactions is relatively narrow, which is mostly restricted to alkenes and alkynes. Second, the fluorosulfonyl radical precursors are still limited to the above four types. The development of novel and efficient radical precursors would provide new approaches for the construction of sulfonyl fluorides in a more sustainable way. Therefore, this highly reactive intermediate is worthy of further investigation. Besides that, enantioselective radical fluorosulfonylation involving the fluorosulfonyl radical to deliver chiral sulfonyl fluorides remains undeveloped. The exploration of enantioselective radical flurosulfonylation is highly in demand. Given these challenges and opportunities, further progress can be expected in the near future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 22101199), the Natural Science Foundation of Zhejiang Province (LQ21B020002), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005), and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD04) is gratefully acknowledged.References
- (a) A. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dong and J. E. Moses, The growing applications of SuFEx click chemistry, Chem. Soc. Rev., 2019, 48, 4731–4758 RSC; (b) C. Lee, A. J. Cook, J. E. Elisabeth, N. C. Friede, G. M. Sammis and N. D. Ball, The Emerging Applications of Sulfur(VI) Fluorides in Catalysis, ACS. Catal., 2021, 11, 6578–6589 CrossRef CAS PubMed; (c) T. S.-B. Lou and M. C. Willis, Sulfonyl fluorides as targets and substrates in the development of new synthetic methods, Nat. Rev. Chem., 2022, 6, 146–162 CrossRef CAS.
- J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry, Angew. Chem., Int. Ed., 2014, 53, 9430–9448 CrossRef CAS PubMed.
- (a) L. Xu and J. Dong, Click Chemistry: Evolving on the Fringe, Chin. J. Chem., 2020, 38, 414–419 CrossRef CAS; (b) Q. Zheng, J. Dong and K. B. Sharpless, Ethenesulfonyl Fluoride (ESF): An On-Water Procedure for the Kilogram-Scale Preparation, J. Org. Chem., 2016, 81, 11360–11362 CrossRef CAS PubMed.
- (a) J. Chen, B.-Q. Huang, Z.-Q. Wang, X.-J. Zhang and M. Yan, Asymmetric Conjugate Addition of Ethylene Sulfonyl Fluorides to 3-Amido-2-oxindoles: Synthesis of Chiral Spirocyclic Oxindole Sultams, Org. Lett., 2019, 21, 9742–9746 CrossRef CAS PubMed; (b) J. Leng and H.-L. Qin, 1-Bromoethene-1-sulfonyl fluoride (1-Br-ESF), a new SuFEx clickable reagent, and its application for regioselective construction of 5-sulfonylfluoro isoxazoles, Chem. Commun., 2018, 54, 4477–4480 RSC; (c) R. Xu, T. Xu, M. Yang, T. Cao and S. Liao, A rapid access to aliphatic sulfonyl fluorides, Nat. Commun., 2019, 10, 3752 CrossRef PubMed; (d) X. Zhang, W.-Y. Fang, R. Lekkala, W. Tang and H.-L. Qin, An Easy, General and Practical Method for the Construction of Alkyl Sulfonyl Fluorides, Adv. Synth. Catal., 2020, 362, 3358–3363 CrossRef CAS.
- (a) L. Wang and J. Cornella, A Unified Strategy for Arylsulfur(VI) Fluorides from Aryl Halides: Access to Ar-SOF3 Compounds, Angew. Chem., Int. Ed., 2020, 59, 23510–23515 CrossRef CAS PubMed; (b) S. W. Wright and K. N. Hallstrom, A Convenient Preparation of Heteroaryl Sulfonamides and Sulfonyl Fluorides from Heteroaryl Thiols, J. Org. Chem., 2006, 71, 1080–1084 CrossRef CAS PubMed; (c) L. Zhang, X. Cheng and Q.-L. Zhou, Electrochemical Synthesis of Sulfonyl Fluorides with Triethylamine Hydrofluoride, Chin. J. Chem., 2022, 40, 1687–1692 CrossRef CAS.
- (a) G. Laudadio, A. de A. Bartolomeu, L. M. H. M. Verwijlen, Y. Cao, K. T. de Oliveira and T. Noël, Sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride, J. Am. Chem. Soc., 2019, 141, 11832–11836 CrossRef CAS; (b) A. Shavnya, S. B. Coffey, K. D. Hesp, S. C. Ross and A. S. Tsai, Reaction of Alkyl Halides with Rongalite: One-Pot and Telescoped Syntheses of Aliphatic Sulfonamides, Sulfonyl Fluorides, and Unsymmetrical Sulfones, Org. Lett., 2016, 18, 5848–5851 CrossRef CAS PubMed; (c) A. Shavnya, K. D. Hesp and A. S. Tsai, A Versatile Reagent and Method for Direct Aliphatic Sulfonylation, Adv. Synth. Catal., 2018, 360, 1768–1774 CrossRef CAS.
- (a) Y. Liu, D. Yu, Y. Guo, J. C. Xiao, Q. Y. Chen and C. Liu, Arenesulfonyl Fluoride Synthesis via Copper-Catalyzed Fluorosulfonylation of Arenediazonium Salts, Org. Lett., 2020, 22, 2281–2286 CrossRef CAS PubMed; (b) T. Zhong, J. Yi, Z. Chen, Q. Zhuang, Y. Li, G. Lu and J. Weng, Photoredoxcatalyzed Aminofluorosulfonylation of Unactivated Olefins, Chem. Sci., 2021, 7, 9359–9365 RSC; (c) P. J. Sarver, N. B. Bissonnette and D. W. C. MacMillan, Decatungstate-Catalyzed C(sp3)–H Sulfinylation: Rapid Access to Diverse Organosulfur Functionality, J. Am. Chem. Soc., 2021, 143, 9737–9743 CrossRef CAS PubMed; (d) Z. Ma, Y. Liu, X. Ma, X. Hu, Y. Guo, Q.-Y. Chen and C. Liu, Aliphatic sulfonyl fluoride synthesis via reductive decarboxylative fluorosulfonylation of aliphatic carboxylic acid NHPI esters, Org. Chem. Front., 2022, 9, 1115–1120 RSC; (e) V. T. Nguyen, G. C. Haug, V. D. Nguyen, N. T. H. Vuong, G. B. Karki, H. D. Arman and O. V. Larionov, Functional group divergence and the structural basis of acridine photocatalysis revealed by direct decarboxysulfonylation, Chem. Sci., 2022, 13, 4170–4179 RSC.
- (a) T. Guo, G. Meng, X. Zhan, Q. Yang, T. Ma, L. Xu, K. B. Sharpless and J. Dong, A New Portal to SuFEx Click Chemistry: A Stable Fluorosulfuryl Imidazolium Salt Emerging as an “F–SO2+” Donor of Unprecedented Reactivity, Selectivity, and Scope, Angew. Chem., Int. Ed., 2018, 57, 2605–2610 CrossRef CAS; (b) H. Zhou, P. Mukherjee, R. Liu, E. Evrard, D. Wang, J. M. Humphrey, T. W. Butler, L. R. Hoth, J. B. Sperry, S. K. Sakata, C. J. Helal and C. W. Am Ende, Introduction of a Crystalline, Shelf-Stable Reagent for the Synthesis of Sulfur(VI) Fluorides, Org. Lett., 2018, 20, 812–815 CrossRef CAS PubMed.
- X. Q. Zeng, H. Beckers and H. Willner, Thermally Persistent Fluorosulfonyl Nitrene and Unexpected Formation of the Fluorosulfonyl Radical, J. Am. Chem. Soc., 2013, 135, 2096–2099 CrossRef CAS PubMed.
- X. Nie, T. Xu, J. Song, A. Devaraj, B. Zhang, Y. Chen and S. Liao, Radical Fluorosulfonylation: Accessing Alkenyl Sulfonyl Fluorides from Alkenes, Angew. Chem., Int. Ed., 2021, 60, 3956–3960 CrossRef CAS PubMed.
- X. Nie and S. Liao, Radical Fluorosulfonylation: Accessing Alkenylsulfonyl Fluorides from Alkenes and Alkynes, Synlett, 2022, 401–408 CAS.
- X. Nie, T. Xu, Y. Hong, H. Zhang, C. Mao and S. Liao, Introducing A New Class of Sulfonyl Fluoride Hubs via Radical Chloro-Fluorosulfonylation of Alkynes, Angew. Chem., Int. Ed., 2021, 60, 22035–22042 CrossRef CAS PubMed.
- D. Chen, X. Nie, Q. Feng, Y. Zhang, Y. Wang, Q. Wang, L. Huang, S. Huang and S. Liao, Electrochemical Oxo-Fluorosulfonylation of Alkynes under Air: Facile Access to β-Keto Sulfonyl Fluorides, Angew. Chem., Int. Ed., 2021, 60, 27271–27276 CrossRef CAS PubMed.
- Q. Feng, Y. Fu, Y. Zheng, S. Liao and S. Huang, Electrochemical Synthesis of β-Keto Sulfonyl Fluorides via Radical Fluorosulfonylation of Vinyl Triflates, Org. Lett., 2022, 24, 3702–3706 CrossRef CAS.
- P. Wang, H. Zhang, X. Nie, T. Xu and S. Liao, Photoredox catalytic radical fluorosulfonylation of olefins enabled by a bench-stable redox-active fluorosulfonyl radical precursor, Nat. Commun., 2022, 13, 3370 CrossRef CAS.
- W. Zhang, Z. Zou, W. Zhao, S. Lu, Z. Wu, M. Huang, X. Wang, Y. Wang, Y. Liang, Y. Zhu, Y. Zheng and Y. Pan, Integrated redox-active reagents for photoinduced regio- and stereoselective fluorocarboborylation, Nat. Commun., 2020, 11, 2572 CrossRef CAS PubMed.
- W. Zhang, H. Li, X. Li, Z. Zou, M. Huang, J. Liu, X. Wang, S. Ni, Y. Pan and Y. Wang, A practical fluorosulfonylating platform via photocatalytic imidazolium-based SO2F radical reagent, Nat. Commun., 2022, 13, 3515 CrossRef CAS.
- P. Wang, H. Zhang, M. Zhao, S. Ji, L. Lin, N. Yang, X. Nie, J. Song and S. Liao, Radical Hydro-Fluorosulfonylation of Unactivated Alkenes and Alkynes, Angew. Chem., Int. Ed., 2022, 61, e202207684 Search PubMed.
- N. L. Frye, C. G. Daniliuc and A. Studer, Radical 1-Fluorosulfonyl-2-alkynylation of Unactivated Alkenes, Angew. Chem., Int. Ed., 2022, 61, e202115593 CAS.
- J. Cui, S. Ke, J. Zhao, S. Wu, W. Luo, S. Xu, X. Su and Y. Li, Photocatalytic access to aromatic keto sulfonyl fluorides from vinyl fluorosulfates, Org. Chem. Front., 2022, 9, 3540–3545 RSC.
| This journal is © the Partner Organisations 2022 |